Abstract
It’s estimated that 18 Americans die every day waiting for an organ donation. And even if a patient receives the organ that s/he needs, there is still >10% chance that the new organ will not work. The field of tissue engineering and regenerative medicine aims to actively use a patient’s own cells, plus biomaterials and factors, to grow an organ or to restore normal functions of that organ, which would eliminate the need for donors and the risk of organ rejection. In this review, we summarized recent advances in fabricating synthetic cells and engineering tissue, with a specific focus on cardiac regenerative medicine and tissue engineering. At the end, we pointed to challenges and future directions for the field.
Introduction: Building Blocks for Regenerative Medicine
Our body, built by Mother Nature, is continuously challenged by diseases and common wear and tear. Like a house, after many years, our body needs repair or even a flip. Regenerative medicine is a promising strategy to repair and rebuild our own body, by creating new tissues to restore or replace organ function lost [1]. Regenerative medicine also empowers scientists and bioengineers to manufacture tissues and organs in the laboratory implantation. Importantly, regenerative medicine holds the potential to solve the problem of organ shortages. There is a huge gap between the number of available donor organs and the number of patients on the organ transplantation waiting list. Unlike other organs and tissues, the heart has very limited regenerative potential [2]. This limitation is partially due to the lack of resident cardiac stem cells, and genetic/epigenetic roadblocks that limit adult cardiomyocytes from proliferating a short period of time after birth [3].
Organs, tissues, cells, and proteins are the main building “legos” for regenerative medicine. Among them, organs are ready-to-use building blocks. Successful organ transplantation started in 1954 with the first successful kidney transplant [4,5] performed by Dr. Joeseph E. Murray and his team at Boston. After that, successful transplantation of pancreas, liver, and heart took place. Approximately half million Americans benefit from a transplant each year. Nowadays, in the US more than 120,000 people are waiting to receive a life-saving organ transplant. Many of these individuals will die before a suitable donor organ is available.
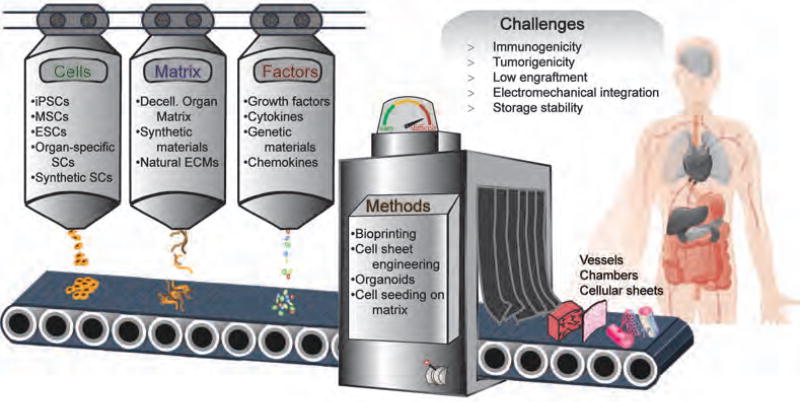
Instead of replacing the entire organ, stem cell therapy holds the promise to replace the lost cells is an effective way to repair the tissue [6]. Generation of transplantable organs in vitro using stem cells and scaffolding materials is also a desirable approach for organ replacement [7]. While, injected stem cells can promote healing and regeneration in situ in the targeted organs [8], typically a more intentional assembly of raw materials (cells, matrix, factors) and fabrication methods is needed to create functional tissue constructs (Fig. 1).
Figure 1. Assembly of raw materials to tissues for organ regeneration.
Functional tissue constructs could be created by cells, matrix and factors through different methods, including bioprinting, cell sheet engineering, cell seeding in matrix, etc. However, there are still many challenges hindering the clinical application of tissue engineering, such as immunogenicity, tumorigenicity, low engraftment, electromechanical integration and storage stability. iPSCs: induced Pluripotent Stem Cells, MSCs: Mesenchymal Stem Cells, ESCs: Embryonic Stem Cells.
