Abstract
The 22q11.2 deletion syndrome (22q11.2 DS) places affected individuals at an increased risk for neurodevelopmental/cognitive, behavioral and social–emotional difficulties. Poor cognitive functioning and intellectual disabilities, attention and executive functioning deficits, learning disorders, emotional dysregulation and impairments in social processing are common among individuals with 22q11.2 DS. Identifying risk and protective/resilience factors that can be detected in early life and can predict neurodevelopmental outcomes for people with 22q11.2 DS is of significant clinical relevance and might allow for early detection and intervention. Given the focus of this review, we will discuss the possible contributing factors that influence the neurodevelopmental outcome in 22q1.2 DS, the cognitive phenotype in 22q11.2 DS, the different developmental trajectories across life span, and the implications for clinical practice and management.
Keywords: 22q11.2 DS, contributing factors, neurodevelopmental outcome, practice and management
1 | INTRODUCTION
Chromosome 22q11.2 deletion syndrome (22q11.2 DS), a neurogenetic condition, is the most common microdeletion syndrome estimated to affect about 1 in 2,000 live births (McDonald-McGinn et al., 2015) and involving haploinsufficiency of ~50 genes resulting in a multi-system disorder. Phenotypic expression is highly variable and ranges from severe life-threatening conditions to only a few associated features. Most common medical problems include congenital heart disease (CHD) (in particular conotruncal anomalies), palatal abnormalities (most frequently velopharyngeal incompetence), immunodeficiency, hypocalcemia due to hypoparathyroidism, severe feeding/gastrointestinal differences, and subtle dysmorphic facial features (McDonald-McGinn & Sullivan, 2011; Philip & Bassett, 2011). This wide phenotypic variability and the considerable morbidity associated with 22q11.2 DS poses significant challenges for both individual and population-based healthcare management. In light of these challenges, the International 22q11.2 DS Consortium has developed practical guidelines for managing patients with 22q11.2 DS that emphasizes the multi-system nature of the condition and includes recommendations for assessment and management (Bassett et al., 2011; Fung et al., 2015).
Not only medical issues but also developmental/educational and behavioral/psychiatric aspects of 22q11.2 DS are major concerns for most families with a child/adolescent/adult with 22q11.2 DS (Swillen & McDonald-McGinn, 2015). The syndrome places individuals at an increased risk for neurodevelopmental/cognitive, behavioral, and social–emotional difficulties. Deficits common in 22q11.2 DS include poor cognitive functioning and intellectual disabilities, attention and executive functioning deficits, learning disorders, emotional dysregulation and impairments in social processing. Early detection of emerging problems will allow for early intervention. The ability to identify risk and protective/resilience factors predictive of neurodevelopmental outcomes in 22q11.2 DS is therefore of obvious clinical relevance (Swillen, 2016).
2 | FACTORS CONTRIBUTING TO THE NEURODEVELOPMENTAL OUTCOME IN 22Q11.2 DS
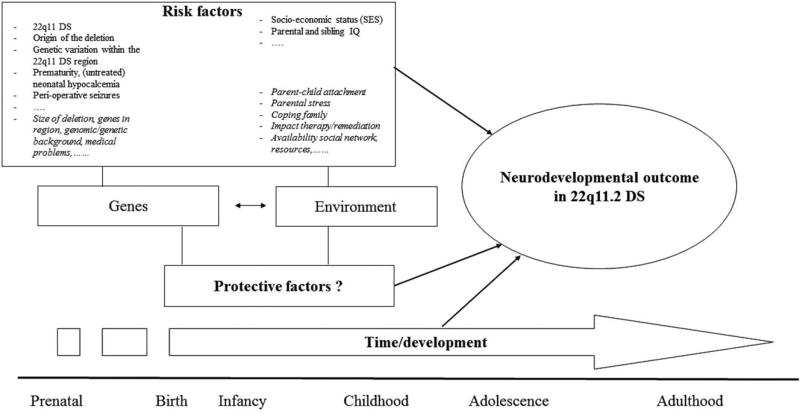
Neurodevelopmental outcome in 22q11.2 DS is the result of the dynamic interaction of multiple factors: person-specific risk and protective factors, family and environmental risk and protective factors, and time/development itself (see Figure 1). These factors act as independent, cumulative, and synergistic influences.
