Abstract
Objective
Immunotherapy using vitamin D (vitD3) and phenylbutyrate (PBA), may support standard drug regimens used to treat infectious diseases. We investigated if vitD3+PBA enhanced clinical recovery from pulmonary tuberculosis (TB).
Methods
A randomized controlled trial was conducted in Addis Ababa, Ethiopia. Patients with smear-positive or smear-negative TB received daily oral supplementation with 5000IU vitD3 and 2×500mg PBA or placebo for 16 weeks, together with 6-months chemotherapy. Primary endpoint: Reduction of a clinical composite TB score at week 8 compared with baseline using modified intention-to-treat (mITT, n=348) and per-protocol (n=296) analyses. Secondary endpoints: Primary and modified TB scores (week 0, 4, 8, 16, 24), sputum conversion, radiological findings and plasma 25(OH)D3 concentrations.
Results
Most subjects had low baseline plasma 25(OH)D3 levels that increased gradually in the vitD3+PBA group compared with placebo (P < 0.0001) from week 0 to 16 (mean 34.7 vs 127.4 nmol/l). In the adjusted mITT analysis, the primary TB score was significantly reduced in the intervention group at week 8 (−0.52, 95% CI −0.93, −0.10; P = 0.015) while the modified TB score was reduced at week 8 (−0.58, 95% CI −1.02, −0.14; P = 0.01) and 16 (−0.34, 95% CI −0.64, −0.03; P = 0.03). VitD3+PBA had no effect on longitudinal sputum-smear conversion (P = 0.98). Clinical adverse events were more common in the placebo group (24.3%) compared with the vitD3+PBA group (12.6%).
Conclusion
Daily supplementation with vitD3+PBA may ameliorate clinical TB symptoms and disease-specific complications, while the intervention had no effect on bacterial clearance in sputum.
Trial registration
ClinicalTrials.gov NCT01698476
Keywords: clinical trial, tuberculosis, vitamin D, phenylbutyrate, host defense
Introduction
New drug regimens for tuberculosis (TB) are necessary both to prevent TB disease and to treat ongoing active TB [1]. In contrast to single molecular targets, targeting multiple pathways in an attempt to treat chronic infections may reduce the risk for drug-resistance and clinical complications. Immunomodulatory compounds such as vitamin D3 (vitD3) and phenylbutyrate (PBA) are attractive therapeutic candidates with the ability to regulate various axes of the immune system [2]. In vitro, vitD3 can enhance macrophage-mediated killing of Mycobacterium tuberculosis (Mtb) by inducing the antimicrobial peptide LL-37 [3, 4] and autophagy [5]. LL-37 may also exert chemotactic functions to activate migration of immune cells to the site of infection [6]. Similarly, PBA, which is a histone deacetylase (HDAC) inhibitor, induces LL-37 in different cell types [7] and autophagy in macrophages [8] but also exhibits direct bacteriostatic effects on Mtb [9]. Accordingly, the combination of vitD3 and PBA has been shown to enhance intracellular Mtb-killing in vitro [8] and ex vivo [10, 11]. While both vitD3 and PBA are potent inducers of innate mucosal immunity, these compounds also possess important anti-inflammatory properties including inhibition of dendritic cell maturation, Th1/Th17 cell proliferation and cytokine production [2, 12]. Thus, hypothetically vitD3+PBA has the potential to reduce bacterial growth and simultaneously resolve pathological inflammation in the Mtb-infected lung.
To test if adjunct therapy with vitD3+PBA could support clinical recovery in pulmonary TB, we performed a randomized controlled trial (RCT) in Ethiopia. As large bolus doses of vitD3 generally fail to improve TB outcomes [13–15], we used daily dosing of vitD3 in combination with PBA for 16 weeks of chemotherapy. Bacteriological confirmation of pulmonary TB mostly involves detection of acid-fast bacilli (AFB) or Mtb-growth in sputum. However, about 20–50% of pulmonary TB patients are smear-negative [16], but qualify for initiation of standard chemotherapy based on a high clinical suspicion and radiographic findings according to the World Health Organization (WHO) criteria [1]. Thus, we designed a longitudinal study to exploit if vitD3+PBA could improve clinical status in smear-positive and smear-negative TB patients by limiting bacterial load and pathological inflammation in the lung. The response to adjunct vitD3+PBA therapy was evaluated using a numerical composite TB score, assessing the reduction in clinical symptoms during the initial 8-weeks intensive-phase treatment with standard drugs. This is a validated score composed of eleven clinical variables used to measure TB patients’ clinical status at repeated visits [17] and as an outcome measure in clinical trials [13, 18, 19]. Symptoms effectively improve during the initial 8-weeks intensive-phase standard treatment as a result from rapidly reduced bacterial loads in the lung and bacterial clearance from sputum in about 80% of TB patients [1, 20], and therefore this time-point was used to assess the primary outcome.
Materials and Methods
For details on the Methods, please see the online Supporting Information.
Study design
A randomized, double-blinded, placebo-controlled trial was conducted at the Chest Unit, Department of Internal Medicine, Black Lion University Hospital in Addis Ababa, Ethiopia in collaboration with eleven health centers after ethical approval in Ethiopia and Sweden (Supporting Information). The study was registered at www.clinicaltrials.gov, NCT01698476, prior to inclusion of the first patient.
Patients
Inclusion criteria: HIV-negative patients >18 years, with newly diagnosed pulmonary TB (<5 days chemotherapy). Diagnoses were made from: a) positive sputum-smear microscopy or Mtb-culture, and/or b) clinical symptoms and chest X-ray findings consistent with TB, i.e. clinical TB defined according to WHO criteria. Exclusion criteria: HIV-infection, multidrug-resistant TB (MDR-TB) or extrapulmonary TB, anti-TB treatment in the past 2 years, hypercalcemia (serum calcium >3.0 mmol/l), pregnancy and breast-feeding, liver or renal diseases, malignancies, or treatment with cardiac glycosides. All patients provided written and signed informed consent before enrolment.
Interventions
This was a two-arm intervention trial using daily adjunct therapy with vitD3+PBA during the first 16 weeks of 6-months standard chemotherapy including a fixed-dose-combination of isoniazid, rifampicin, pyrazinamide, and ethambutol for 8 weeks (intensive-phase treatment) and isoniazid and rifampicin for an additional 16 weeks. Patients were randomized to receive daily oral supplementation using the following dosing scheme [10, 21]: 1) 5000 IU vitD3 (five tablets once daily) and 500 mg PBA (one tablet twice daily), or 2) vitD3 placebo and PBA placebo tablets. VitD3 tablets were used instead of oil, to control for variations in self-dosage of the oil preparation. Good manufacturing practice-produced vitD3 tablets (Vigantoletten) and matching placebo were donated by Merck Serono (Darmstadt, Germany); PBA (Sodium Phenylbutyrate) and matching placebo were obtained from Scandinavian Formulas (PA, USA).
Randomization and masking
Subjects were randomized in a one-to-one allocation ratio using computer-generated randomization codes and block randomization with a block size of ten (Karolinska Trial Alliance, Stockholm, Sweden), to ensure that in each block, five subjects were randomized to vitD3+PBA and the other five subjects to placebo. Pharmacists at the Black Lion Hospital prepared the study medication and provided the randomization codes that assigned the patients to vitD3+PBA or placebo treatment. Patients were recruited by senior consultants and a health officer, and they were all blinded to the randomization.
