Abstract
The present study reports two cases of concurrently diagnosed T-lymphoblastic lymphoma (T-LBL) and chronic myeloid leukemia (CML). The literature review revealed that myeloid leukemia may appear secondary to Hodgkin lymphoma or non-Hodgkin lymphoma. However, simultaneous bi-lineage hematologic malignancies are rarely seen and the prognosis is worse than single lineage lymphoma or myeloid leukemia. There were no standard therapies. All simultaneous bi-lineage malignancies of myeloid leukemia and lymphoma reported in Pubmed were combined with the present two cases, to analyses its pathogenesis, features and treatment. It was concluded that the prognosis of bi-lineage hematologic malignancies was poor, however allogeneic hematopoietic stem cell transplantation could improve survival (P=0.033).
Keywords: concurrent bi-lineage hematologic malignancies, myeloid leukemia, lymphoma, pathogenesis, prognosis, allogeneic hematopoietic stem cell transplantation
Introduction
Lymphoma, classified into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) with various subtypes respectively, originates from precursor cells in primary lymph organs or from mature cells located in the peripheral lymphoid organs, arising from a clone expansion of B-or-T lymphocytes transformed during the pathways of lymphocyte differentiation (1,2). Chronic myeloid leukemia (CML) and acute myeloid leukemia (AML) are clonal expansion of hematopoietic progenitor cells characterized by exaggerated proliferation of granulocytic lineage while CML undergoes a chronic course relatively. Lymphoma and myeloid leukemia are different malignancy originating from two lineages and possess disparate cytogenetic, cell phenotype and biological process. Generally, lymphoma combining with myeloid leukemia is rarely seen except when CML in blast crisis with a bare possibility occurs acute lymphocyte mutation. It is more rarely seen that simultaneous bi-lineage malignancies without history treatment at initial diagnosis. Shen et al reviewed 24 patients with CML and T-lymphoblastic cell NHL (T-LBL) in the lymph node between 1980 and 2016, but most of those patients experienced chronic history of CML followed by T-LBL afterwards (3). Some scholars reported NHL or HL developing into leukemia during remission or treatment (4–7). The cause about the bi-lineage hematologic malignancies is unclear yet. Lam et al analysedrisk factors ofsecondary acute myeloid leukemia/myelodysplastic syndrome among survivors of NHL (6). Eichenauer et al reported therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with HL (4). To our best knowledge, the therapy-related secondary tumor has been frequently reported in patients who received various chemotherapy regimens or radiotherapy or transplantation, however, there is no systematic summary to individual cases about simultaneous bi-lineage hematologic malignancies without previous therapy. So, we summary simultaneous lymphoma and myeloid leukemia through literature searching on PubMed (ncbi.nlm.nih.gov/pubmed) with the term ‘myeloid leukemia’ or ‘myelogenous leukemia’ combined with ‘lymphoma’ and ‘simultaneous’ or ‘concurrent’ or ‘coinstantaneous’ or ‘co-existence’ to explore the features, prognosis and treatment. In the meantime, we present our two cases diagnosed with concurrent T-LBL and CML.
Patients and methods
Case report
Case 1
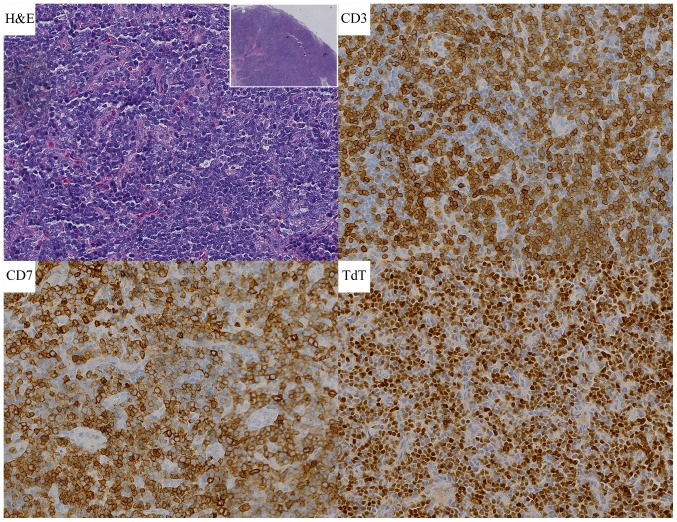
