Abstract
PURPOSE
In this study, the shear bond strengths (SBS) of luting cements to fixed superstructure metal surfaces under various seating forces were investigated.
MATERIALS AND METHODS
Seven different cements [Polycarboxylate (PCC), Glass-Ionomer (GIC), Zinc phospahate (ZPC), Self-adhesive resin (RXU), Resin (C&B), and Temporary cements ((RXT) and (TCS))] were bonded to a total number of 224 square blocks (5×5×3 mm) made of one pure metal [Titanium (CP Ti) and two metal alloys [Gold-Platinum (Au-Pt) and Cobalt-Chrome (Co-Cr)] under 10 N and 50 N seating forces. SBS values were determined and data were analyzed with 3-way ANOVA. Pairwise comparisons and interactions among groups were analyzed with Tukey's simultaneous confidence intervals.
RESULTS
Overall mean scores indicated that Co-Cr showed the highest SBS values (1.96±0.4) (P<.00), while Au-Pt showed the lowest among all metals tested (1.57±0.4) (P<.00). Except for PCC/CP Ti, RXU/CP Ti, and GIC/Au-Pt factor level combinations (P<.00), the cements tested under 10 N seating force showed no significantly higher SBS values when compared to the values of those tested under 50 N seating force (P>.05). The PCC cement showed the highest mean SBS score (3.59±0.07) among all cements tested (P<.00), while the resin-based temporary luting cement RXT showed the lowest (0.39±0.07) (P<.00).
CONCLUSION
Polycarboxylate cement provides reliable bonding performance to metal surfaces. Resin-based temporary luting cements can be used when retrievability is needed. GIC is not suitable for permanent cementation of fixed dental prostheses consisting of CP Ti or Au-Pt substructures.
Keywords: Metal alloy, Cement, Bond strength, Seating force
INTRODUCTION
Implant-supported fixed dental prostheses have become a widely used dental treatment option in current dentistry. These restorations are either cemented or screw retained on the implant abutments. However, the retention and long-term stability of the restorations play an important role in the success of these treatments.1 Although no consensus exists on which method of retention is superior, cemented restorations seem to become popular because of advantages such as an improvement of esthetics because of the lack of visible screw-access openings, elimination of loosening or breakage of the restoration retaining screws, and simple cementation procedure similar to that of tooth-supported restorations.2,3
The following factors influence the retention in cement-supported implant-supported fixed restorations: taper or parallel, surface area and height, surface finish or roughness, the type of cement, and the cement film thickness. The decision to select the correct cement is controversial as a great variety of cements are on the dental market.4 Some authors recommend the use of provisional cements to facilitate retrievability without damaging the restoration or the implant and its abutment; however, low mechanical properties and high solubility are the disadvantages of these cement types.5,6 On the other hand, permanent cements for implant-supported restorations may also cause difficulties to retrieve. The retention of residual cement in the soft tissues due to problems in removing excess cement may result in periimplant tissue inflammation.3,7 For all these reasons, it is preferable to use a cement that provides adequate retention of the restoration and yet allows retrieval.3,8
The luting cement should completely fill the space between the restoration and the abutment with no marginal discrepancy. Although complete seating of the restoration with low film thickness is reported as an important factor during cementation, the results of the bond strength studies related to the cement film thickness are contradictory. 9,10,11,12,13,14 Furthermore, the cement film thickness is strongly influenced by the type of luting cement and the seating force applied. It is well known that the amount of force required to allow maximum seating is cement-specific.15,16,17 The forces routinely used for crown cementation lie within a range of 10 N to 60 N and a seating force higher than 100 N may present a risk of pulpal damage and crown deformation.18 On the other hand, it was confirmed that an increased seating force from 5 N or 20 N to 100 N improve the seating of crowns during cementation.19
A strong and durable bond between a metal framework and a luting agent is also important to withstand various changes in the oral environment.20 The bond strength of cements to a metal substructure may vary depending on the alloy type. It has been reported that adhesive resin cements successfully bond to base metal alloys; however, strong bond to noble metal alloys needed surface treatment such as heat treatment, electroplating, ion-coating or application of metal primers.21
This study investigated the shear bond strengths of different luting cements to implant-supported fixed superstructure alloys under two different seating forces. The null hypotheses to be tested were: 1- a change in seating force does not affect the shear bond strengths of luting cements to implant-supported fixed restoration substructures; and 2- the type of the metal substructure does not influence the shear bond strength of cements to metal surfaces.