FIGURE 1.
Possible factors contributing to neurodevelopmental outcome in 22q11.2 DS. Neurodevelopmental outcome in 22q11.2 DS is the result of the dynamic interaction of multiple factors: person-specific risk and protective factors, family and environmental risk and protective factors, and time/development itself. These factors act as independent, cumulative and synergistic influences
Known person-specific risk factors with implications for brain development and neurodevelopmental outcome in 22q11.2 DS include having the 22q11.2 deletion syndrome (by definition), origin of the deletion (inherited deletions may result in a more-severe cognitive phenotype, related to a combination of socio-economic factors and heritable components contributed by the unaffected parent) (De Smedt, 2007; Swillen et al., 1997), genetic variation within the 22q11.2 region (Gothelf et al., 2005; Raux et al., 2007), variants in genes on the intact 22q11.2 (McDonald-McGinn et al., 2013), prematurity, untreated neonatal hypocalcemia (Cheung, George, & Andrade, 2014), perioperative seizures (McDonald-McGinn et al., 2015) and undetected and untreated thyroid disease in children with 22q11 DS (Shugar et al., 2015). However, much work is still to be done in identifying other possible risk factors contributing to the neurodevelopmental outcome in 22q11.2 DS such as size of the deletion, genes within the region (COMT, PRODH, TBX1, CRKL1, etc.), the remainder of the genome/genetic background, impact of medical problems (e.g. type and severity of CHD, number of hospitalizations, medical stress, ands preverbal trauma), personality and temperament, the level of stress sensitivity and anxiety, and the presence of developmental disorders such as intellectual disability (ID), attention hyperactivity disorder, autism spectrum disorder (ASD), and psychosis.
Family and environmental factors contributing to the neurodevelopmental outcome in 22q11.2 DS include socio-economic status (De Smedt, 2007; Shashi et al., 2010) and parental and sibling IQ (Olszewski, Radoeva, Fremont, Kates, & Antshel, 2014). As of yet, many risk and protective environmental factors are understudied within the context of 22q11.2 DS such as parent–child interaction (attachment), parental stress/resilience, parenting style and coping the impact of therapy/remediation/anticipatory guidance, quality of life, availability of social network support and resources.
Finally, time/development itself has its impact, and contributes to the changing and mixed neurodevelopmental outcome in individuals with 22q11.2 DS (Swillen, 2016; Swillen & McDonald-McGinn, 2015).
3 | COGNITIVE DEVELOPMENT IN 22Q11.2 DS
One of the first and most important questions parents and caregivers of children with 22q11.2 DS ask is what the impact of the 22q11.2 deletion will be on the global cognitive development. Knowledge of the cognitive capacities of a child are of great clinical relevance since it plays a key role when planning intervention and re-evaluating an individualized educational plan (IEP). In this respect, it is important to keep in mind that both genes and environmental factors play essential roles in shaping brain development and growth and neurodevelopmental outcome throughout life; in the end each infant/child/adolescent/adult with 22q11.2 DS is unique (Swillen, 2016).
3.1 | Infancy and early childhood (0–4 years)
Very few studies have been published on developmental outcome in young children with 22q11.2 DS. During infancy and toddlerhood, gross/fine and neuromotor difficulties, expressive language delays and speech problems dominate (Gerdes et al., 1999; Solot et al., 2001; Swillen et al., 2005). Roizen et al. (2007) reported retrospective data from 88 parents with a child with 22q11.2 DS about developmental milestones. Compared to sibling and community control participants, expressive language and gross motor milestones were more delayed than other areas of development. Since all above-mentioned studies were cross-sectional and included small samples, there is an urgent need for prospective, longitudinal studies using large samples on early neurodevelopmental outcome in 22q11.2 DS.