Obtaining Cells and Tissues from the Nature
Cardiac cell therapy using adult stem cells started from the early 2000s. Studies from several groups claimed that transplants of bone-marrow cells or c-Kit+ cardiac progenitor cells can regenerate the infarcted rodent heart [9]. About a decade later, this work was translated to the clinic, with phase I clinical trials in patients [10,11]. As the field progressed, a paradigm change occurred as scientists started to realize that the injected cells hardly form any new cardiac tissue but instead promote endogenous repair via paracrine mechanisms [12]. In 2017, Nature Biotechnology expressed a severe concern on the none-to-marginal benefits of cardiac cell therapy trials and argued that cardiac cell therapy is “far from getting approval” and “much more preclinical data needs to be performed before any new clinical trials”[13]. In summary, most scientists in the field now recognize that cardiac stem cells (CSCs) are very rare and the therapeutic benefits of CSCs are mainly from pro-survival, proangiogenic, and anti-inflammatory effects rather than direct cardiomyogenic differentiation [14]. As a result, there is a strong movement toward investigating the potential of pluripotent cell types (e.g., ESC and iPSC) to become cardiac cell types necessary for regeneration. Substantial challenges exist in this realm as well including teratoma formation, karyotypic abnormalities, immune rejection, immature phenotypes, cardiomyocyte heterogeneity, etc. [15]. Due to the low cell retention rate, a large number of stem cells need to be injected. This not only raises costs but also represents a safety concern to patients.
Building synthetic and artificial cells
As a “live drug”, stem cells need to be carefully processed and preserved before clinical application. Studies indicate that the quality of stem cells can directly affect their therapeutic benefits in vivo. To switch from the concept of “obtain from nature” to “learn from nature (and build)”, efforts have been made to create synthetic or artificial stem cells. An artificial cell is an engineered entity that mimics one or many functions of a biological (natural) cell. The first artificial cell was developed by Professor Thomas Chang at McGill University in the 1960s [16]. It is important to note that the term “artificial cell” does not refer to a specific physical or morphological identity. Instead, certain functions or structures of biological cells can be replaced or mimicked with an engineered entity. Often, artificial cells are biological or polymeric blocks that encapsulate biologically active materials. These materials include nano-/micro-particles, liposomes, micelles etc.
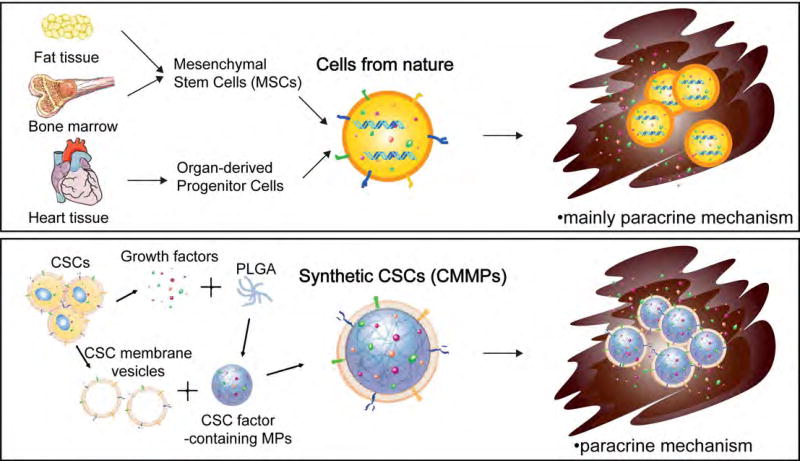
We now know that most adult stem cell types exert their beneficial effects through paracrine mechanisms (via secretion of soluble factors). In addition, studies further suggest that cell-cell contact between the injected CSCs and injured cardiomyocytes plays an important role in tissue regeneration [17]. Based on these principles, we designed a cell-mimicking microparticle (CMMP) that recapitulates the secretion and biointerfacing of natural stem cells during tissue repair. Our overall hypothesis is that CMMPs can act as “synthetic stem cells”, harnessing the power of both stem cell membrane and secretome, to induce tissue regeneration. The process starts with Poly Lactic-co-Glycolic Acid (PLGA) as the building material. PLGA microparticles were fabricated using the conditioned media from cardiac stem cells (CSC) which contains the various regenerative factors secreted by CSCs. After that, the microparticles are coated with the cell membranes of CSCs to become the final product synthetic CSCs (Fig. 2). The therapeutic potency of synthetic CSCs was confirmed in a mouse model of myocardial infarction [18,19]. The scientific premise is that the synthetic CSC technology overcomes several major challenges of the status quo of cell therapy practice, namely cryopreservation stability, standardization, and “off the shelf” feasibility. In addition, using synthetic entities will likely mitigate the tumorigenicity and immunogenicity risks associated with stem cell transplantation.