MATERIALS AND METHODS
The study cements used to bond to one pure metal and two metal alloy surfaces were three water-based cements [Polycarboxylate cement (PCC), (Durelon, 3M ESPE, St. Paul, MN, USA); Glass-ionomer cement (GIC), (Ketac-CEM, 3M ESPE); and Zinc phospahate cement (ZPC) (Hoffmann's Harmonic Shades, Hoffmann, Berlin, Germany)], two resin-based cements [Self-adhesive resin cement (RXU), (RelyX U200, 3M ESPE) and Resin cement (C&B), (C&B, BISCO, Inc., Schaumburg, IL, USA)], and two temporary cements [RelyX Temp NE (RXT), (3M ESPE) and Telio CS Cem Implant (TCS), (Ivoclar Vivadent; Schaan, Liechtenstein)] (Table 1).
Table 1. The study cements, their type, composition and manufacturer.
| Materials | Manufacturer | Type | Lot No. | Composition |
|---|---|---|---|---|
| Hoffmann's Harmonic Shades (ZPC) | Hoffmann Dental Manufaktur GmbH, Berlin, Germany | Zinc-phosphate cement | 5826 | O-phosphoric acid, zinc oxide, magnesium oxide |
| Ketac Cem Radiopaque (GIC) | 3M ESPE, St. Paul, MN, USA | Glass-ionomer cement | 526731 | Copolymer acrylic acid-maleic acid (< 20 wt%), glass powder (80 – 90 wt%) |
| Durelon (PCC) | 3M ESPE, St. Paul, MN, USA | Polycarboxylate cement | 538749 | Polyacrylic acid (40 – 50 wt%), water (50 – 65 wt%), zinc oxide (85 – 95 wt%), stannous fluoride (1 – 10 wt%), tin dioxide (1 – 5 wt%) |
| Rely X U200 (RXU) | 3M ESPE, St. Paul, MN, USA | Resin-based, self-adhesive, dual cure | 586 495 | GPDM, self-etching/adhering acidic monomer, 7 wt% (46 vol%) barium glass, FAS glass, fumed silica |
| C&B (C&B) | BISCO, Inc., Schaumburg, IL, USA | Self-cured composite cement | 1400003636 | Fused silica, bisphenol A glycidylmethacrylate, triethylene glycol dimethacrylate, sodium fluoride |
| RelyX Temp NE (RXT) | 3M ESPE, St. Paul, MN, USA | Resin based temporary luting cement | 5547792 | Resin, reaction products with acrylic acid (60 – 70 wt%), nonanoic acid (30 – 40 wt%), silane treated silica (1 – 5 wt%), Zinc oxide (80 – 90 wt%), white mineral oil (5 – 15 wt%), petrolatum (1 – 5 wt%) |
| Telio CS Cem Implant (TCS) | Ivoclar Vivadent AG, Schaan, Liechtenstein | Resin based temporary luting cement | T22772 | Bismethacrylates (approx. 52 wt%), Ytterbium trifluoride (10 – < 25 wt%) |
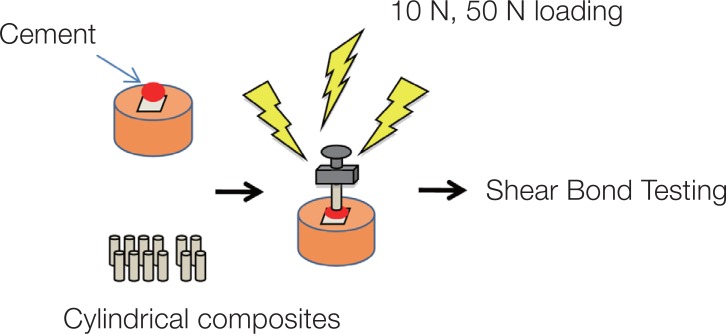
Thirty-two square blocks (5 × 5 × 3 mm) were prepared from pure titanium (CP-Ti) (Eutitan, Eukamed, Essen, Germany), gold-platinum alloy (Au-Pt) (Platin Lloyd 100, BEGO, Bremen, Germany), and cobalt-chrome alloy (Co-Cr) (Wirebond C, BEGO Medical, Bremen, Germany) for each cement group. The top surfaces of metal blocks were polished with #600 SiC papers in order to receive standard bonding surfaces without any further treatment.22,23 After application of each cement group, the specimen surfaces were repolished again for the application of the other cement groups. All cements were mixed according to the manufacturers' instructions, using supplied dispensers and/or auto mixing syringe tips when applicable. Mixing of the encapsulated cements was performed for the recommended time. Cylindrical composite (Beautifil II, Shofu Dent. Corp, Kyoto, Japan) specimens (2.1 mm in diameter, 3 mm in height) were bonded to the polished metal surfaces with one of the study cements in a special alignment apparatus, where a load of 10 N was applied on the first sixteen specimens and a load of 50 N was applied on the second sixteen specimens for 10 minutes each (Fig. 1).17,24,25 The specimens were irradiated (Optilux 500, Kerr/Demetron, Danbury, CT, USA) for 40 seconds from three sides (buccal, lingual, and occlusal) for a total of 120 seconds and were then stored in deionized water at 37℃ for 24 hours.