3.2 | From preschool to adolescence (4–18 years)
The majority of studies have focused on childhood and adolescence, and very little is known on the intellectual functioning of adults with 22q11.2 DS (Henry et al., 2002; Van Amelsvoort et al., 2004). The (neuro)cognitive phenotype in 22q11 DS is variable, mixed and complex. In childhood and early adolescence borderline intellectual function (IQ 75–85) and mild ID (IQ 55–75) is quite common (De Smedt, 2007; Swillen et al., 1997). A small percentage of children and adolescents fall into the low average intelligence range (IQ 85–100), but also moderate to severe ID (35–55) is possible. Thus, learning difficulties are very common in preschool and primary school, especially within the domains of mathematics (De Smedt et al., 2009a, 2009b; Tobia, Brigstocke, Hulme, & Snowling, 2017; Wang, Woodin, Kreps-Falk, & Moss, 2000) and language comprehension (Glaser et al., 2002; Van den Heuvel et al., 2016). Cognitive deficits are seen in the majority (90–100%) of individuals with 22q11DS with impairments in sustained attention, executive function, memory, and visual-spatial perception (Antshel, Fremont, & Kates, 2008; Campbell & Swillen, 2005; Gur et al., 2014). In a recent cross-sectional study (Gur et al., 2014), the cognitive functions of 137 subjects (aged 8–21) were compared with youth with a developmental delay and medical comorbidities and with typically developing controls. Greatest deficits were observed in the domains of complex cognition (verbal language-mediated reasoning, non-verbal reasoning, and spatial processing). These impairments do continue into adulthood, with challenges in the transition to adulthood including the management of medical and psychiatric comorbidities (Bassett et al., 2005), and the provision of educational and vocational supports (Butcher et al., 2012; Fung et al., 2015).
4 | DIVERGENT COGNITIVE TRAJECTORIES IN 22Q11.2 DS
Longitudinal studies in 22q11.2 DS have found a negative correlation between age and IQ scores, particularly a decline in Verbal IQ, suggesting that at least some of these individuals show a gradual decline in cognitive development as they grow into adulthood (Gothelf et al., 2005; Green et al., 2009). Recent longitudinal studies indicate that cognitive development is variable with divergent trajectories (Duijff et al., 2012; Green et al., 2009) and that the level of intellectual ability is not necessarily stable across the lifespan of the patient (Chawner et al., 2017; Swillen & McDonald-McGinn, 2015). Although IQ is generally considered to be a more or less stable trait in the typically developing youth, an average decline of 7 full-scale IQ points is observed in individuals with 22q11.2DS between 8 and 24 years of age (Vorstman et al., 2015). However, there seem to be different subgroups within the 22q11.2 DS population with some children’s IQ decreasing over time, while others seem to make progress (Chawner et al., 2017; Duijff et al., 2012; Swillen, 2016).
5 | IMPLICATIONS FOR CLINICAL PRACTICE
Although there are some guidelines and recommendations relevant for all patients, treatment must be targeted to best suit the individual, incorporating age or developmental stage, and the specific constellation of associated features, severity, and need for treatment (Bassett et al., 2011; Fung et al., 2015). Given the increased risk for impaired neurodevelopmental outcome and deficits in several neuropsychological domains (such as attention, executive functions, visual-spatial abilities, and emotion recognition) early neurodevelopmental follow-up and intervention is warranted for individuals with 22q11.2 DS. It will be key to find a balance between follow-up and intervention, and to monitor in a flexible way the changing and increasing environmental demands with age.
Because of the delays in many developmental domains “a holistic and multidisciplinary approach” is required in follow-up and in intervention programs. This means that different caregivers (parents, teachers) and therapists from different disciplines (pediatrician, speech-language pathologist, physiotherapist and/or occupational therapist and early childhood specialists) should work together to integrate findings. Management of 22q11.2 DS requires an individualized, multidisciplinary and coordinated care plan that takes into account the associated features of the individual. This comprehensive care is provided in multidisciplinary 22q11.2 DS clinics. Because of the complexity of 22q11DS in many cases, when geographically and economically feasible, we recommend that all affected individuals be evaluated periodically at a comprehensive care center.