Figure 2. Comparison between natural cells and artificial cells for tissue engineering.
Natural stem cells have certain limitations in clinical usage. Artificial cells keep the membranes and growth factors from the parental stem cells with the genetic materials removed. They could circumvent the shortages of natural stem cells. Importantly, the main regenerative mechanisms of adult stem cells and artificial cells are both from paracrine effect. CSCs: Cardiac Stem Cells, PLGA: Poly Lactic-co-Glycolic Acid, CMMPs: Cell-Mimicking Microparticles.
Despite the proof of concept for synthetic CSCs, several obstacles may hinder the further development of this technology. The efficacy of stem cell transplantation suffers from low retention/engraftment and poor targeting to the heart [20]. As a non-living entity, synthetic CSCs cannot migrate, therefore may suffer more from these drawbacks. In addition, synthetic CSCs have not yet been endowed with the capacity to contract with stimulus and so cannot contribute to the function of the failing muscle mass. Future efforts should also be focused on developing compatible strategies to more efficiently deliver synthetic CSCs. Recent advances in targeting intravascular exosomes and stem cells can be adopted to deliver synthetic CSCs to the injured heart [21,22]. It is noteworthy that unlike natural stem cells, synthetic stem cells are unable to multiply. Nevertheless, adult stem cells undergo very limited proliferation after they are injected into the heart. The prevailing wisdom that one only needs a small amount of “seed” stem cell engraftment and they will turn into a large graft after multiplication is not the case, at least for adult stem cells [23,24]. Like any chemical drugs, repeated stem cell injections are needed for the functional benefits[25]. In this regard, synthetic stem cells offer an important alternative to natural stem cells.
Building functional tissues and artificial organs
As synthetic cells evolve to harbor more complex functionality, including the possibility of contraction, it will be important to consider organization such that cardiac functionality can ultimately be coordinated with the host. In the absence of coordinated function, aberrant function including accelerated idioventricular rhythm and ventricular tachycardia of host tissue can ensue [26]. The first step toward productive organization is to form 3D versus 2D structures to better represent the structural and functional complexity of tissue. This is especially important in the context of cardiovascular tissue as stiffness can be more easily modulated to mimic that of the native heart with development or disease and mechanical and electrical stimulation akin to that experienced in the heart can be imposed. Indeed, mechanical and electrical stimulation in the context of 3D model systems has been one of the more successful approaches to improving the organization of engineered cardiac tissue. In this way, maturation in terms of reduced calcium sensitivity, frequency-dependent acceleration of relaxation and enhanced post-rest potentiation can be observed in addition to expression of sarcomeric structural maturation [27].
Another successful approach to organization of engineered cardiac tissue is the intentional placement of cardiac cells and particular extracellular matrix proteins using 3D bioprinting. 3D bioprinting is arguably the most promising means to advance tissue and organ scale cardiovascular tissues in the future. There are several different 3D bioprinting technologies, each with distinct advantages and disadvantages [28]. Methodologies that excel in resolution (i.e., multiphoton-based 3D bioprinting and laser-assisted bioprinting) are limited in the thickness of the tissue to be created, time to create tissue and the complexity of inner void space. These methodologies have been used to generate cardiac patches via deposition of extracellular matrix proteins in a gridded pattern such that cardiomyocytes seeded on the structures undergo alignment, spontaneous and synchronous beating with signal propagation from one side of the patch to the other [29]. Methodologies that excel in speed and generation of complex geometries (i.e., extrusion and photolithography) fall short in the limit of resolution. These methodologies are being harnessed to 3D print vascularized networks (at least at the scale of the arteriole) together with myocardium and valve structures as a prelude to an intact heart [30].