Fig. 1. Test set-up of the study.
Each specimen was locked in a special device, which was seated on the compression load cell of a universal testing machine (Bisco Bond Tester, Bisco, Schaumburg, IL, USA) and SBS was tested using a half circle edge with a 2.5-mm diameter at a crosshead speed of 0.5 mm/min until failure occurred. Special caution was taken to avoid applying any stress to the specimens. The load at failure was recorded in Newton (N). Bond strength was calculated in MPa by dividing the load at failure by the bonding area (in mm2).
Data were analyzed with 3-way ANOVA. Pairwise comparisons and interactions among groups were analyzed with Tukey's simultaneous confidence intervals. In the analysis, “factor” represented metal, cement, and seating force, and “level” represented the types of each. The overall significance level was set at α = .05. Minitab statistical software, version 13.0 for Windows (Minitab Ltd., Coventry, UK), was used for the calculations.
RESULTS
According to the results of 3-way ANOVA, statistically significant differences among the groups tested were observed (P < .00). Overall Mean SBS (MPa) scores and SD of the cement/metal/seating force groups of the study are presented in Table 2. Table 3 shows Mean SBS (MPa) and SD values of factor level combinations of the groups.
Table 2. Overall mean shear bond strength scores (MPa) and standard deviation of the cement/metal/seating force groups of the study.
| Cement | (Mean ± SD) | Metal | (Mean ± SD) | Force (N) | (Mean ± SD) |
|---|---|---|---|---|---|
| PCC | 3.59 ± 0.07 | Co-Cr | 1.96 ± 0.04 | 10 | 1.87 ± 0.03 |
| GIC | 1.17 ± 0.07 | Au-Pt | 1.57 ± 0.04 | 50 | 1.64 ± 0.03 |
| ZPC | 1.41 ± 0.07 | CP Ti | 1.74 ± 0.04 | ||
| RXU | 3.28 ± 0.07 | ||||
| TCS | 0.52 ± 0.07 | ||||
| RXT | 0.39 ± 0.07 | ||||
| C&B | 1.94 ± 0.07 |
Table 3. Mean and standard deviation shear bond strength values (MPa) for factor level combinations of cement/metal/seating force groups.
| Cements∖Force | Co-Cr | Au-Pt | CP Ti | |||
|---|---|---|---|---|---|---|
| 10 N (n-16) | 50 N (n-16) | 10 N (n-16) | 50 N (n-16) | 10 N (n-16) | 50 N (n-16) | |
| PCC | 3.69 ± 0.9 | 4.52 ± 1.5 | 4.08 ± 0.4 | 3.42 ± 1 | 3.83 ± 1.2* | 2.04 ± 0.4* |
| RXU | 2.62 ± 0.8 | 2.14 ± 0.6 | 2.32 ± 0.6 | 2.90 ± 0.5 | 5.71 ± 2.2* | 4.02 ± 1.5* |
| C&B | 1.51 ± 0.4 | 2.41 ± 0.6 | 2.96 ± 0.4 | 2.53 ± 0.5 | 1.44 ± 0.5 | 0.80 ± 0.2 |
| ZPC | 1.84 ± 0.6 | 1.73 ± 0.4 | 0.43 ± 0.1 | 0.46 ± 0.1 | 2.26 ± 0.7 | 1.71 ± 0.7 |
| GIC | 2.45 ± 0.8 | 3.27 ± 0.9 | 1.28 ± 0.2* | 0* | 0 | 0 |
| TCS | 0.50 ± 0.3 | 0.28 ± 0.2 | 0.50 ± 0.1 | 0.26 ± 0.09 | 0.47 ± 0.1 | 0.34 ± 0.08 |
| RXT | 0.24 ± 0.1 | 0.20 ± 0.1 | 0.44 ± 0.1 | 0.47 ± 0.1 | 0.75 ± 0.2 | 1.01 ± 0.4 |
For the (*) marked groups, 10 N seating force caused significantly higher SBS values when compared to the SBS values obtained under 50 N seating force.