Neuro-motor deficits, especially in the domains of balance and coordination, occur early and require remediation (Sobin, Monk, Kiley-Brabeck, Khuri, & Karayiorgou, 2006; Van Aken, Caeyenberghs, Smits-Engelsman, & Swillen, 2009). Awareness of the neurodevelopmental, cognitive and social features in 22q11 DS is crucial to avoid situations in which the environmental expectations exceed the abilities of the child. Beside this awareness and the stimulation of the child’s development in different areas, it is also very important to pay attention to the parent–child interaction/relation. The early attachment between parent and child with a 22q11.2 DS can be complicated or disturbed by the major medical and developmental problems these children experience during their first months (years) of life. Feeding problems, frequent hospital appointments and possible hospitalizations, underlying developmental disorders such as ASD or attention deficit hyperactivity disorder can all result in a high burden for parents and/or children with 22q11.2 DS (Mercer-Rosa et al., 2015). Acknowledging this burden and supporting parents to talk about this is crucial. In our experience parents often benefit from psychological guidance, interventions aimed at the parent/child interaction and contact with other parents.
In Table 1, a summary is given of the developmental features and treatment recommendations in 22q11.2 DS during infancy and early childhood (0–6 years).
TABLE 1.
Treatment recommendations for improving neurodevelopmental outcome in 22q11 DS during infancy and early childhood (0–6y)
| Developmental area | Developmental features | Treatment recommendations |
|---|---|---|
| Motor development | Hypotonia and neuromotor deficits | Physiotherapy, occupational therapy, and sensory integration therapy from early age on |
|
| ||
| Feeding | Poor sucking, nasal reflux, and oral motor coordination problems | Medical guidance/monitoring of feeding problems Feeding advice (feeding specialist with expertise in 22q11 DS) |
|
| ||
| Speech and language | Impaired speech and language development, hypernasality, high-pitched voice, and compensatory speech | Speech and language therapy, total communication approach (verbal, non-verbal, and sign language in combination with oral speech) (Solot et al., 2001) |
| In the case of severe hypernasality, a pharyngoplasty is sometimes required | ||
|
| ||
| Neurodevelopment/Cognitive development | Varying degree of impairment (from borderline development to mild–moderate ID) | Educational monitoring |
| Early childhood specialist | ||
| Anticipatory guidance | ||
|
| ||
| Social–emotional development and social skills | Emotionally reactive | Provide a secure and highly structured environment |
| Problems with regulation of emotion and behavior | Infant mental health intervention | |
| Socially withdrawn, poor peer relations, self-directed behavior | Play therapy (structured play to promote social play) | |
| Social anxiety and general anxieties | Structured (social) group experience | |
|
| ||
| Attention | Easily distracted, impulsiveness | Structured (learning) environment |
| Environment free from stimuli | ||
| Use visual aids to improve sustained attention (sand timer; time-timer, etc.) | ||
Type of education should be chosen depending on the overall cognitive capacities (borderline intelligence vs. ID) of primary-aged children and adolescents with 22q11.2 DS. In some instances, additional learning and educational support (starting from an IEP) will suffice, in other situations children are better off following special education with IEPs that are adapted to the individual needs of the child/adolescent. Implementation of the IEP, and tight control over the quality of service delivery are critical. Besides, a global intellectual delay and slow maturing, many children and adolescents with the 22q11.2 DS show a cognitive profile of strengths and weaknesses. Typically, areas of relative strengths are reading (decoding), spelling, and (auditory/verbal) rote memory (Antshel et al., 2008; Campbell & Swillen, 2005; Moss et al., 1999). Areas of relative weaknesses are reading and language comprehension, arithmetics, visual-spatial memory, working memory, and executive skills (planning, problem-solving, cognitive flexibility, and monitoring) (Antshel et al., 2008; Bearden et al., 2001; Campbell & Swillen, 2005; De Smedt, Swillen, Verschaffel, and Ghesquiere, 2009b; Moss et al., 1999; Simon, Bearden, Mc-Ginn, and Zackai, 2005; Stoddard, Beckett, and Simon, 2011; Wong, Riggins, Harvey, Cabaral, and Simon, 2014; Woodin et al., 2001).