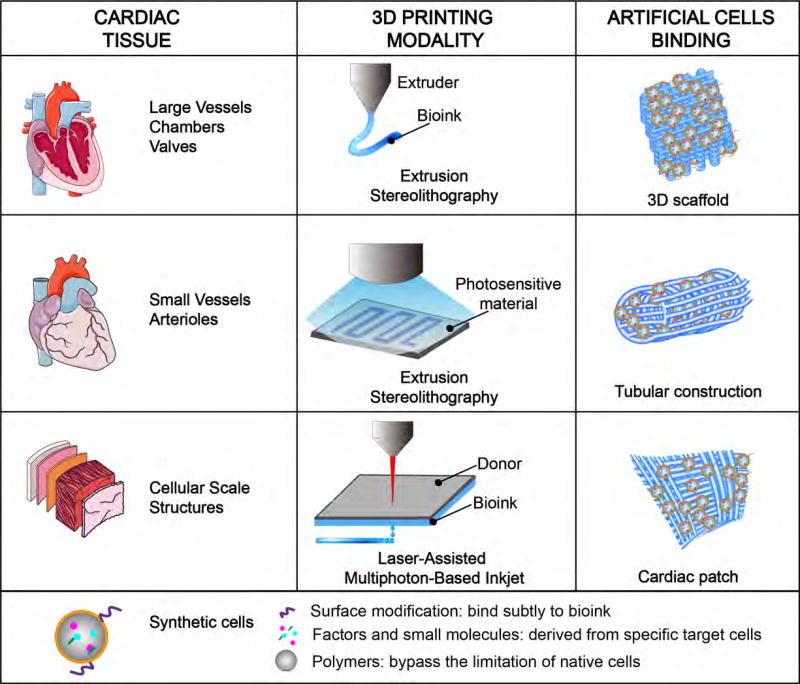
The challenges of 3D bioprinting cardiac tissues may be diminished with the addition or substitution of synthetic stem cells. A cardiac cell patch needs to be freshly prepared and maintained under physiological conditions for cell viability. This limits the storage/shipping feasibility as a therapeutic product. Besides, one challenge with native cells, especially cardiomyocytes are the limited propensity to migrate following specification. Limited migration means limited organization following printing, especially with extrusion-based modalities and in the absence of high resolution bioprinted chambers. One way around this challenge is to print stem cells and then drive specification in the 3D bioprinted entity. This approach suffers the possibility of differentiation of unwanted cell types. Instead, one could imagine a synthetic cell with contractile machinery that also expresses specific cell surface molecules that can bind exquisitely to the 3D bioink. In this way, one might prescribe orientation and arrangement without the variability and limitations of native cells (Fig. 3).
Figure 3. 3D bioprinting of cardiac tissue.
Extrusion stereolithography is mostly used for the construction of chambers, valves, small and large vessels of heart, while laser-assisted multiphoton-based inkjet is more suitable for the construction of cellular scale structures. With synthetic cells, the limitation of 3D bioprinting could be greatly diminished due to the stability and artificial characterization of synthetic cells compared to native stem cells.
Another challenge of 3D printed cardiac tissues is the inherent low viscosity of the bioink. High viscosity inks that include synthetic polymers and/or are heavily crosslinked are not conducive to cell health and organization. In fact, Hinton et al developed a 3D bioprinting technology termed freeform reversible embedding of suspended hydrogels (FRESH) in order to address the limited intersection between printability, viscosity and cell health [31]. This method is quite promising for hydrogel materials and has been used successfully to print whole heart tissue with intact chambers and valvular structures. But one might alternatively avoid the limitations of low viscosity ink with the inclusion of synthetic cells which might tolerate high stiffness, limited pore size and lack of adhesion epitope more successfully. Synthetic cells might also be engineered to bind and release factors for survival, movement or differentiation of cardiac cell types.
The success of grand ideas of cardiac tissue engineering, those that could include new technologies such as 3D bioprinting and synthetic cells, rely heavily on basic studies to elucidate critical interactions between cells, the scaffolds they come into contact with and the signals provided exogenously or from each other. For example, of the cues that can potentially guide stem cell behavior in the context of the heart, we know a quite a bit about the soluble cues that can trigger signaling pathways critical for mesoderm and mature cardiac cell specification. We know quite a bit less about the pathways linked to extracellular matrix (ECM) interaction. For example, we have no idea why binding of different ECM receptors (i.e., integrins)[32] with varying valency yields discrete cardiac differentiation outcomes [33]. And almost completely unknown is the relationship between metabolism or the immune response and stem cell fate. When cues of this type are more clearly defined they can be incorporated effectively into technologies to advance cardiac tissue engineering.