Outcome measures
The primary endpoint was clinical recovery, assessed as the reduction/change of clinical symptoms at week 8 compared to week 0 (baseline). As it is not possible to record improvement of TB disease using a single symptom or laboratory result, we used a previously validated clinical TB score [13, 17]. This is a numerical composite TB score (2-point scale: symptom absent (0p) or present (1p), max 13p) that included self-reported clinical symptoms (cough, night sweats, and chest pain), as well as different variables monitored by the study physician upon clinical examination (anemia, hemoptysis, dyspnea, tachycardia, positive findings at lung auscultation, fever, low body mass index (BMI), and low mid-upper arm circumference (MUAC)). The primary TB score was also grouped into different severity classes as mild (SC-I: 0–5p), moderate (SC-II: 6–7) and severe (SC-III: ≥8p) disease [17].
Secondary endpoints included longitudinal assessments of the primary and a modified TB score (week 0, 4, 8, 16, and 24), sputum-smear microscopy (week 0–4, and 8) and Mtb-culture (week 0 and 8) conversion, chest X-ray (week 0, 4, 8, 16 and 24), and levels of 25-hydroxyvitamin D3 (25(OH)D3) in plasma (week 0, 4, 8 and 16). The modified TB score (3-point scale: symptom absent (0p), improved (1p) or no change/worsened (2p), max 22p) was generated using a more spread grading scale of the primary TB score, aiming to detect and include small but important changes in clinical symptoms (Table S1).
Procedures
Sputum and blood samples were collected for the described laboratory analyses. Sputum-smear microscopy and sputum-culture, erythrocyte sedimentation rate (ESR), total and differential counts, CD4 T cell counts (BD Biosciences, NJ, USA) and blood chemistry analyses were conducted at ICL, which is a Randox International Quality Assessment Scheme (RIQAS)-accredited and Centers for Disease Control and Prevention (CDC)-certified commercial laboratory in Addis Ababa, Ethiopia. Adverse events (AEs) included examinations of TB-specific clinical complications (week 4, 8, 16 and 24) and blood chemistry analysis (week 0, 4, 8 and 16) to measure liver and kidney function, and calcium/phosphate homeostasis. QuantiFERON-TB Gold in-Tube (Cellestis; Statens Serum Institut, Denmark) was assessed in whole blood samples at the Armauer Hansen Research Institute (AHRI) in Adddis Ababa, Ethiopia, according to the manufacturer’s instructions, for detection of Mtb-specific IFN-γ release in vitro. Levels of 25(OH)D3 in plasma samples were analyzed at the Department of Clinical Chemistry, Karolinska University Hospital in Stockholm, Sweden using a chemiluminescence immunoassay (CLIA) on a LIAISON-instrument (DiaSorin Inc., Stillwater, MN, USA), detectable range 7.5–175 nmol/l, CV 2–5%. Plasma 25(OH)D3 concentrations were used to determine vitD3 status and to monitor treatment adherence.
Statistical analysis
The sample size calculation was based on a previous study demonstrating that standard TB care will reduce the primary clinical TB score from 6.5 to 3.2 during the initial 8-weeks intensive-phase treatment [17]. To reduce the TB score an additional 25% above the effect of standard chemotherapy at 8-weeks (calculating with a mean TB score of 3.2 in the placebo group and a standard deviation of 2.3 in both intervention groups), a sample size of 131 patients/group was required (80% power, P < 0.05, two-sided test). The power calculations included 8-weeks data alone. But as described in the original study protocol, analyses of the primary endpoint was based on a comparison of the change in the TB score between baseline (week 0) and week 8 in the two study groups, which likely increased the power of the analyses. Assuming a dropout rate of approximately 15%, the sample size was increased to 300 patients. To compensate for the proportion of patients with sputum-negative clinical TB to patients with sputum-smear positive TB, the sample size was increased another 20%, resulting in 360 patients.
Results were analyzed following the intention-to-treat (ITT)-concept, using multiple imputation by chained equations to impute outcomes for persons lost to follow-up. We applied modified ITT (mITT) analysis, which is commonly used in antimicrobial/anti-infective trials, when test results obtained after randomization show that some patients were misdiagnosed and/or ineligible. mITT allows for these randomized subjects to be excluded from the analysis in a justified way. Per-protocol analyses included all subjects who completed the study treatment. Primary and secondary analyses were conducted using linear regression, ordinal logistic regression (AFB-grading and radiology) and logistic regression (Mtb-culture conversion). Both crude and adjusted analyses were made. The covariates adjusted for were age, gender, smear-positivity and baseline value of the outcome. Those variables were selected a priori to increase the precision of our estimates, since we believed them to be associated with the outcome [22]. Time to sputum-microscopy conversion was shown using a Kaplan-Meier-plot and a log rank test. A P-value < 0.05 was considered significant. Analyses were conducted using IBM SPSS Statistics 20.0 and Stata 13 (StataCorp, College Station, Texas, USA).
Results
Enrolment
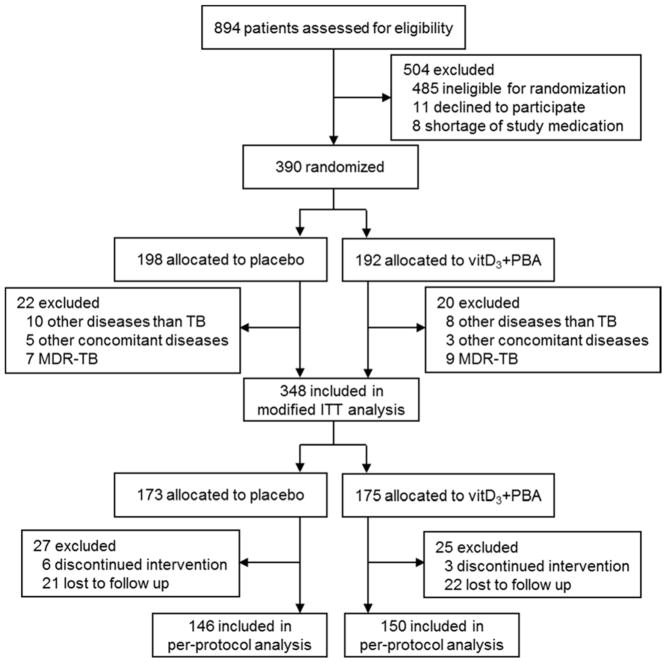
We screened 894 patients for eligibility from January 2013-May 2015 as described in the trial profile (Fig. 1). Most patients ineligible for randomization were HIV-infected (n=418). After randomization of 390 patients, laboratory testing confirmed that 42 enrolled patients did not fulfill the pre-defined exclusion criteria (other pulmonary diseases (n=18), other concomitant diseases (n=8) and MDR-TB (n=16)). The remaining 348 subjects constituted the mITT cohort, allocated to vitD3+PBA (n=175) or placebo (n=173) treatment. A total of 52 patients discontinued the intervention or were lost to follow-up (dropout rate=14.9%). Thus, 296 patients completed the treatment per-protocol, allocated to vitD3+PBA (n=150) or placebo (n=146) treatment.