It is our clinical experience that many children with 22q11.2 DS are helped with:
a highly structured learning environment,
the utilization of concrete (visual) materials and experiences,
a step-by-step approach with much repetition and rehearsal,
an encouraging and reinforcing learning environment with clear learning goals and frequent feedback, and
instructions on how to learn and how to memorize (with visual aids and schemes) and pre-teaching (for learning new material).
Given the changing cognitive phenotype with age and the possible cognitive decline in a subgroup, the cognitive abilities of children and adolescents should be followed-up and re-evaluated on a regular basis. Additionally, in case of a changing developmental/cognitive trajectory, realistic expectations and an adapted learning environment will be necessary to provide a good balance between the individual’s capacities and the environmental demands. In this way, anticipatory guidance can be implemented at home and in school thereby averting unnecessary stress. The emergence of social deficits by the end of primary school age, can represent a major source of disability in individuals with 22q11.2 DS. Awareness for and psychoeducation about these issues for parents and school teams is recommended. Interventions should focus on appropriate adaptation of social demands by use of sociocognitive remediation programs (Glaser et al., 2012, Mariano, Tang, Kurtz, & Kates, 2015) and/or cognitive-behavioral therapy to improve social skills (Mariano et al., 2015), although studies are needed on the long-term effects of these interventions. As part of anticipatory care, individuals with 22q11.2 DS should be screened for social processing deficits and anxiety and mood disorders throughout the lifetime.
6 | CONCLUSION
There is a wide variability in cognitive abilities and profile in children and adolescents with 22q11.2 DS ranging from borderline intelligence to mild–moderate ID. This profile is often colored by a complex associated medical phenotype which frequently results in multiple hospitalizations beginning at an early age. It is clear that appropriate neurodevelopmental and educational support from a very young age on is critical to children affected with this condition. Children with a parent who also has 22q11.2DS are at greater risk for a poorer long-term outcome, and these families need more intense support and follow-up throughout life (Swillen & McDonald-McGinn, 2015).
In this context, many risk and protective environmental factors are still understudied in 22q11.2 DS. Future studies should focus on parent–child interaction (attachment), parental stress/resilience, parenting style and coping, the impact of therapy/remediation/anticipatory guidance, quality of life, availability of social network support and resources.
Early educational intervention is strongly recommended, and should include training of both verbal (language, articulation, and reading comprehension) and non-verbal areas (motor skills, visual-spatial skills and memory, mathematical training, attention, and social skills). Educational and neurodevelopmental specialists are important members of the professional multidisciplinary team which provides services to a child with 22q11.2 DS. Recognizing areas of strengths and weaknesses may help to guide educational approaches and identify additional resources that can effectively support the learning and neurodevelopmental process. Additionally, a better understanding of the challenges, these families face and the underlying neuropsychological processes, will lead to the development of appropriate and targeted intervention and proactive treatment that will help children, adolescents, and adults achieve their full potential.
Acknowledgments
Funding information
International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome, Grant/Award Number: 5U01MH101722-04
Biographies

A. Swillen is professor at the Department of Human Genetics, KU Leuven and at the Department of Rehabilitation Sciences, KU Leuven (University of Leuven, Belgium). Trained as an educational psychologist, she is also affiliated to the Center of Human Genetics Leuven (University Hospital Gasthuisberg), an international center of excellence in the field of clinical and molecular genetics. She has more than 25 years of clinical expertise and research in 22q11.2 DS. She can be reached by ann.swillen@uzleuven.be.

E. Moss is the former Director of Outpatient Neuropsychology at The Children’s Hospital of Philadelphia (CHOP), and is now in independent research and clinical practice. He has been working with individuals affected with 22q11.2 DS for over 20 years, through the Department of Clinical Genetics at CHOP. kidbrains@gmail.com.

S. Duijff is a pediatric psychologist at the Wilhelmina Children’s Hospital/University Medical Centre Utrecht (UMCU). She also works as a researcher within the 22q11DS lab of the Department of Psychiatry, UMCU in The Netherlands. She is one of the founding members of the Dutch multidisciplinary 22q11.2DS expertise outpatient clinic in Utrecht and in the past 15 years has gained a strong expertise in the diagnostic assessment of children, adolescents and young adults with 22q11.2DS with a special interest in infant mental health. s.duijff@umcutrecht.nl.