On April 27, 2009, a 43-year-old Chinese male was admitted hospital because of finding a cervical mass for 10 days. On physical examination, multiple enlarged lymph nodes no bigger than 4×2 cm were found in bilateral cervical, submandibular and submental region. Other physical findings were unremarkable. The chest and abdomen CT scan was normal except splenomegaly. A complete blood count revealed leucocyte count 43.81×109/l with 3.6% blasts, 7.2% promyelocytes, erythrocyte count 4.45×1012/l, hemoglobin level 136.0 g/l, platelet count 123×109/l, aneutrophils count 25.06×109/l, β-microglobulin level 2.01 mg/l, lactate dehydrogenase (LDH) level 382 U/l. A subsequent bone marrow aspiration showed malignant proliferation of the myeloid Department with myeloblasts >10% and that ratios of neutrophilic myelocyte, metamyelocyte and segmented neutrophil all increased. The chromosome indicated 46,XY,t(9,22). The FISH test for BCR/ABL was positive with a rate of 7%. Biopsy of the right cervical lymph node reveal T-LBL with lymphoma cells expressing CD3, CD4, CD45, TdT (terminal deoxynucleotidyl transferase), but negative for CD20, Pax-5, CD79a, ALK, MPO, Ki-67 level is 90% (Fig. 1). So ultimate diagnosis was T-LBL in stage II according to the Ann Arbor classification, the IPI (8) being 2, combining with CML in blastic phase. The patient was treated with Hyper-CVAD A (cyclophosphamide, vincristine, adriamycin and dexamethasone) scheme one cycle and imatinib 600 mg qd. Then MOAP (mitoxantrone, vincristine, arabinoside and prednisone) five cycles and intrathecal injection four times. The patients obtained nearly complete remission with bone marrow blasts and promyelocytes reduced to 0.4%. Afterwards the patient accepted haploidentical hematopoietic stem cell transplantation on December 15, 2009. Until now (June 2017), the patient had obtained continuous complete remission (CR) for over 8 years.
Figure 1.
Case 1 histological findings. H&E stain of cervical lymph node section showing destruction of normal structure (magnification, ×40) and numerous lymphoblastic lymphoma cells (magnification, ×400). Lymph node with T-lymphoblastic cell non-Hodgkin's lymphoma stained with CD3, CD7 and TdT (magnification, ×400). CD, cluster of differentiation; H&E, hematoxylin and eosin.
Case 2
On December 12, 2012, a 44-year-old Chinese male was complained of finding a cervical mass with exacerbation for more than 20 days. On physical examination, several enlarged lymph nodes were observed in the bilateral neck, right collarbone and axillary. In addition, the patient's left pharyngeal cavity was inflamed with a random-shaped neoplasm. A complete blood test: leucocyte count 25.1×109/l, erythrocyte count 3.34×1012/l, hemoglobin 103.0 g/l, platelet 123×109/l, neutrophils 19.6×109/l, β-microglobulin 2.0 mg/l, LDH 638 U/l. the blasts, promyelocytes and metamyelocytes appeared in the peripheral blood. Bone marrow analysis revealed granulocyte proliferation with hyperactivity, blasts and promyelocytes accounted for 7.6% (Figs. 2 and 3). The fluorescence in situ hybridization (FISH) test for BCR/ABL was positive with a rate of 70.2%. So, CML was diagnosed. The biopsy of left cervical lymph node conformed to T-LBL with lymphoma cells expressing CD20, CD3, CD21 (part of the FDC were destroyed), CD10, Bcl-2, TdT, CD43, CD7, CD2 and CD34, while MPO, CD30, ALK, EMA were negative (Fig. 4). The Ki-67 labeling index was 50 to 60%. According to these findings, a preliminary diagnosis was: CML in chronic phase, mydoid sarcoma (MS) and T-LBL. Without treatment, the patient left the hospital. On March 2, 2013, the patient re-hospitalized. Repeated examination was the same as it was before except lymph nodes bigger. From March 5, 2013 to July 8, 2013, the patient was treated with Hyper-CVAD A and B alternately for six cycles, and intrathecal injection for 11 times and reached partial remission, but he didn't take imatinib for lack of money during this period. Since August 2, 2013, two cycles of Hyper-CVAD B were given again with taking imatinib 400 mg qd. However the disease progressed. The patient did not continue treatment later, and succumbed on March 12, 2014.
Figure 2.
Flow cytometer analysis of bone marrow aspirate. (A) CD45/SSC gating and (B) data presentation. (C) The percentage of granulocyte increase and express CD33, CD15, HLA-DR+. (D) Blasts are CD33+, CD15+ and HLA-DR+. CD, cluster of differentiation.
Figure 3.
Intracellular antigen determination. MPO + occupy (A) 83.63% of live and (B) 89.44% of blast.
Figure 4.