Fig. 1.
Trial profile. Consort flow diagram of patients with suspected pulmonary TB, from screening to analysis. Patients ineligible for randomization included HIV infection (n=418), relocation after diagnosis (n=17), age <18 years (n=16), non-TB pleural effusions (n=16), TB relapse (n=9), too weak/old (n=4), >5 days into TB chemotherapy (n=2), pregnancy (n=2) and mental health problems (n=1). Diseases other than TB included pulmonary fibrosis (n=14), cancer (n=2), and pulmonary thromboembolism (n=2) while other concomitant diseases included HIV infection (n=2), liver disease (n=3) and renal disease (n=3). Discontinued intervention included patients with liver toxicity (n=3), adherence failure (n=5) and cancer (n=1). Lost to follow up included patients who withdraw their consent (n=30), moved from study area (n=12), or were imprisoned (n=1). Patients who dropped out from the placebo treatment at week 0: n=11, week 4: n=9, week 8: n=5 and week 16: n=2. Patients who dropped out the vitD3+PBA treatment at week 0: n=14, week 4: n=5, week 8: n=5 and week 16: n=1.
Baseline characteristics
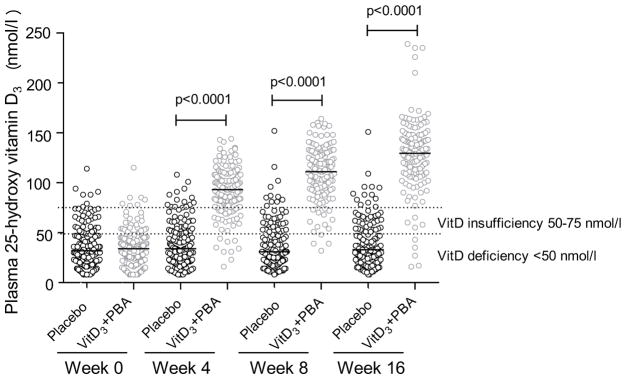
Baseline data are presented in Table 1. About 81% of all patients had a positive sputum-smear and/or Mtb-culture result, out of which 10–12% were discordant samples (Table 1). Additional 19% of the patients had a clinical TB diagnosis of whom 94% had a positive QuantiFERON result (Supporting Information). Around 50% of the patients had a BMI below 18 kg/m2 while 54% had a MUAC below 22 cm, indicating underweight (Table 2). The primary TB score had a mean of 5–6p, grouping 49.5% of the patients into severity class II–III. Plasma 25(OH)D3 concentrations were low, around 35 nmol/l. As an international reference for 25(OH)D3 concentrations in blood, we followed the Endocrine Society’s Clinical Practice Guideline defining vitD3 deficiency as a 25(OH)D3 levels below 50 nmol/l [23]. Accordingly, most TB patients were vitD3 deficient (80.6%) or insufficient (15.0%) at baseline. From Table 1, it is also evident that the distribution of gender was slightly skewed. Gender was one of the covariates adjusted for in the primary and secondary analyses.
Table 1.
Baseline characteristics
| Variables (mITT, n=348)a | Placebo (n=173) | VitD3+PBA (n=175) |
|---|---|---|
| Gender (M/F) (no/%) | 110 (64)/63 (36) | 91 (52)/84 (48) |
| Age (mean) | 30.63 | 30.31 |
| Sputum-smear status (no/%)b | ||
| pos | 120 (69.4) | 116 (66.2) |
| neg | 53 (30.6) | 59 (33.7) |
| Sputum-culture status (no/%)b | ||
| pos | 124 (71.7) | 119 (68.0) |
| neg | 25 (14.4) | 30 (17.1) |
| NDA | 25 (14.4) | 26 (14.9) |
| Clinical TB (no/%) | 31 (17.9) | 35 (20.0) |
| Pos QuantiFERON (no/%)c | 30 (96.8) | 32 (91.4) |
| Duration of cough (weeks) | 4 (3–8) | 4 (3–8) |
| History of contact (no/%) | 35 (20.2) | 45 (25.7) |
| Histrory of TB treatment (no/%)d | 17 (9.8) | 8 (4.6) |
| History of smoking (no/%) | 23 (13.3) | 33 (18.9) |
| BCG vaccination (no/%) | 50 (28.9) | 52 (29.7) |
| Weight loss (no/%) | 127 (73.4) | 111 (63.4) |
| Weight loss (kg) | 5 (3–8) | 5 (3–10) |
| BMI (median) | 18 (17–19) | 18 (17–20) |
| MUAC (median) | 22 (20–23) | 22 (21–24) |
| Pulse rate/min (median) | 87 (80–94) | 83 (78–93) |
| Respiratory rate/min (median) | 20 (18–24) | 20 (18–24) |
| WBC (median) | 7 (6–9) | 8 (5–10) |
| ESR (median) | 42 (30–52) | 46 (31–55) |
| Hemoglobin (median) | 12 (12–15) | 13 (12–14) |
| Calcium (median) | 9 (8–9) | 9 (8–10) |
| Albumin (median) | 4 (3–4) | 4 (3–4) |
| TB score (median/IQR) | ||
| Primary TB score | 6 (4–7) | 5 (4–7) |
| Modified TB score | 9 (7–12) | 9 (6–12) |
| Primary TB score: severity class (no/%) | ||
| SC-I: 0–5 | 83 (48.0) | 93 (53.1) |
| SC-II: 6–7 | 56 (32.4) | 44 (25.1) |
| SC-III: ≥8 | 34 (19.6) | 38 (21.7) |
| 25(OH)D3 nmol/l (mean) | 35.47 | 34.72 |
| Deficiency <50 nmol/l (no/%) | 135 (78.0) | 144 (83.2) |
| Insufficiency 50–75 nmol/l | 31 (17.9) | 21 (12.1) |
| Sufficiency >75 nmol/l | 7 (4.0) | 8 (4.6) |
mITT, modified intention-to-treat; NDA, no data available; BCG, Bacillus Calmette Guerin; IQR, interquartile range; BMI, Body Mass Index; MUAC, Mid-Upper-Arm-Circumference; WBC, white blood cell; ESR, erythrocyte sedimentation rate; SC, severity class; 25(OH)D3, 25-hydroxyvitamin D
Data are n (%), mean or median (IQR).
Sputum-microscopy and sputum-culture positivity were not always overlapping, but around 10–12% of the samples were discordant.
Three patients had a negative QuantiFERON; vitD3+PBA (n=2) and placebo (n=1), and one patient had no QuantiFERON test taken, vitD3+PBA (n=1).
Treatment with anti-TB drugs >2 years before study enrollment.
Table 2.