Summary and future directions
Building a body or an organ isn’t an easy job. Efforts should be focused on the fabrication of functional cardiac tissues to replace a portion of the heart’s function. The points to consider for cardiac tissue engineering include the choice of cell types—adult, embryonic, induced stem cells—or synthetic stem cells for heart regeneration, the choice of scaffolding materials—natural materials versus synthetic materials, and the choice of manufacturing methods—additive versus subtractive fabrication. The challenges include vascularization of the tissue constructs, electrical and mechanical functions of the tissue, long-term storage stability of the tissue engineering products, integration of the engineered tissues with the host, and availability of clinically-relevant large animal models for translation. Looking forward, we envision that synthetic cells will play an important role in tissue engineering and great efforts will be focused on the design and delivery approaches of synthetic cells. Collaboration among stem cell biologists, biomedical engineers, and physician scientists will likely to bring new solutions to those challenges and push artificial cell therapy to the clinic.
Acknowledgments
This work is supported by National Institute of Health grants HL123920 and HL137093 to K.C. and HL137204 and HL131017 to B.M.O.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest
The authors declare no competing interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Cossu G, Birchall M, Brown T, De Coppi P, Culme-Seymour E, Gibbon S, Hitchcock J, Mason C, Montgomery J, Morris S, et al. Lancet Commission: Stem cells and regenerative medicine. Lancet. 2017 doi: 10.1016/S0140-6736(17)31366-1. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 2*.Tzahor E, Poss KD. Cardiac regeneration strategies: Staying young at heart. Science. 2017;356:1035–1039. doi: 10.1126/science.aam5894. This review paper summarized the recent development in the field of cardiac regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, et al. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–227. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- 4.Harrison JH, Merrill JP, Murray JE. Renal homotransplantation in identical twins. Surg Forum. 1956;6:432–436. [PubMed] [Google Scholar]
- 5.Merrill JP, Murray JE, Harrison JH, Guild WR. Successful homotransplantation of the human kidney between identical twins. JAMA. 1956;160:277–282. doi: 10.1001/jama.1956.02960390027008. [DOI] [PubMed] [Google Scholar]
- 6.Fish KM, Ishikawa K, Hajjar RJ. Stem cell therapy for acute myocardial infarction: on the horizon or still a dream? Coron Artery Dis. 2018;29:89–91. doi: 10.1097/MCA.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Ogle BM, Bursac N, Domian I, Huang NF, Menasche P, Murry CE, Pruitt B, Radisic M, Wu JC, Wu SM, et al. Distilling complexity to advance cardiac tissue engineering. Sci Transl Med. 2016;8:342–313. doi: 10.1126/scitranslmed.aad2304. This review paper summarized the challenges and possible solutions to cardiac tissue engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Yang R, He Z, Gao WQ. Generation of functional organs from stem cells. Cell Regen. 2013;2:1. doi: 10.1186/2045-9769-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 10.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase I trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marban L, Mendizabal A, Cingolani E, Johnston PV, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisen J, Giacca M, Hare JM, Houser S, Lee RT, et al. Cardiomyocyte regeneration: a consensus statement. Circulation. 2017;136:680–686. doi: 10.1161/CIRCULATIONAHA.117.029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A futile cycle in cell therapy. Nat Biotechnol. 2017;35:291. doi: 10.1038/nbt.3857. [DOI] [PubMed] [Google Scholar]
- 14.Kelkar AA, Butler J, Schelbert EB, Greene SJ, Quyyumi AA, Bonow RO, Cohen I, Gheorghiade M, Lipinski MJ, Sun W, et al. Mechanisms contributing to the progression of ischemic and nonischemic dilated cardiomyopathy: possible modulating effects of paracrine activities of stem cells. J Am Coll Cardiol. 2015;66:2038–2047. doi: 10.1016/j.jacc.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Duelen R, Sampaolesi M. Stem cell technology in cardiac regeneration: a pluripotent stem cell promise. E Bio Medicine. 2017;16:30–40. doi: 10.1016/j.ebiom.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang TMS. Semipermeable microcapsules. Science. 1964;146:524–525. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 17.Xie Y, Ibrahim A, Cheng K, Wu Z, Liang W, Malliaras K, Sun B, Liu W, Shen D, Cheol Cho H, et al. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells. 2014;32:2397–2406. doi: 10.1002/stem.