Case 2 histological findings. H&E stain of cervical lymph node section showing destruction of normal structure (magnification, ×40) and numerous lymphoblastic lymphoma cells (magnification, ×400). Lymph node with T-lymphoblastic cell non-Hodgkin's lymphoma stained with CD3, CD7 and TdT (magnification, ×400). CD, cluster of differentiation; H&E, hematoxylin and eosin.
Summary to the two cases
Our two cases were admitted because of a cervical mass, and then found superficial lymphadenopathy with the peripheric blood leucocyte soaring. Biopsy of the cervical lymph node prove T-LBL depending on immunohistochemistry and typical morphology. The FISH test for BCR/ABL of bone marrow was positive with rates of 7 and 70.2%. So the two cases were diagnosed T-LBL with CML finally. In terms of treatment, case 1 experienced durable complete remission until present through chemotherapy combining imatinib and then haploidentical hematopoietic stem cell transplantation. However the second patient soon died after chemotherapy and taking imatinib.
Methods
We here summary all concurrent myeloid leukemia and lymphoma from 1976 to present (Table I) to analyze the features, prognosis and treatment. Statistical analyses were performed using IBM SPSS statistics software, version 21.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). OS distributions were estimated using the Kaplan-Meier curve analysis, time-to-event distributions were compared using the log-rank test and two-tailed significance-level of 0.05 was considered statistically significant.
Table I.
Review of patients with myeloid leukemia and lymphoma from 1976 to present.
| Case | First author, year | Sex | Age (years) | Involvement sites | Initial diagnosis | Treatment | Follow-up (months) | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| 1 | Kapadia, 1976 | F | 64 | Lymph nodes and liver and marrow | NHL-PDL and AML | CTx | 11 | (27) |
| 2 | Youness, 1978 | M | 67 | Spleen and marrow | NHL-PDL and AML | CTx | 5 | (28) |
| 3 | Ramji, 1988 | NC | NC | NC | T-NHL and CML | NC | NC | (29) |
| 4 | Ohtsu, 1988 | M | 49 | NC | ATL and AML | NC | 6 | (30) |
| 5 | Tsukasaki, 1995 | F | 36 | NC | ATL and AML | NC | 30b | (11,16) |
| 6 | Abe, 1999 | F | 82 | Gallbladder and marrow | MALT and AML | Untreated | 3 | (9) |
| 7 | Morales, 1999 | M | 63 | Lymph nodes and marrow | T- NHL and CML | CTx | NC | (31) |
| 8 | Montefusco, 2001 | M | 64 | Spleen and marrow | NHL-LG and AML | CTx and hydroxyurea | 25 | (32) |
| 9 2002 | Zámecníková, | M | 34 | Lymph nodes and liver and marrow | DLBCL and CML | CTx and RT | 4 | (2) |
| 10 | Au, 2003 | M | 67 | Mediastinal lymph nodes and marrow | B-NHL and CML | CTx and RT and hydroxyurea | 144b | (33) |
| 11 | Lamb, 2005 | M | 9 | Lymph nodes and spleen and marrow | T-LBL and AML | CTx and allo-HSCT | 48b | (34) |
| 12 | Metzgeroth, 2007 | M | 58 | Lymph nodes and spleen and marrow | T-NHL and AEL | Imatinib | 18b | (12) |
| 13 | Capovilla, 2008 | M | 33 | Lymph nodes and spleen and marrow | T-LBL and CEL | Imatinib | 12b | (35) |
| 14 | Li, 2011 | M | 12 | Lymph nodes and marrow | T-LBL and AML | CTx | 4 | (36) |
| 15 | Chang, 2012 | M | 41 | Lymph nodes and skin lesions and marrow | T-LBL and AML | CTx and allo-HSCT | 14a | (13) |
| 16 | Sharkunov, 2012 | F | N | NC | HL and CML | CTx and imatinib | NC | (37) |
| 17 | Wan, 2012 | M | 43 | Lymph nodes and marrow | T-LBL and CML | CTx and allo-HSCT | 19b | (38) |
| 18 | VanCrombrugge, 2012 | M | 47 | Sinonasal and adjacent and marrow | NK-NHL IVB and AML | CTx | Approximately 2 months | (10) |
| 19 | Kunitomi, 2014 | F | 62 | Lymph nodes and marrow | EBV(+)DLBCL and AML | CTx | 34 | (39) |
| 20 | Dong, 2016 | M | 25 | Lymph nodes and marrow | T-LBL and AML | CTx and allo-HSCT | 34b | (40) |
| 21 | Shen, 2016 | M | 28 | Lymph nodes and spleen and marrow | T-LBL and CML | CTx | 3 months and lost follow-up | (3) |
| 22 | Dai, 2017 | F | 37 | Lymph nodes and skull and marrow | DLBCL and AML | CTx andimatinib | Approximately 2 months | (41) |
| 23 | Our case | M | 43 | Lymph nodes and marrow | T-LBL and CML | CTx and allo-HSCT andimatinib | 98b | |
| 24 | Our case | M | 44 | Lymph nodes and marrow | T-LBL and CML | CTx and imatinib | 15 |
The patient succumbed of GVHD and related infections, but he remained free from both malignancies for at least 11 months after transplantation.