Baseline data in clinical TB scores
| Variables (mITT, n=348)a | Placebo (n=173) | VitD3+PBA (n=175) |
|---|---|---|
| Cough (0/1) | 8/165 | 3/172 |
| Night sweats (0/1) | 30/143 | 29/146 |
| Chest pain (0/1) | 61/112 | 63/112 |
| Conjunctiva pallor (0/1) | 157/16 | 146/28 |
| Anemia (Hb, mg/dl) (0/1) | 119/54 | 116/59 |
| Hemoptysis (0/1) | 145/28 | 149/26 |
| Dyspnea (0/1) | 102/71 | 102/73 |
| Tachycardia (0/1) | 81/92 | 78/97 |
| Lung auscultations (0/1) | 116/57 | 106/69 |
| Fever (0/1) | 166/7 | 169/6 |
| BMI <18 (0/1) | 77/96 | 95/80 |
| MUAC <220 cm (0/1) | 71/102 | 90/85 |
mITT, modified intention-to-treat; Hb, hemoglobin; BMI, Body Mass Index; MUAC, Mid-Upper-Arm-Circumference
Clinical symptoms in the primary TB score are reported as absent (0) or present (1). Data are numbers.
Primary endpoint: Clinical TB score
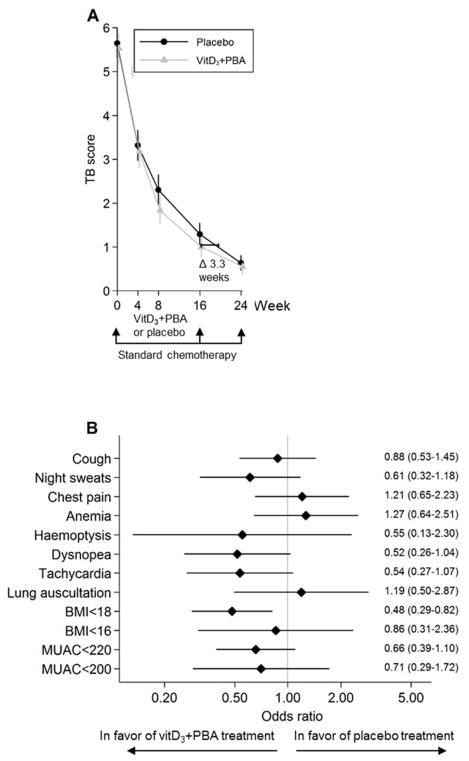
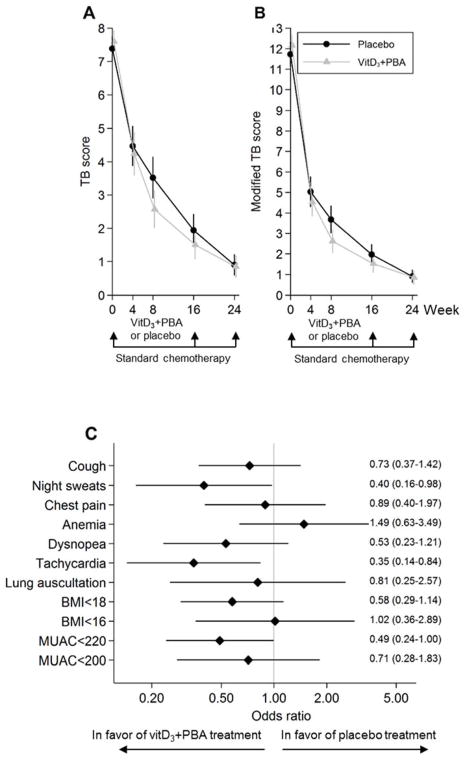
In the TB score, cough, night sweats, chest pain, tachycardia, low BMI, and low MUAC were the most common clinical symptoms, while conjunctiva pallor, hemoptysis, and fever were less frequent (Table 2). Longitudinal assessments of the primary TB score are illustrated in Figure 2a and Figure S1, and the differences and 95% CI are shown in Table 3. In the adjusted mITT analysis, the primary TB score was significantly reduced at week 8 (P = 0.015) and the modified TB score was significantly reduced at weeks 8 (P = 0.01) and 16 (P = 0.03) in the vitD3+PBA group compared with placebo. Similarly, in the adjusted per-protocol analysis, we observed a significant reduction of both the primary (P = 0.022) and the modified (P = 0.016) TB score at week 8. Overall, the odds ratios of individual clinical symptoms predominantly favored the treatment group (Fig. 2b).
Fig. 2.
Primary efficacy analyses. (a) The primary clinical TB score was assessed at baseline and at weeks 4, 8, 16, and 24 after initiation of anti-TB chemotherapy. Adjunct vitD3+PBA treatment was provided during the first 16 weeks of standard care. The efficacy analysis included comparison of the vitD3+PBA and placebo treatment between week 0 and week 8. Crude data from the mITT cohort are presented as the mean and 95% CI. The blue line (circles) represents placebo while the red line (triangles) represents vitD3+PBA treatment. The horizontal bar indicate the estimated difference (given a linear reduction of the TB score) in weeks that it would take to reduce the primary TB score in the placebo group to a level comparable to the TB score in the vitD3+PBA group assessed at the end of adjunct treatment at week 16. (b) Forrest plot showing the odds ratio of the individual diseases symptoms included in the primary efficacy analysis. The estimate and 95% CI at week 8 are shown.
Table 3.
Clinical TB score in vitD3+PBA versus placebo
| Crude | Adjusteda | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Endpoint | Week | n | Difference | 95% CI | P Value | Difference | 95% CI | P Value |
| All patients (mITT) | ||||||||
| Primary TB score | 4 | 348 | −0.07 | (−0.49 to 0.35) | 0.741 | −0.16 | (−0.54 to 0.23) | 0.425 |
| 8 | 348 | −0.42 | (−0.90 to 0.06) | 0.089 | −0.52 | (−0.93 to −0.10) | 0.015 | |
| 16 | 348 | −0.17 | (−0.65 to 0.31) | 0.483 | −0.32 | (−0.65 to 0.00) | 0.051 | |
| Modified TB score | 4 | 348 | −0.13 | (−0.80 to 0.54) | 0.700 | −0.24 | (−0.77 to 0.29) | 0.375 |
| 8 | 348 | −0.48 | (−1.15 to 0.20) | 0.165 | −0.58 | (−1.02 to −0.14) | 0.010 | |
| 16 | 348 | −0.19 | (−0.88 to 0.49) | 0.580 | −0.34 | (−0.64 to −0.03) | 0.030 | |
| Patients (per-protocol) | ||||||||
| Primary TB score | 4 | 320 | −0.10 | (−0.54 to 0.33) | 0.635 | −0.17 | (−0.55 to 0.25) | 0.454 |
| 8 | 309 | −0.42 | (−0.90 to 0.06) | 0.087 | −0.47 | (−0.86 to −0.07) | 0.022 | |
| 16 | 302 | −0.22 | (−0.71 to 0.28) | 0.386 | −0.30 | (−0.61 to 0.01) | 0.055 | |
| Modified TB score | 4 | 320 | −0.21 | (−0.86 to 0.45) | 0.533 | −0.28 | (−0.82 to 0.26) | 0.315 |
| 8 | 309 | −0.53 | (−1.23 to 0.17) | 0.134 | −0.62 | (−1.11 to −0.11) | 0.016 | |
| 16 | 302 | −0.27 | (−1.02 to 0.47) | 0.474 | −0.40 | (−0.81 to 0.02) | 0.060 | |
| Patients with 25(OH)D3 ≤50 nmol/l + TB score >5 (per-protocol) | ||||||||
| Primary TB score | 4 | 125 | −0.38 | (−1.10 to 0.34) | 0.296 | −0.46 | (−1.18 to 0.26) | 0.209 |
| 8 | 120 | −1.12 | (−1.90 to −0.34) | 0.005 | −1.11 | (−1.89 to −0.34) | 0.005 | |
| 16 | 118 | −0.62 | (−1.29 to 0.05) | 0.070 | −0.61 | (−1.21 to 0.00) | 0.051 | |
| Modified TB score | 4 | 125 | −0.84 | (−1.74 to 0.06) | 0.066 | −0.89 | (−1.79 to 0.01) | 0.053 |
| 8 | 120 | −1.43 | (−2.39 to −0.47) | 0.004 | −1.37 | (−2.28 to −0.47) | 0.003 | |
| 16 | 118 | −0.91 | (−1.83 to 0.01) | 0.052 | −0.83 | (−1.61 to −0.06) | 0.036 | |
CI, confidence interval; mITT, modified intention-to-treat; 25(OH)D3, 25-hydroxyvitamin D
Data are adjusted for gender, age, and TB score and sputum-smear positivity at baseline.