Among the metal surfaces tested, overall mean SBS scores revealed that Co-Cr alloy showed the highest (1.96 ± 0.4) and the Au-Pt showed the lowest (1.57 ± 0.4) SBS values (P < .00) regardless of cements and seating force (Table 2). Also, pairwise comparisons indicated that there were significant differences among three metal surfaces tested (Co-Cr > CP Ti > Au-Pt) (P < .00). The interactions showed that difference between levels of seating force tested (10 N and 50 N) was significant only for PCC/CP Ti, RXU/CP Ti, and GIC/Au-Pt factor level combinations (10 N > 50 N) (P < .00); for all other combinations, SBS values of the seating force levels were not significantly different (P > .05) (Table 3).
Among levels of cements tested, overall mean SBS scores revealed that PCC showed the highest SBS values (3.59 ± 0.04) (P < .00), while the RXT and TCS showed the lowest (0.52 ± 0.07 and 0.39 ± 0 .07) (P < .00) (Table 2). According to the pairwise comparisons, SBS values of the resin cements (RXU and C&B) were significantly lower than the value of the PCC (P < .00). On the other hand, the SBS values of ZPC and GIC were significantly lower than those of the resin cements (RXU and C&B) (P < .00). Interactions showed that the SBS value of RXU/CP Ti factor level combination was significantly higher than that of C&B /CP Ti factor level combination (P < .00). Also, the SBS value for GIC/Co-Cr factor level combination was significantly higher than that of ZPC/Co-Cr factor level combination (P < .00). Overall RXU/CP Ti factor level combination revealed the highest SBS value among all factor level combinations (P < .00) whilst no bond was observed for the GIC/CP Ti factor level combination (Table 3).
All the groups tested in this study showed adhesive failure with no resin residues on the metal surfaces.
DISCUSSION
In this in vitro study, the bond strengths of luting cements to metal surfaces under different seating forces were investigated. For this aim, the shear bond strengths of seven different implant luting cements to one pure metal and two metal alloy surfaces, which are usually used to fabricate implant supported fixed restoration substructures, were measured and compared under two different seating forces (10 N and 50 N). Interactions between different factors were also analyzed.
Interaction effects represent the combined effects of factors on the dependent measure. When an interaction effect is present, the impact of one factor depends on the levels of the other factors.26 For this reason, pairwise comparisons among all factor level combinations were calculated by using Tukey's simultaneous confidence intervals. In the analysis, “factor” represented metal, cement, and seating force and “level” represented the types of each factor. According to the results, pairwise comparison revealed that 10 N seating force caused significantly higher SBS values when compared to the SBS values obtained under 50 N seating force (P < .00). However, interaction analysis showed that this difference was significant only for PCC/CP Ti, RXU/CP Ti, and GIC/Au-Pt factor level combinations. Therefore, the first hypothesis of the study was partly rejected.