Footnotes
CONFLICT OF INTEREST
None.
References
- Antshel KM, Fremont W, Kates WR. The neurocognitive phenotype in velo-cardio-facial syndrome: A developmental perspective. Developmental Disabilities Research Reviews. 2008;14(1):43–51. doi: 10.1002/ddrr.7. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, Husted J, Weksberg R, Caluseriu O, Webb GD, Gatzoulis MA. Clinical features of 78 adults with 22q11 deletion syndrome. American Journal of Medical Genetics Part A. 2005;138(4):307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, Vorstman J. International 22q11.2 deletion syndrome consortium. Practical guidelines for managing patients with 22q11.2 deletion syndrome. The Journal of Pediatrics. 2011;159(2):332–339. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, Cannon TD. The neurocognitive phenotype of the 22q11.2 deletion syndrome: Selective deficit in visual-spatial memory. Journal of Clinical and Experimental Neuropsychology. 2001;23(4):447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Butcher NJ, Chow EW, Costain G, Karas D, Ho A, Bassett AS. Genetics in Medicine. 2012;14(10):836–43. doi: 10.1038/gim.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Swillen A. The cognitive spectrum in velo-cardio-facial syndrome. In: Murphy KC, Scambler PJ, editors. Velo-cardio-facial syndrome. Cambridge, MA: Cambridge University Press; 2005. pp. 147–164. [Google Scholar]
- Chawner SJ, Doherty JL, Moss H, Niarchou M, Walters JTR, Owen MJ, van den Bree MBM. Childhood cognitive development in 22q11.2 deletion syndrome: Case-control study. The British Journal of Psychiatry. 2017;211(4):223–230. doi: 10.1192/bjp.bp.116.195651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EN, George SR, Andrade DM. Neonatal hypocalcemia, neonatal seizures, and intellectual disability in 22q11.2 deletion syndrome. Genetics in Medicine. 2014;16:40–44. doi: 10.1038/gim.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt B. Intellectual abilities in a large sample of children with velo-cardio-facial syndrome: An update. Journal of Intellectual Disability Research. 2007;51:666–670. doi: 10.1111/j.1365-2788.2007.00955.x. [DOI] [PubMed] [Google Scholar]
- De Smedt B, Reynvoet B, Swillen A, Verschaffel L, Boets B, Ghesquière P. Basic number processing and difficulties in single-digit arithmetic: Evidence from velo-cardio-facial syndrome. Cortex. 2009a;45(2):177–188. doi: 10.1016/j.cortex.2007.06.003. [DOI] [PubMed] [Google Scholar]
- De Smedt B, Swillen A, Verschaffel L, Ghesquiere P. Mathematical learning disabilities in children with 22q11.2 deletion syndrome: A review. Developmental Disabilities Research Reviews. 2009b;15:4–10. doi: 10.1002/ddrr.44. [DOI] [PubMed] [Google Scholar]
- Duijff SN, Klaassen PW, de Veye HF, Beemer FA, Sinnema G, Vorstman JA. Cognitive development in children with 22q11.2 deletion syndrome. The British Journal of Psychiatry. 2012;200(6):462–468. doi: 10.1192/bjp.bp.111.097139. [DOI] [PubMed] [Google Scholar]
- Fung WL, Butcher NJ, Costain G, Andrade DM, Boot E, Chow EW, Bassett AS. Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genetics in Medicine. 2015;17(8):599–609. doi: 10.1038/gim.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes M, Solot C, Wang PP, Moss E, LaRossa D, Randall P, Zackai EH. Cognitive and behavior profile in preschool children with chromosome 22q11 deletion. American Journal of Medical Genetics. 1999;85(2):127–133. [PubMed] [Google Scholar]
- Glaser B, Lothe A, Chabloz M, Dukes D, Pasca C, Redoute J, Eliez S. Candidate socioemotional remediation program for individuals with intellectual disability. American Journal on Intellectual and Developmental Disabilities. 2012;117(5):368–383. doi: 10.1352/1944-7558-117.5.368. [DOI] [PubMed] [Google Scholar]