Secondary endpoints: Sputum-conversion analyses and 25(OH)D3 levels in plasma
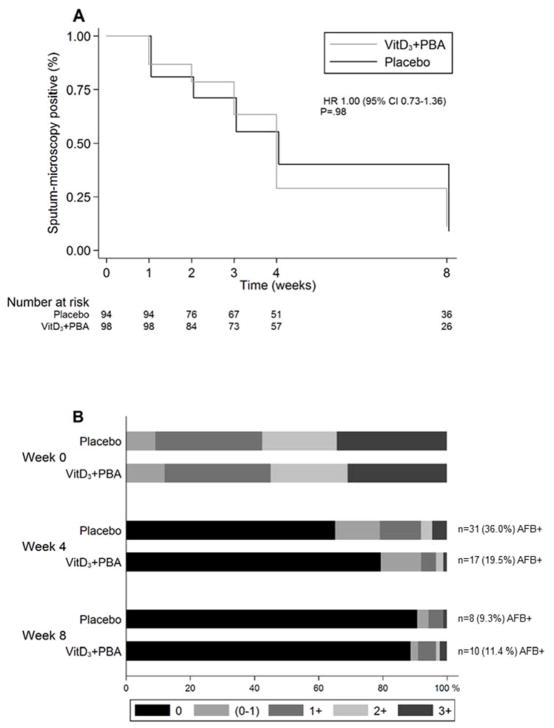
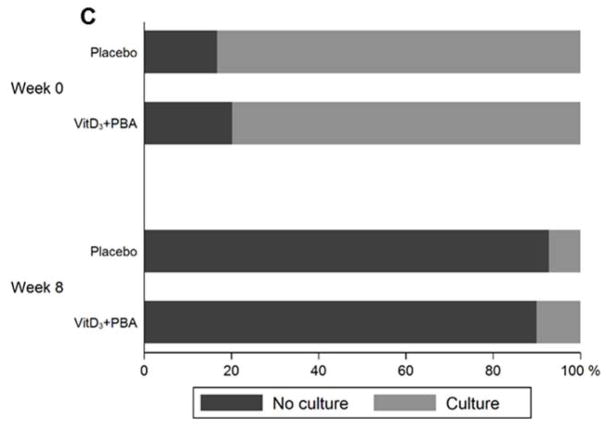
Sputum-conversion rates and AFB-grading are illustrated in Figure 3 and the odds ratio and 95% CI are shown in Table 4. Longitudinal analysis showed no significant effect of vitD3+PBA treatment on the time to sputum-microscopy conversion in smear-positive patients (P = 0.98) (Fig. 3a). However, AFB-grading demonstrated a significant reduction of smear-positive TB in the intervention group at week 4 using both mITT (P = 0.017 and P = 0.037) and per-protocol analyses (P = 0.024 and P = 0.038), although this difference was no longer detected at week 8 (Fig. 3b and Table 3). Neither, could we detect enhanced Mtb-culture conversion (Fig. 3c) or radiological improvement (Fig. S2).
Fig. 3.
Sputum-smear conversion analyses. (a) Longitudinal analysis of time to sputum-smear conversion after initiation of anti-TB chemotherapy in patients who were sputum-microscopy positive at enrolment. Crude data are presented in a Kaplan-Meier curve. The blue line represents placebo while the red line represents vitD3+PBA treatment. The hazard ratio (HR) and 95% CI is shown. (b) AFB-grading among sputum-smear positive TB patients at baseline compared to week 4 and 8 after initiation of anti-TB chemotherapy. AFB-positivity (+) was graded using microscopy as no AFB (negative), scanty (0–1), +1, +2, or +3 AFB. Data are shown in a bar graph with a colour scale from 0 (red) to 3+ (blue) AFB. The numbers and proportion of AFB+ TB patients in the placebo vs vitD3+PBA group at week 4 and 8 are also indicated in the graph. Patients with a negative sputum-smear result at baseline were excluded from the conversion analysis. (c) Sputum-culture conversion among both Mtb-culture positive and negative TB patients at baseline compared to week 8 after initiation of anti-TB chemotherapy. Bar graph showing negative Mtb-culture (red) vs positive Mtb-culture (blue).
Table 4.
Sputum-smear conversion in vitD3+PBA versus placebo
| Crude | Adjusteda | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Endpoint | Week | n | OR | 95% CI | P Value | OR | 95% CI | P Value |
| Smear-positive patients (mITT) | ||||||||
| Sputum-positivity | 4 | 199 | 0.45 | (0.23 to 0.87) | 0.017 | 0.49 | (0.25 to 0.94) | 0.037 |
| 8 | 199 | 1.07 | (0.43 to 2.65) | 0.879 | 1.05 | (0.42 to 2.63) | 0.904 | |
| Smear-positive patients (per-protocol) | ||||||||
| Sputum-positivity | 4 | 173 | 0.46 | (0.23 to 0.90) | 0.024 | 0.48 | (0.24 to 0.95) | 0.038 |
| 8 | 174 | 1.29 | (0.48 to 3.42) | 0.616 | 1.29 | (0.48 to 3.47) | 0.584 | |
| Smear-positive patients with elevated 25(OH)D3 levels at week 4 (per-protocol) | ||||||||
| Sputum-positivity | 4 | 170 | 0·98 | (0·97–1·00) | 0·005 | 0·99 | (0·97–1·00) | 0·008 |
OR, odds ratio; CI, confidence interval; mITT, modified intention-to-treat
Data are adjusted for gender, age and sputum-smear positivity at baseline.