In a study, it was reported that when cementing a crown by finger pressure, the peak force ranged between 6 N and 77 N.25 10 N seating force was described as “low force” in literature.17 On the other hand, although several studies claim that high seating forces induce reduction in cement film thickness and this condition affects its cohesive strength,2,18,27,28,29 Jorgensen stated that increasing the applied force above 50 N does not improve the seating of a crown.27 Referring to these reports, this study was conducted under two different seating forces 10 N and 50 N as low and high force and no significant difference was observed between SBS values of 10 N and 50 N seating force with the exception of PCC/CP Ti, RXU/CP Ti, and GIC/Au-Pt factor level combinations. Therefore, it may be concluded that the two different seating forces tested in this study might not be significantly different to alter the film thickness of the cements. However, the differences observed for the three combinations might be due to the variances in the physical, chemical, or mechanical properties of the cements such as the thixotropic property of polycarboxylate cement, composition of self-adhesive resin cement, and bonding properties of glass-ionomer cement. The thixotropic property of polycarboxylate cement may lead to decrease in material thickness under increased seating forces and this may also decrease its cohesive properties.30 Also, during setting, polycarboxylate cement bonds to metal substructures by chelation of metallic ions.31 At this point, together with the decreased cohesive properties, a lower bond strength of polycarboxylate cement to titanium dioxide (TiO2) than chromium oxide (Cr2O3) might have decreased the bond strength to CP Ti under 50 N seating force.30,32,33,34,35,36
The increase in seating force might also lead to a possible reduction in cement film thickness depending on the composition and alter the cohesive strength of the self-adhesive resin cement because it is reported that self-adhesive resin cements exhibit lower mechanical properties than conventional resin cements.37 Although resin cements containing phosphoric groups are able to produce strong chemical bonds with the superficial oxide layer of titanium,38 the decrease in SBS value of RXU/CP Ti factor level combination under 50 N seating force might be due to this decrease in the cohesive strength and surface wettability of the RXU.32,33,34,35,36,37,38,39 Also, the glass-ionomer cement establishes no chemical bond with noble metal alloys.2,28 The bond strength was very low (1.28 ± 0.2 MPa) under 10 N. However, no bonding performance was received under 50 N seating force application.
Noble alloys have a number of advantages over base metal alloys such as biocompatibility, esthetics, and strong bonding to ceramics, but base metals possess higher free surface energy compared to noble alloys resulting in a thicker oxide layer formation and higher reactivity.2,32,33,34,35 Among metals tested in this study, overall mean SBS scores showed that Co-Cr had the highest and Au-Pt had the lowest SBS values (P < .00) regardless of cements and seating force. Therefore, the second null hypothesis, which claims that the type of the metal substructure does not influence the shear bond strength of cements, was also rejected. The improved bond strength to Co-Cr may be attributed to thick oxide layer formation on the surface, enabling it to produce a strong chemical bond with the cement. Titanium dioxide (TiO2) and chromium oxide (Cr2O3) both have the same analogous characteristics; however, variances in the composition might alter the bond quality in some aspects.34,36
Interaction analysis showed that RXU/CP Ti factor levels combination revealed the highest SBS value among all other factor level combinations tested (P < .00). It was previously found that the acidic monomers containing phosphoric groups and carboxylic acid derivatives monomers are able to bond chemically with the superficial oxide layer of base metals by Bolger's mechanism through the electrostatic interaction between the acids of the monomers and the OH groups of the superficial oxide layer.27,35 Fonseca et al. demonstrated that the chemical bond between monomers and the oxides present at the surface of CP Ti were stronger than those that occurred at the surface of Ni-Cr alloy.20 Kern and Thompson also verified that the oxides on the titanium surface are more stable than the surface oxides of other metals and establish stable chemical bonds with monomers containing phosphoric groups.40 RXU self-adhesive resin cement is composed of phosphorylated methacrylate monomers with an acidic nature.41 Therefore, both the phosphorylated methacrylates monomers in RXU and the stable oxide layer of titanium might have led to the establishment of a strong bond between cement and metal. However, C&B cement does not contain phosphorylated methacrylate monomers;42 so, the lower SBS values obtained from C&B than that of RXU may be explained by the lack of these bond strengthening effects of phosphoric groups between the cement and the oxide layer of the metals tested. 43
Among the cements used in this study, the results showed that PCC had the highest (P < .00), and the provisional cements RXT and TCS had the lowest mean SBS scores (P < .00). McIntyre et al. stated that polycarboxylate cements form strong chemical bonds with stainless steel surfaces, weaker bonds with gold and other noble metal alloys, and no bonds with porcelain.44 Also, Mehl et al. reported that PCC cements establish chemical bonds to the metal surfaces of the Ti abutment and the Cr-Co crown.2 These studies support the findings of this in vitro study. This current study exhibited lower SBS value for ZPC cement when compared to those of polycarboxylate and resin luting cements (P < .00). Our results also displayed similar findings with the study that reported that ZPC cement had no adhesive properties and its retentiveness depended on mechanical interlocking over the substrate irregularities.15 In this present study, GIC showed significantly lower SBS value for Au-Pt but significantly higher SBS value for Co-Cr when compared to that of ZPC. GIC has low tensile strength and fracture resistance and the studies confirmed that it establishes no chemical bond with noble metal alloys.2,28,45,46 However, it has been reported that the passive oxide layer formed on the surface increases the bond strength of glassionomer cements to base metal alloys.47 These reports correlate with the results of our study. On the other hand, in this study, SBS value for GIC/CP Ti factor levels combination could not be obtained. This might be explained by the issue that the difference in chemical composition and characteristic of the oxide film formed on metal alloys may affect the affinity of its bonding to cement.33,35,47
CONCLUSION
Under the limitations of this study, PCC cement provided reliable bonding performance to metal alloy surfaces. However, the best bonding performance was achieved between RXU and CP Ti surfaces. Therefore, resin based cements can be recommended for permanent cementation of Ti based implant-supported fixed dental prostheses. However, ZPC, RXT, and TCS can be used for cementation of implant-retained restorations when retrievability is essential. On the other hand, GIC is not suitable for permanent cementation of implant-supported fixed dental prostheses consisting of CP Ti or Au-Pt substructures.