- Glaser B, Mumme DL, Blasey C, Morris MA, Dahoun SP, Antonarakis SE, Eliez S. Language skills in children with velocardiofacial syndrome (deletion 22q11.2) The Journal of Pediatrics. 2002;140(6):753–758. doi: 10.1067/mpd.2002.124774. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, Reiss AL. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nature Neuroscience. 2005;8(11):1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, Eliez S. Psychiatric Disorders and Intellectual Functioning Throughout Development in Velocardiofacial (22q11.2 Deletion) Syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(11):1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Gur RE, Yi JJ, McDonald-McGinn DM, Tang SX, Calkins ME, Whinna D, Gur RC. Neurocognitive development in 22q11.2 deletion syndrome: Comparison with youth having developmental delay and medical comorbidities. Molecular Psychiatry. 2014;2014:1–7. doi: 10.1038/mp.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JC, Van Amelsvoort T, Morris RG, Owen MJ, Murphy DG, Murphy KC. An investigation of the neuropsychological profile in adults with velo-cardio-facial syndrome (VCFS) Neuropsychologia. 2002;40(5):471–478. doi: 10.1016/s0028-3932(01)00136-1. [DOI] [PubMed] [Google Scholar]
- Mariano M, Tang K, Kurtz M, Kates WR. Cognitive remediation for adolescents with 22q11 DS: A preliminary study examining effectiveness, feasibility, and fidelity of a hybrid strategy, remote and computer-based intervention. Schizophrenia Research. 2015;166(1–3):283–289. doi: 10.1016/j.schres.2015.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Fahiminiya S, Revil T, Nowakowska BA, Suhl J, Bailey A, Jerome-Majewska LA. Hemizygous mutations in SNAP29 unmask autosomal recessive conditions and contribute to atypical findings in patients with 22q11.2DS. Journal of Medical Genetics. 2013;50(2):80–90. doi: 10.1136/jmedgenet-2012-101320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Medicine (Baltimore) 2011;90(1):1–18. doi: 10.1097/MD.0b013e3182060469. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA, Bassett AS. 22q11.2 deletion syndrome. Nature Reviews Disease Primers. 2015;1:1–19. doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer-Rosa L, Paridon SM, Fogel MA, Rychik J, Tanel RE, Zhao H, Goldmuntz E. 22q11.2 deletion status and disease burden in children and adolescents with tetralogy of Fallot. Circulation. Cardiovascular Genetics. 2015;8(1):74–81. doi: 10.1161/CIRCGENETICS.114.000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA, Wang PP. Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. The Journal of Pediatrics. 1999;134(2):193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- Olszewski AK, Radoeva PD, Fremont W, Kates WR, Antshel KM. Is child intelligence associated with parent and sibling intelligence in individuals with developmental disorders? An investigation in youth with 22q11.2 deletion (velo-cardio-facial) syndrome. Research in Developmental Disabilities. 2014;35(12):3582–3590. doi: 10.1016/j.ridd.2014.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N, Bassett A. Cognitive, behavioural and psychiatric phenotype in 22q11.2 deletion syndrome. Behavior Genetics. 2011;41(3):403–412. doi: 10.1007/s10519-011-9468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raux G, Bumsel E, Hecketsweiler B, van Amelsvoort T, Zinkstok J, Manouvrier-Hanu S, Campion D. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Human Molecular Henetics. 2007;16(1):83–91. doi: 10.1093/hmg/ddl443. https://www.ncbi.nlm.nih.gov/pubmed/17135275. [DOI] [PubMed] [Google Scholar]
- Roizen NJ, Antshel KM, Fremont W, AbdulSabur N, Higgins AM, Shprintzen RJ, Kates WR. 22q11.2 DS syndrome: Developmental milestones in infants and toddlers. Journal of Developmental and Behavioral Pediatrics. 2007;28(2):119–124. doi: 10.1097/01.DBP.0000267554.96081.12. [DOI] [PubMed] [Google Scholar]