Most subjects had low plasma 25(OH)D3 levels at baseline that increased significantly (P < 0.0001) in the vitD3+PBA group compared with placebo at week 4 (mean 38.6 vs 91.5 nmol/l), week 8 (mean 38.4 vs 109.4 nmol/l), and week 16 (mean 40.1 vs 127.4 nmol/l) (Fig. 4), which indicated good adherence. Baseline levels of 25(OH)D3 also increased significantly (P = 0.0014) in the placebo group at week 16 (mean 35.5 vs 39.9 nmol/l), although this increase was modest compared with the increase in the vitD3+PBA group at week 16 (4.4 nmol/l vs 92.7 nmol/l). Thus, vitD3 deficiency in the vitD3+PBA group was rapidly corrected and most patients reached optimal 25(OH)D3 levels >75 nmol/l within 4 weeks of vitD3+PBA supplementation. Interestingly, patients who raised their baseline 25(OH)D3 levels at week 4, regardless of treatment allocation, were significantly more likely to have reduced AFB in sputum compared with patients who maintained low 25(OH)D3 levels (P = 0.005 and P = 0.008) (Table 4). Thus, the odds of an AFB-positive sputum sample was reduced with 2% per unit increase in 25(OH)D3 concentration.
Fig. 4.
VitD3 analysis. Plasma levels of 25(OH)D3 in the placebo compared to the vitD3+PBA group at baseline and at weeks 4, 8, and 16 after initiation of anti-TB chemotherapy. Data are shown in a scatter dot plot with blue symbols for placebo and red symbols for vitD3+PBA treatment. The solid line indicates the median, and the dashed lines mark the thresholds for vitD3 deficiency and insufficiency.
Subgroup analyses: Clinical TB score in patients with vitD3 deficiency and moderate-to-severe TB
A sub-group analysis showed that vitD3+PBA treatment was most beneficial in vitD3 deficient patients with moderate-to-severe disease (TB score>5 i.e. severity class II–III) (P for interaction = 0.016) (Table S2). The primary and modified TB scores in this group are illustrated in Figure 5 and the differences and 95% CI are shown in Table 3. Per-protocol analyses revealed a significant reduction in the primary TB score at week 8 (P = 0.005), while the modified TB score was significantly reduced at weeks 8 (P = 0.004 and P = 0.003) and 16 (P = 0.036) in the vitD3+PBA group. Furthermore, vitamin D responders ie. TB patients with 25(OH)D3 levels ≤50 nmol/l at baseline and >75 nmol/l at week 16, revealed a significant decrease in both primary and modified TB scores at week 8 (P = 0.034 and P = 0.014) and week 16 (P = 0.021 and P = 0.023) (Table S2).
Fig. 5.
Subgroup analyses. Longitudinal assessment of (a) the primary or (b) the modified TB score in TB patients with 25(OH)D3 levels ≤50 nmol/l and a TB score>5 at baseline. The primary clinical TB score and the modified TB score were assessed at baseline and at weeks 4, 8, 16, and 24. Crude data from this cohort are presented as the mean and 95% CI. The blue line (circles) represents placebo while the red line (triangles) represents vitD3+PBA treatment. (c) Forrest plot showing the odds ratio of the individual diseases symptoms included in the primary TB score for TB patients with 25(OH)D3 levels ≤50 nmol/l and a TB score>5 at baseline. The estimate and 95% CI at week 8 are shown.
Adverse events
The major clinical AEs observed at follow-up were reported as TB-specific clinical complications listed in Table 5. The most common manifestations in the treatment group were chest pain and anemia while the placebo group patients commonly experienced chest pain, dyspnea, and dyspepsia. Significantly fewer clinical complications were reported in the vitD3+PBA group compared with placebo (22 vs 42; P = 0.006). No clinically relevant changes in blood chemistry (calcium, phosphate, albumin, or creatine) related to the intervention were observed (Table S3 and S4).
Table 5.
Adverse events
| Manifestation (no/%) | Placebo (n=173) | VitD3+PBA (n=175) |
|---|---|---|
| Chest pain | 8 (4.6) | 6 (3.4) |
| Dyspnea | 9 (5.2) | 2 (1.1) |
| Anemia | 3 (1.7) | 5 (2.8) |
| Numbness | 2 (1.2) | 2 (1.1) |
| Dyspepsia | 4 (2.3) | 0 |
| Night sweats | 1 (0.6) | 3 (1.7) |
| Hemoptysis | 3 (1.7) | 0 |
| Flank pain | 3 (1.7) | 1 (0.6) |
| Pneumonia | 2 (1.2) | 0 |
| Arthralgia | 1 (0.6) | 1 (0.6) |
| Exacerbated asthma | 1 (0.6) | 0 |
| Oral rash | 1 (0.6) | 0 |
| Skin rash | 2 (1.2) | 1 (0.6) |
| Diarrhea | 1 (0.6) | 0 |
| Ear discharge | 1 (0.6) | 0 |
| Breast abscess | 0 | 1 (0.6) |
| Total AEsa | 42 (24.3) | 22 (12.6) |
AE = adverse event
All AEs were grade 1 or mild, apart from the oral rash that was classified as a grade 2 AE. All AEs were experienced by different individuals.
Discussion
In this trial, we tested if daily adjunct therapy with vitD3+PBA could improve clinical symptoms in smear-positive as well as smear-negative patients with pulmonary TB. Supplemented patients had an enhanced clinical recovery assessed as a reduction in clinical TB score during the first 8-weeks of intensive-phase treatment. The intervention did not influence time to sputum-microscopy conversion, although the odds of an AFB-positive result was significantly lower in the vitD3+PBA group at week 4. The intervention was particularly effective in patients with low 25(OH)D3 levels and an elevated TB score at enrolment, suggesting that disease amelioration was more efficient in vitD3 deficient patients with more pronounced clinical symptoms. Moreover, it was safe to administer vitD3+PBA daily for 16 weeks and clinical AEs were more common in the placebo group. We conclude that adjunct therapy with vitD3+PBA may contribute to reduced disease severity and reduced clinical complications in patients with pulmonary TB, while the treatment had less effects on bacterial clearance in vivo.
Our study has several limitations. A randomized study does not exclude the possibility of chance imbalances at baseline. In our study, this imbalance was observed in the somewhat skewed distribution of gender in the placebo compared with the vitD3+PBA group. However, the adjusted analysis corrected for this imbalance. Furthermore, designing a 2-arm intervention trial enabled an increased sample size per group, but prevented assessment of the individual effects of vitD3 or PBA. Although the synergistic or additive effects of these compounds have been well-described in vitro [7–9] and ex vivo [10, 11], additional clinical studies will contribute to an increased understanding of vitD3+PBA treatment effects in vivo.
Furthermore, we used a semi-soft endpoint as primary outcome, a TB score that is a rapid, low-cost method for clinical monitoring of TB in resource-poor settings [17]. This validated score has been successfully used to follow prognosis and treatment outcome especially in smear-negative TB patients [13, 18, 19]. The score correlated with grade of smear-positivity [17], and an elevated TB score at week 8 (i.e. SC-III) was associated to higher mortality and poor prognosis [19]. It is possible that smear-negative TB patients have a milder form of disease, including lower bacterial loads and less severe symptoms. Nevertheless, many of these patients start chemotherapy in line with WHO guidelines. Only including sputum-positive TB patients, representing 40–70% of all cases, may generate a selection bias that is not representative of standard clinical care. Thus, we maintain that a composite clinical score has advantages in measuring TB outcomes, particularly in routine clinical practice where sputum results are frequently negative.