Increasing the seating force do not significantly change the bonding performance of the cements to metal surfaces except for PCC/CP Ti, RXU/CP Ti, and GIC/Au-Pt cement/metal combinations.
References
- 1.Lee A, Okayasu K, Wang HL. Screw- versus cement-retained implant restorations: current concepts. Implant Dent. 2010;19:8–15. doi: 10.1097/ID.0b013e3181bb9033. [DOI] [PubMed] [Google Scholar]
- 2.Mehl C, Harder S, Steiner M, Vollrath O, Kern M. Influence of cement film thickness on the retention of implant-retained crowns. J Prosthodont. 2013;22:618–625. doi: 10.1111/jopr.12058. [DOI] [PubMed] [Google Scholar]
- 3.Mehl C, Harder S, Shahriari A, Steiner M, Kern M. Influence of abutment height and thermocycling on retrievability of cemented implant-supported crowns. Int J Oral Maxillofac Implants. 2012;27:1106–1115. [PubMed] [Google Scholar]
- 4.Hebel KS, Gajjar RC. Cement-retained versus screw-retained implant restorations: achieving optimal occlusion and esthetics in implant dentistry. J Prosthet Dent. 1997;77:28–35. doi: 10.1016/s0022-3913(97)70203-8. [DOI] [PubMed] [Google Scholar]
- 5.Breeding LC, Dixon DL, Bogacki MT, Tietge JD. Use of luting agents with an implant system: Part I. J Prosthet Dent. 1992;68:737–741. doi: 10.1016/0022-3913(92)90194-f. [DOI] [PubMed] [Google Scholar]
- 6.Heinemann F, Mundt T, Biffar R. Retrospective evaluation of temporary cemented, tooth and implant supported fixed partial dentures. J Craniomaxillofac Surg. 2006;34:86–90. doi: 10.1016/S1010-5182(06)60019-X. [DOI] [PubMed] [Google Scholar]
- 7.Chaar MS, Att W, Strub JR. Prosthetic outcome of cement-retained implant-supported fixed dental restorations: a systematic review. J Oral Rehabil. 2011;38:697–711. doi: 10.1111/j.1365-2842.2011.02209.x. [DOI] [PubMed] [Google Scholar]
- 8.Mehl C, Harder S, Schwarz D, Steiner M, Vollrath O, Kern M. In vitro influence of ultrasonic stress, removal force preload and thermocycling on the retrievability of implant-retained crowns. Clin Oral Implants Res. 2012;23:930–937. doi: 10.1111/j.1600-0501.2011.02236.x. [DOI] [PubMed] [Google Scholar]
- 9.Good ML, Mitchell CA, Pintado MR, Douglas WH. Quantification of all-ceramic crown margin surface profile from tryin to 1-week post-cementation. J Dent. 2009;37:65–75. doi: 10.1016/j.jdent.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Ohnishi E, Kaneshima T, Mine A, Yatani H. Porcelain veneer bonding to enamel with plasma-arc light resin curing. Dent Mater J. 2002;21:61–68. doi: 10.4012/dmj.21.61. [DOI] [PubMed] [Google Scholar]
- 11.Carter SM, Wilson PR. The effects of die-spacing on post-cementation crown elevation and retention. Aust Dent J. 1997;42:192–198. doi: 10.1111/j.1834-7819.1997.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 12.Dixon DL, Breeding LC, Lilly KR. Use of luting agents with an implant system: Part II. J Prosthet Dent. 1992;68:885–890. doi: 10.1016/0022-3913(92)90544-k. [DOI] [PubMed] [Google Scholar]
- 13.Vermilyea SG, Kuffler MJ, Huget EF. The effects of die relief agent on the retention of full coverage castings. J Prosthet Dent. 1983;50:207–210. doi: 10.1016/0022-3913(83)90015-x. [DOI] [PubMed] [Google Scholar]
- 14.Hembree JH, Jr, Cooper EW., Jr Effect of die relief on retention of cast crowns and inlays. Oper Dent. 1979;4:104–107. [PubMed] [Google Scholar]