The OS was longer that corresponding time and the death was not observed. AEL, acute eosinophilic leukemia; AML, acute myeloid leukemia; ATL, adult T-cell leukemia; B-NHL, B-cell non-Hodgkin-lymphoma; CEL, chronic eosinophilic leukemia; CML, chronic myeloid leukemia; DLBCL, diffuse large B cell lymphoma; MALT, mucosa-associated lymphoid tissue; NHL-LG, non-Hodgkin lymphoma low-grade; PDL, poorly differentiated lymphoma; T-NHL, T-cell non-Hodgkin-lymphoma. CTx, chemtherapy; RT, radiotherapy; CR, complete remission; GVHD, graft-versus-host disease, allo-HSCT, allogeneic hematopoietic stem cell transplantation; NC, not clear; (+), positive.
Patient characteristics
From the statistics we conclude that the patients ranged between 9 and 82 years old (median, 43 years). The male/female ratio was 2.83:1 (17:6). 16 patients were involved lymphadenectasis in bilateral cervical, submandibular, submental and mediastinal region accompanying fatigue and fever, among of which skull or liver or spleen or skin lesions was involved in 1 patient, 2 patients, 4 patients and 1 patient respectively. 2 patients was involved spleen without lymphadenectasis. Abe et al (9) reported a patient was admitted in the hospital because of progressive jaundice with MALT lymphoma in gallbladder and AML in marrow. Van Crombrugge et al (10) reported a patient referred to hospital because of severe headache and progressive facial pain and ultimately diagnosed NK cell lymphoma in sinonasal and AML. The remaining patients were failed to get information. We sum up total cases of different simultaneous lymphoma and myeloid leukemia (Fig. 5). Simultaneous AML and lymphoma is more than simultaneous CML and lymphoma being 14 and 10 respectively. The number of simultaneous T-cell non-Hodgkin-lymphoma is 15 and the number of simultaneous B-cell non-Hodgkin-lymphoma is 5. For the treatment, 10 patients were treated with chemotherapy, 2 patients were treated with chemotherapy andradiotherapy, 2 patients were treated with with single imatinib, 5 patients were treated with with chemotherapy and transplantation and 1 patient was untreated.
Figure 5.
Summary of cases of simultaneous lymphoma and myeloid leukemia. T-LBL, T-lymphoblastic lymphoma; ATL, Adult T-Cell Leukemia/Lymphoma; CML, chronic myeloid leukemia; T-NHL, T-cell non-Hodgkin-lymphoma; MALT, mucosa-associated lymphoid tissue; PDL, poorly differentiated lymphoma; DLBCL, diffuse large B cell lymphoma; HL, Hodgkin lymphoma.
Survival and statistical analysis
In the 24 patients, 21 patients were available to analyze survival and the median survival was 15 months (Fig. 6). We performed univariate analysis to evaluate the prognostic factors. There was no statistical significance for sex (P=0.301) and for age (P=0.168) which was set 43.5 years as cut-off based on the ROC curve. There was no survival difference between B cell lymphoma companying myeloid leukemia and T cell lymphoma companying myeloid leukemia (P=0.158; Fig. 7A). Similarly, no survival difference was received between AML companying lymphoma and CML companying lymphoma (P=0.167; Fig. 7B). Because of concurrent T-LBL and myeloid leukemia being relatively common, we performed statistical analysis between T-LBL combining CML and T-LBL combining AML, but there was no statistical difference in survival (P=0.485; (Fig. 7C). For the treatment, chemotherapy together with transplantation are superior to other treatment without transplantation (P=0.033; Fig. 7D). The median survival was unreached for patients with transplantation and 11 months for those without transplantation.
Figure 6.

Overall survival for 21 patients with simultaneous bi-lineage malignancies. The median survival was 15 months.
Figure 7.
(A-C) The comparison of OS for different kinds of concurrent bi-lineage malignancy and there was no difference in survival. (D) Treatment with HSCT can improve survival compared with that without HSCT. OS, overall survival. HSCT, hematopoietic stem cell transplantation.