It is difficult to show an effect of vitD3+PBA on top of the highly effective standard chemotherapy. In this study, an additional 25% reduction of the TB score in the intervention group compared with the standard drugs at 8 weeks, was considered a significant effect. This change in TB score has previously been used to define clinical improvement [17]. The intervention had a significant effect on the composite TB scores, but not on any given symptom alone. The modified TB score was significantly reduced at both weeks 8 and 16 compared with week 8 for the primary TB score, also showing lower P-values. This indicated that using a more nuanced grading scale (3-point instead of 2-point scale) of the validated primary TB score may increase the likelihood to detect changes in clinical symptoms among the study subjects. About 3–4 weeks extra time was required to reduce the primary TB score in the placebo group to a level comparable with the vitD3+PBA group at the end of adjunctive therapy at week 16. At the end of standard chemotherapy at week 24, most patients had a TB score below 1, which suggested that the majority of clinical symptoms had disappeared due to the successful effects of lengthy 6-months standard care. Importantly, the reduction in both TB scores were more powerful in the subgroup analyses including one third of the patients with vitD3 deficiency and more advanced TB disease, which strengthen the results of the primary analysis. Altogether, these data support the clinical relevance of our findings, although continued investigations will need to validate their applicability. Importantly, for a common infectious disease such as TB, even a small-to-moderate clinical effect on top of already existing standard treatment, may have significant positive effects on treatment outcome [24].
This study failed to show significant effects of vitD3+PBA treatment on sputum conversion rates. The sensitivity and specificity of sputum-smear microscopy is limited, although this is the most common method for TB diagnosis and to follow treatment outcome [25]. Microscopy targets the most infectious cases with a threshold for Mtb detection of <10 000 bacilli/ml of sputum, and therefore fails to diagnose clinical TB in many smear-negative patients [25]. Sputum-culture is more sensitive, but time-consuming and prevents grading of the bacterial load. Possibly, the standard anti-TB drugs are so effective to reduce bacterial growth that the potential anti-mycobacterial effects of vitD3+PBA will be masked. Consequently, TB trial results could be misinterpreted if the primary effect of vitD3+PBA is to modulate inflammatory responses or in other ways affect physiological processes that will improve clinical but not bacteriological outcomes [12, 26]. A recently described role for parent vitD3, the 25(OH)D3 proform and the active 1,25(OH)D3 metabolite, is to stabilize the endothelium, which is typically activated and destabilized during inflammation [27]. Interestingly, such vascular stabilization occurs independently from the antimicrobial effector functions triggered via intracellular vitD3 receptor signaling. Such effects may be better assessed using clinical improvement, resolution of inflammation and prevention of relapse.
This study also has several strengths. The majority of TB patients had a vitD3 deficiency at baseline that was rapidly corrected upon vitD3+PBA treatment. Compelling evidence suggests that a low vitD3 status may enhance susceptibility to active TB [28, 29]. Importantly, basal 25(OH)D3 levels can vary substantially between different populations and therefore TB patients may respond differently to vitD3 supplementation. TB patients in Tanzania [30], India [31], and Guinea-Bissau [13] had higher 25(OH)D3 levels (ranges: 62–91 nmol/l), while patients in South Africa [28], Bangladesh [11], Pakistan [18], and the UK [14] were mostly vitD3 deficient (ranges: 20–34 nmol/l). Therefore, screening for vitD3 deficiency before start of standard treatment may increase the likelihood of successful adjunctive therapy with vitD3 and/or PBA.
Another strength was that daily doses of vitD3 was administered together with PBA instead of using a bolus regimen. Due to the short half-life of parent vitD3 (12–24h), even large bolus doses are rapidly cleared from the circulation. Moreover, the cellular availability of vitD3 and its proform is very different since 25(OH)D3 is tightly bound to the vitD3 binding protein, reducing cellular entry and activation compared with vitD3 [32]. While daily dosing will sustain stable and physiological concentrations of circulating vitD3, high-dose, long-interval dosing will result in large fluctuations in circulating vitD3 concentrations [32]. The unfavorable consequences of such pharmacological dosing is underappreciated, as this will severely reduce a continuous supply of bioavailable intact vitD3 as the major source for cellular uptake and conversion to the active metabolite that can maintain optimal functions of vitD3-induced systems.
Until 2017, eleven randomized trials have been published investigating the therapeutic potential of adjunctive vitD3 treatment in TB [11, 13–15, 18, 31, 33–37], but consensus on the potential beneficial effects is still lacking. Most trials were too small to demonstrate statistical power, the dosage regimen of vitD3 was highly variable, as were baseline concentrations of 25(OH)D3. The primary endpoint was mainly time to sputum conversion, while treatment efficacy including smear-negative patients have rarely been reported. Importantly, most studies used bolus doses of vitD3, which have consistently failed to support clinical and microbiological efficacy in TB [13–15, 31, 36, 37]. VitD3 given at an early stage of chemotherapy (0, 14, 28, and 42 days) resulted in enhanced sputum conversion only in patients with the Taq1 tt genotype of the VDR [14], while vitD3 provided at later time-points (0, 5, and 8 months) failed to increase 25(OH)D3 levels and accordingly had no effect compared to placebo [13]. Two doses of 200 000 IU vitD3 (0 and 4 weeks) showed significant effects on weight gain, BMI, and pulmonary involvement, but had no overall effect on the clinical TB score or smear conversion [18]. However, patients with 25(OH)D3<30 nmol/l at enrolment revealed significantly lower TB scores and a clear trend towards enhanced bacterial sputum clearance [18]. Similarly, daily vitD3+PBA treatment reduced both primary and modified TB scores more robustly in vitD3 deficient patients with moderate-to-severe TB disease. Likewise, vitD3 supplementation did not affect the time to first exacerbation in patients with COPD, but subgroup analysis revealed significant effects in vitD3 deficient patients [38, 39]. Consistently, a recent meta-analysis provided evidence that daily-weekly administration of vitD3 reduced the risk of acute respiratory tract infections, particularly among individuals with low vitD3 levels [40]. Altogether, these studies underline that the protective effects of vitD3 supplementation is most likely affected by baseline vitD3 status.
Conclusion
Our results suggest that a physiological dosing schedule based on daily supplementation with vitD3 in combination with PBA can be used to ameliorate clinical symptoms and TB-specific AEs, primarily in vitD3 deficient TB patients. Therefore, although vitD3+PBA may not be applicable as a therapeutic intervention to a broad range of TB patients, supplementation may turn out promising for certain high-risk groups with vitD3 deficiency, immunodeficiency diseases, MDR-TB or latent TB. In contrast to treatment of active TB, there is a possibility that nutritional supplementation will have a greater impact on the prevention of disease among individuals with latent TB and vitD3 deficiency [41]. Such prophylactic studies are complicated to implement, but would shed additional light on the potential benefit of vitD3+PBA immunotherapy.
Supplementary Material
Acknowledgments
We thank the local study team at the Black Lion Hospital and the Armauer Hansen Research Institute (AHRI), as well as all the nurses and administrative staff at the collaborative health centers in Addis Ababa, Ethiopia. We also thank the members of our Data Monitoring and Safety Board. Finally, we would like to sincerely thank all the patients who participated in this trial. We also thank BioScience Writers (BSW) in Houston, Texas, for assistance with language editing of this manuscript.