- 15.Haddad MF, Rocha EP, Assunção WG. Cementation of prosthetic restorations: from conventional cementation to dental bonding concept. J Craniofac Surg. 2011;22:952–958. doi: 10.1097/SCS.0b013e31820fe205. [DOI] [PubMed] [Google Scholar]
- 16.Bagheri R. Film thickness and flow properties of resin-based cements at different temperatures. J Dent (Shiraz) 2013;14:57–63. [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson PR. Low force cementation. J Dent. 1996;24:269–273. doi: 10.1016/0300-5712(95)00074-7. [DOI] [PubMed] [Google Scholar]
- 18.Goracci C, Cury AH, Cantoro A, Papacchini F, Tay FR, Ferrari M. Microtensile bond strength and interfacial properties of self-etching and self-adhesive resin cements used to lute composite onlays under different seating forces. J Adhes Dent. 2006;8:327–335. [PubMed] [Google Scholar]
- 19.Piemjai M. Effect of seating force, margin design, and cement on marginal seal and retention of complete metal crowns. Int J Prosthodont. 2001;14:412–416. [PubMed] [Google Scholar]
- 20.Fonseca RG, de Almeida JG, Haneda IG, Adabo GL. Effect of metal primers on bond strength of resin cements to base metals. J Prosthet Dent. 2009;101:262–268. doi: 10.1016/S0022-3913(09)60050-0. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida K, Kamada K, Tanagawa M, Atsuta M. Shear bond strengths of three resin cements used with three adhesive primers for metal. J Prosthet Dent. 1996;75:254–261. doi: 10.1016/s0022-3913(96)90481-3. [DOI] [PubMed] [Google Scholar]
- 22.Matinlinna JP, Lung CYK, Tsoi JKH. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent Mater. 2018;34:13–28. doi: 10.1016/j.dental.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Özcan M, Matinlinna J. Surface conditioning protocol for the adhesion of resin-based cements to base and noble alloys: How to condition and why? J Adhes Dent. 2015;17:372–373. doi: 10.3290/j.jad.a34787. [DOI] [PubMed] [Google Scholar]
- 24.Cherkasski B, Wilson PR. The effect of oscillation, low seating force and dentine surface treatment on pulpward pressure transmission during crown cementation: a laboratory study. J Oral Rehabil. 2003;30:957–963. doi: 10.1046/j.1365-2842.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- 25.Black S, Amoore JN. Measurement of forces applied during the clinical cementation of dental crowns. Physiol Meas. 1993;14:387–392. doi: 10.1088/0967-3334/14/3/018. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery DC. Design and analysis of experiments. 8th ed. NC: John Wiley & Sons; 2012. pp. 174–188. [Google Scholar]
- 27.Jørgensen KD. Factors affecting the film thickness of zinc phosphate cements. Acta Odontol Scand. 1960;18:479–490. [Google Scholar]
- 28.D'Souza R, Shetty O, Puppala P, Shetty N. A better bond: Luting simplified. Int J Prosthet Rest Dent. 2012;2:77–81. [Google Scholar]
- 29.Mitchell CA, Abbariki M, Orr JF. The influence of luting cement on the probabilities of survival and modes of failure of cast full-coverage crowns. Dent Mater. 2000;16:198–206. doi: 10.1016/s0109-5641(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 30.Sen D. Cementation. 1st ed. Istanbul: Quintessence Publishing; 2012. pp. 9–31. [Google Scholar]
- 31.Mansour A, Ercoli C, Graser G, Tallents R, Moss M. Comparative evaluation of casting retention using the ITI solid abutment with six cements. Clin Oral Implants Res. 2002;13:343–348. doi: 10.1034/j.1600-0501.2002.130401.x. [DOI] [PubMed] [Google Scholar]