- Shashi V, Keshavan MS, Kaczorowski J, Schoch K, Lewandowski KE, McConkie-Rosell A, Kwapil TR. Socioeconomic status and psychological function in children with chromosome 22q11.2 deletion syndrome: Implications for genetic counseling. Journal of Genetic Counseling. 2010;19(5):535–544. doi: 10.1007/s10897-010-9309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugar AL, Shapiro JM, Cytrynbaum C, Hedges S, Weksberg R, Fishman L. An increased prevalence of thyroid disease in children with 22q11.2 deletion syndrome. American Journal of Medical Genetics Part A. 2015;167(7):1560–4. doi: 10.1002/ajmg.a.37064. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Mc-Ginn DM, Zackai E. Visuospatial and numerical cognitive deficits in children with chromosome 22q11.2 deletion syndrome. Cortex. 2005;41(2):145–155. doi: 10.1016/s0010-9452(08)70889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solot C, Gerdes M, Kirschner RE, McDonald-McGinn DM, Moss E, Woodin M, Wang PP. Communication issues in 22q11.2 deletion: Children at risk. Genetics in Medicine. 2001;3(1):67–71. doi: 10.1097/00125817-200101000-00015. [DOI] [PubMed] [Google Scholar]
- Stoddard J, Beckett L, Simon TJ. Atypical development of the executive attention network in children with chromosome 22q11.2 deletion syndrome. Journal of Neurodevelopmental Disorders. 2011;3(1):76–85. doi: 10.1007/s11689-010-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A. The importance of understanding developmental trajectories: The case of 22q11 DS. Current Opinion in Psychiatry. 2016;29(2):133–137. doi: 10.1097/YCO.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP. Intelligence and psychosocial adjustment in velocardiofacial syndrome: A study of 37 children and adolescents with VCFS. Journal of Medical Genetics. 1997;34(6):453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, McDonald-McGinn D. Developmental trajectories in 22q11.2 deletion syndrome. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 2015;169(2):172–181. doi: 10.1002/ajmg.c.31435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobia V, Brigstocke S, Hulme C, Snowling MJ. Developmental changes in the cognitive and educational profiles of children and adolescents with 22q11.2 deletion syndrome. Journal of Applied Research in Intellectual Disabilities. 2017;31(1):1–5. doi: 10.1111/jar.12344. [DOI] [PubMed] [Google Scholar]
- Van Aken K, Caeyenberghs K, Smits-Engelsman B, Swillen A. The motor profile of primary school-age children with a 22q11.2 deletion syndrome (22q11.2DS) and an age- and IQ-matched control group. Child Neuropsychology. 2009;15(6):532–542. doi: 10.1080/09297040902740678. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel E, Reuterskiöld C, Solot C, Manders E, Swillen A, Zink I. Referential communication abilities in children with 22q11.2 deletion syndrome. International Journal of Speech Language Pathology. 2016;19(5):490–502. doi: 10.1080/17549507.2016.1221456. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort T, Henry J, Morris R, Owen M, Linszen D, Murphy K, Murphy D. Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophrenia Research. 2004;70(2–3):223–232. doi: 10.1016/j.schres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Breetvelt E, Duijff S, Eliez S, Schneider M, Jalbrzikowski M The International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. A cognitive decline precedes the onset of psychosis in patients with the 22q11.2 deletion syndrome. JAMA. 2015;72(4):377–385. doi: 10.1001/jamapsychiatry.2014.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PP, Woodin MF, Kreps-Falk R, Moss EM. Research on behavioral phenotypes: Velocardiofacial syndrome (deletion 22q11.2) Developmental Medicine & Child Neurology. 2000;42:422–427. doi: 10.1017/s0012162200000785. [DOI] [PubMed] [Google Scholar]
- Wong LM, Riggins T, Harvey D, Cabaral M, Simon TJ. Children with chromosome 22q11.2 deletion syndrome exhibit impaired spatial working memory. American Journal on Intellectual and Developmental Disabilities. 2014;119(2):115–132. doi: 10.1352/1944-7558-119.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, Moss E. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genetics in Medicine. 2001;3(1):34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]