Funding
This study was funded by the Swedish Contingency Agency (MSB) and the Swedish International Development Agency (Sida) (2010-7938), the Swedish Research Council (VR) (K2015-56X-20665-08-3), the Swedish Heart and Lung Foundation (HLF) (20140752) and Karolinska Institutet (senior research position of SB). Merck Serono kindly donated Vigantoletten and placebo tablets and also assisted the study with labels. NB was supported in part by the National Institute of Health (NIH) Research Training grant R25TW009337, funded by the Fogarty International Center, the NIH Office of the Director, and the National Institute of Mental Health.
Abbreviations
- TB
tuberculosis
- Mtb
Mycobacterium tuberculosis
- VitD3
vitamin D3
- 25(OH)D3
25-hydroxyvitamin D3
- 1,25(OH)D3
1,25-dihydroxyvitamin D3
- PBA
phenylbutyrate
- HDAC
histone deacetylase inhibitor
- RCT
randomized controlled trial
- AFB
acid-fast bacilli
- WHO
World Health Organization
- HIV
human immunodeficiency virus
- MDR-TB
multidrug-resistant TB
- mITT
modified intention-to-treat
- SC
severity class
- BMI
body mass index
- MUAC
mid-upper arm circumference
- AE
adverse event
- CI
confidence interval
- OR
odds ratio
- HR
hazard ratio
Footnotes
Conflict of interest statement
No conflict of interest to declare.
Author’s contributions
S.B., P.B., J.A., S.A., A.B., E.K., W.A., G.Ad., B.A. and R.R. contributed to study design. S.B., P.B. and J.A. wrote the protocol. S.B. acquired funding. S.B., S.A., N.G. and A.B. acquired ethics permissions. N.G., A.B., S.A., M.T., E.K., W.A. and G.Ad. coordinated the clinical work, collected data and participated in data management. G.As. read chest radiographs. N.G. and A.S. coordinated the laboratory analyses. S.B., N.G., S.A. and G.Ad. supervised data collection. S.B., P.B., J.A. participated in data analysis, and data interpretation. U.H. and A.W. performed the statistical analyses. S.B. wrote the manuscript and did the literature search; all other authors critically reviewed the content and approved the final version.
Additional Supporting Information may be found in the online version of this article:
References
- 1.Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis. 2016;63:e147–95. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Rheum Dis Clin North Am. 2012;38:125–39. doi: 10.1016/j.rdc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 4.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 5.Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Kai-Larsen Y, Agerberth B. The role of the multifunctional peptide LL-37 in host defense. Front Biosci. 2008;13:3760–7. doi: 10.2741/2964. [DOI] [PubMed] [Google Scholar]
- 7.Steinmann J, Halldorsson S, Agerberth B, Gudmundsson GH. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother. 2009;53:5127–33. doi: 10.1128/AAC.00818-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rekha RS, Rao Muvva SS, Wan M, et al. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy. 2015;11:1688–99. doi: 10.1080/15548627.2015.1075110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coussens AK, Wilkinson RJ, Martineau AR. Phenylbutyrate is bacteriostatic against Mycobacterium tuberculosis and regulates the macrophage response to infection, synergistically with 25-hydroxy-vitamin D3. PLoS pathog. 2015;11:e1005007. doi: 10.1371/journal.ppat.1005007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mily A, Rekha RS, Kamal SM, et al. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: a dose finding study for treatment of tuberculosis. BMC Pulm Med. 2013;13:23. doi: 10.1186/1471-2466-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mily A, Rekha RS, Kamal SM, et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: A randomized controlled trial. PloS one. 2015;10:e0138340. doi: 10.1371/journal.pone.0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coussens AK, Wilkinson RJ, Hanifa Y, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci U S A. 2012;109:15449–54. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843–50. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 14.Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–50. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganmaa D, Munkhzul B, Fawzi W, et al. High-Dose vitamin D3 during tuberculosis treatment in Mongolia. A randomized controlled trial. Am J Respir Crit Care Med. 2017;196:628–37. doi: 10.1164/rccm.201705-0936OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–9. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 17.Wejse C, Gustafson P, Nielsen J, et al. TBscore: Signs and symptoms from tuberculosis patients in a low-resource setting have predictive value and may be used to assess clinical course. Scand J Infect Dis. 2008;40:111–20. doi: 10.1080/00365540701558698. [DOI] [PubMed] [Google Scholar]
- 18.Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis. BMC Infect Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janols H, Abate E, Idh J, et al. Early treatment response evaluated by a clinical scoring system correlates with the prognosis of pulmonary tuberculosis patients in Ethiopia: a prospective follow-up study. Scand J Infect Dis. 2012;44:828–34. doi: 10.3109/00365548.2012.694468. [DOI] [PubMed] [Google Scholar]
- 20.Parikh R, Nataraj G, Kanade S, Khatri V, Mehta P. Time to sputum conversion in smear positive pulmonary TB patients on category I DOTS and factors delaying it. J Assoc Physicians India. 2012;60:22–6. [PubMed] [Google Scholar]
- 21.Bergman P, Norlin AC, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ open. 2012;2:e001663. doi: 10.1136/bmjopen-2012-001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–30. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 24.Iacobucci G. Vitamin D supplementation does cut respiratory infections, new study suggests. BMJ. 2017;356:j847. doi: 10.1136/bmj.j847. [DOI] [PubMed] [Google Scholar]
- 25.Keeler E, Perkins MD, Small P, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature. 2006;444(Suppl 1):49–57. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 26.Cegielski P, Vernon A. Tuberculosis and vitamin D: what’s the rest of the story? Lancet Infect Dis. 2015;15:489–90. doi: 10.1016/S1473-3099(15)70163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson CC, Davis CT, Zhu W, et al. Dietary vitamin D and its metabolites non-genomically stabilize the endothelium. PloS one. 2015;10:e0140370. doi: 10.1371/journal.pone.0140370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martineau AR, Nhamoyebonde S, Oni T, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci U S A. 2011;108:19013–7. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 30.Tostmann A, Wielders JP, Kibiki GS, Verhoef H, Boeree MJ, van der Ven AJ. Serum 25-hydroxy-vitamin D3 concentrations increase during tuberculosis treatment in Tanzania. Int J Tuberc Lung Dis. 2010;14:1147–52. [PubMed] [Google Scholar]
- 31.Daley P, Jagannathan V, John KR, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15:528–34. doi: 10.1016/S1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]
- 32.Hollis BW, Wagner CL. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98:4619–28. doi: 10.1210/jc.2013-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 34.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 35.Morcos MM, Gabr AA, Samuel S, et al. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137:157–64. [PubMed] [Google Scholar]
- 36.Ralph AP, Waramori G, Pontororing GJ, et al. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PloS one. 2013;8:e70032. doi: 10.1371/journal.pone.0070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tukvadze N, Sanikidze E, Kipiani M, et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;102:1059–69. doi: 10.3945/ajcn.115.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156:105–14. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 39.Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:120–30. doi: 10.1016/S2213-2600(14)70255-3. [DOI] [PubMed] [Google Scholar]
- 40.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganmaa D, Giovannucci E, Bloom BR, et al. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr. 2012;96:391–6. doi: 10.3945/ajcn.112.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.