- 32.Hattar S, Hatamleh M, Khraisat A, Al-Rabab'ah M. Shear bond strength of self-adhesive resin cements to base metal alloy. J Prosthet Dent. 2014;111:411–415. doi: 10.1016/j.prosdent.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 33.Bauer J, Costa JF, Carvalho CN, Souza DN, Loguercio AD, Grande RH. Influence of alloy microstructure on the microshear bond strength of basic alloys to a resin luting cement. Braz Dent J. 2012;23:490–495. doi: 10.1590/s0103-64402012000500004. [DOI] [PubMed] [Google Scholar]
- 34.Raeisosadat F, Ghavam M, Hasani Tabatabaei M, Arami S, Sedaghati M. Bond strength of resin cements to noble and base metal alloys with different surface treatments. J Dent (Tehran) 2014;11:596–603. [PMC free article] [PubMed] [Google Scholar]
- 35.Kious AR, Roberts HW, Brackett WW. Film thicknesses of recently introduced luting cements. J Prosthet Dent. 2009;101:189–192. doi: 10.1016/S0022-3913(09)60026-3. [DOI] [PubMed] [Google Scholar]
- 36.Anusavice KJ. Phillips' Science of dental materials. 11th ed. Missouri: W.B. Saunders Company; 2003. pp. 621–654. [Google Scholar]
- 37.Sakaguchi RL, Powers JM. Craig's restorative dental materials. 13th ed. Philadelphia: Elsevier Mosby; 2012. pp. 327–347. [Google Scholar]
- 38.Almilhatti HJ, Neppelenbroek KH, Vergani CE, Machado AL, Pavarina AC, Giampaolo ET. Adhesive bonding of resin composite to various titanium surfaces using different metal conditioners and a surface modification system. J Appl Oral Sci. 2013;21:590–596. doi: 10.1590/1679-775720130255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fawzy AS, El-Askary FS. Effect acidic and alkaline/heat treatments on the bond strength of different luting cements to commercially pure titanium. J Dent. 2009;37:255–263. doi: 10.1016/j.jdent.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Kern M, Thompson VP. Durability of resin bonds to pure titanium. J Prosthodont. 1995;4:16–22. doi: 10.1111/j.1532-849x.1995.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 41.Technical data sheet RelyX U200. St. Paul, MN, USA: 3M ESPE; 2011. [Google Scholar]
- 42.Safety data sheet C&B. Schaumburg, IL, USA: BISCO, Inc.; 2015. [Google Scholar]
- 43.Yoshida K, Kamada K, Sawase T, Atsuta M. Effect of three adhesive primers for a noble metal on the shear bond strengths of three resin cements. J Oral Rehabil. 2001;28:14–19. doi: 10.1046/j.1365-2842.2001.00625.x. [DOI] [PubMed] [Google Scholar]
- 44.McIntyre FM, Sorensen SE, Carter JM, Johnson RR. The effect of film thickness on the bond strength of polycarboxylate cement. Int J Prosthodont. 1994;7:461–467. [PubMed] [Google Scholar]
- 45.Orsi IA, Varoli FK, Pieroni CH, Ferreira MC, Borie E. In vitro tensile strength of luting cements on metallic substrate. Braz Dent J. 2014;25:136–140. doi: 10.1590/0103-6440201302290. [DOI] [PubMed] [Google Scholar]
- 46.Subramaniam P, Kondae S, Gupta KK. Retentive strength of luting cements for stainless steel crowns: an in vitro study. J Clin Pediatr Dent. 2010;34:309–312. doi: 10.17796/jcpd.34.4.p5h1068v41ggt450. [DOI] [PubMed] [Google Scholar]
- 47.Hibino Y, Kuramochi K, Hoshino T, Moriyama A, Watanabe Y, Nakajima H. Relationship between the strength of glass ionomers and their adhesive strength to metals. Dent Mater. 2002;18:552–557. doi: 10.1016/s0109-5641(01)00086-0. [DOI] [PubMed] [Google Scholar]