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Tang JA, Shen DL, Caranasos TG, Wang ZG, Vandergriff AC, Allen TA, Hensley MT, Dinh PU, Cores J, Li TS, et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun. 2017;8:13724. doi: 10.1038/ncomms13724. This research paper reported the design and application of synthetic stem cells for cardiac repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo L, Tang J, Nishi K, Yan C, Dinh PU, Cores J, Kudo T, Zhang J, Li TS, Cheng K. Fabrication of synthetic mesenchymal stem cells for the treatment of acute myocardial infarction in mice. Circ Res. 2017;120:1768–1775. doi: 10.1161/CIRCRESAHA.116.310374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Yanamandala M, Zhu W, Garry DJ, Kamp TJ, Hare JM, Jun HW, Yoon YS, Bursac N, Prabhu SD, Dorn GW, 2nd, et al. Overcoming the roadblocks to cardiac cell therapy using tissue engineering. J Am Coll Cardiol. 2017;70:766–775. doi: 10.1016/j.jacc.2017.06.012. This review paper summarized the challenges in cardiac tissue engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Tang J, Su T, Huang K, Dinh P-U, Wang Z, Vandergriff A, Hensley MT, Cores J, Allen T, Li T, et al. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat Biomed Eng. 2018;2:17–26. doi: 10.1038/s41551-017-0182-x. This research paper reported a novel method of targeting cardiac stem cells to the infarct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandergriff AHK, Shen D, Hensley MT, Qian L, Caranasos TG, Cheng K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;7:1869–1878. doi: 10.7150/thno.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Mignone J, MacLellan WR. Cardiac regeneration and stem cells. Physiol Rev. 2015;95:1189–1204. doi: 10.1152/physrev.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixit P, Katare R. Challenges in identifying the best source of stem cells for cardiac regeneration therapy. Stem Cell Res Ther. 2015;6:26. doi: 10.1186/s13287-015-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang XL, Nakamura S, Li Q, Wysoczynski M, Gumpert AM, Wu WJ, Hunt G, Stowers H, Ou Q, Bolli R. Repeated administrations of cardiac progenitor cells are superior to a single administration of an equivalent cumulative dose. J Am Heart Assoc. 2018;7:e007400. doi: 10.1161/JAHA.117.007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. This research paper reported the use of human ESC-derived cardiomyocytes in non-human primates. This study, despite its proof-of-concept nature, paves the ground for the use of pluripotent stem cells for heart repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godier-Furnemont AF, Tiburcy M, Wagner E, Dewenter M, Lammle S, El-Armouche A, Lehnart SE, Vunjak-Novakovic G, Zimmermann WH. Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials. 2015;60:82–91. doi: 10.1016/j.biomaterials.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung JP, Bhuiyan DB, Ogle BM. Solid organ fabrication: comparison of decellularization to 3D bioprinting. Biomater Res. 2016;20:27. doi: 10.1186/s40824-016-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Gao L, Kupfer ME, Jung JP, Yang L, Zhang P, Da Sie Y, Tran Q, Ajeti V, Freeman BT, Fast VG, et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-Dimensionally printed scaffold. Circ Res. 2017;120:1318–1325. doi: 10.1161/CIRCRESAHA.116.310277. This research paper reported the generation of a cardiac patch made exclusively of ECM proteins deposited with high resolution via multiphoton-based 3D printing and capable of aligning cardiomyocytes with synchronous function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borovjagin AV, Ogle BM, Berry JL, Zhang J. From microscale devices to 3D printing: advances in fabrication of 3D cardiovascular tissues. Circ Res. 2017;120:150–165. doi: 10.1161/CIRCRESAHA.116.308538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Hinton TJJQ, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1:e1500758. doi: 10.1126/sciadv.1500758. This research paper reported a facile method for printing low viscosity, cell-laden bioinks that strikes a nice balance between engineered tissue stiffness, printability and cell health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Villa-Diaz LG, Kim JK, Laperle A, Palecek SP, Krebsbach PH. Inhibition of FAK signaling by integrin alpha6beta1 supports human pluripotent stem cell self-renewal. Stem Cells. 2016;34:1753–1764. doi: 10.1002/stem.2349. This research paper reported a compelling link between alpha6beta1 engagement and the maintenance of pluripotency. This study paves the way for future work to identify similar linkages that enable stem cell differentiation. [DOI] [PubMed] [Google Scholar]
- 33.Civitarese RA, Kapus A, McCulloch CA, Connelly KA. Role of integrins in mediating cardiac fibroblast-cardiomyocyte cross talk: a dynamic relationship in cardiac biology and pathophysiology. Basic Res Cardiol. 2017;112:6. doi: 10.1007/s00395-016-0598-6. [DOI] [PubMed] [Google Scholar]