Discussion
Lymphoma and myeloid leukemia derive from different tumour cells, and they mostly happen alone. It was frequently reported that secondary or therapy-related hematologic malignancies, but co-concurrent bi-lineage hematologic malignancies are really rare. We present two cases simultaneous T-LBL and CML and then reviewed all cases available to collect from Pubmed. The characteristics are as follows: The simultaneous neoplasm tended to occur in young to old with a good majority in male. Patients are mostly admitted in the hospital because of enlarged lymph nodes accompanying fatigue, fever and splenomegaly. Simultaneous HL and myeloid leukemia is extremely rare and only one case was reported. Simultaneous AML and lymphoma is more commonly seen than simultaneous CML and lymphoma. Simultaneous T cell lymphoma and myeloid leukemia is more thansimultaneous B cell lymphoma and myeloid leukemia. The number of simultaneous T-LBL and myeloid leukemia is maximum than any other subtypes. There is no statistical difference in survival for different bi-lineage malignancy.
However, due to the rarity of patients with bi-lineage tumors, little is known concerning the pathogenesis. Early in 1998, Tsukasaki et al reported the possible association between adult T-cell leukemia/lymphoma and acute myeloid leukemia. One of the possible mechanism is that immune system is compromised severely in Adult T-Cell Leukemia/Lymphoma (ATL) patients which results in the occurrence of AML. The other possible explanation for the association of ATL and AML is that growth factors such as M-CSF, G-CSF, and GM-CSF produced by the ATL cells support the growth of the AML cells (11). Metzgeroth et al demonstrated the association of the FIP1L1-PDGFRA fusion gene with lymphoblastic T-NHL and eosinophilia-associated acute myeloid leukemia (12). Chang et al (13) and Holroyd et al (14) also recognized that FIP1L1-PDGFA is associated with differentiation into both myeloid and lymphoid lineages. Therefore FIP1L1-PDGFRA fusion gene, growth factors and compromised immune system may lead to the co-concurrent bi-lineage malignancies. Besides, the recently studies have confirmed that retrovirus could cause leukemia and lymphoma in reptiles, primates and mammals (15). Furthermore, hematologic neoplasms have been reported to be complications of ATL (16).
From the research, we can see that simultaneous T-LBL and myeloid leukemia are more commonly seen than other subtypes. The reason is still unclear. For T-LBL, combining cytomorphology and flow cytometric immunophenotyping (FCI) enables the accurate and rapid diagnosis (17). The diagnosis of T-LBL is based on the identification of a neoplastic proliferation of small to medium-sized blasts. Blasts express T-cell lineage markers (CD2, CD3, CD4, CD5, CD7, and/or CD8) as well as markers of precursor T lymphoblasts (CD1a, CD34, CD99, and/or TDT) (18). Concurrent T-LBL and CML is likely to misdiagnosed with 8p11 myeloproliferative syndrome which is characterized in its typical form by the simultaneously or sequentially occurrence of a bcr/abl-negative myeloproliferative disorder and a lymphoma, usually a precursor T lymphoblastic lymphoma (19). The genetic testing can identify.
The prognosis of the simultaneous bi-lineage malignancies is poor with the median survival 15 months in this study. For the treatment, there is not yet consensus with regard to the optimal therapeutic modality due to the limited number of case reports and absence of prospective studies of treatments and outcomes. As we know, in terms of leukemia, hematopoietic stem cell transplantation may be the best choice to reach complete remission. Many studies highlighted the advantages of ASCT to AML (20–22). Meanwhile, some researches show that Allo-geneic BMT treated for young patients is feasible and can result in long-term disease-free survival for advanced LGL or CLL (23). For highly invasive lymphoma, such as T-LBL, allogeneic hematopoietic stem cell transplantation is alternative after reaching complete remission from high dose chemotherapy (24). For bi-lineage hematologic malignancies, chemotherapy is necessary. Withregard to bcr/abl-positive CML, imatinib may improve the survival time even though some reports stated that the targeted drug might lead to the secondary neoplasm. After complete remission, hematopoietic stem cell transplantation is recommended. From our chart, we concluded that those who were treated with transplantation survived longer than those without transplantation (P=0.033). Unfortunately, one patient died for graft-versus-host disease (GVHD) after transplantation. It is obvious that the allogeneic hematopoietic stem cell transplantation is good to the lymphoma with myeloid leukemia, but GVHD should be taken high attention. In recent years, immunotherapies paly crucial roles in hematologic neoplasms. CD19-directed CAR-T cells can reach a complete remission rate of 94% in patients with refractory/relapsed ALL, much higher than that of chemotherapy (25). Bispecific antibodies (BsAbs) can bind simultaneously two different antigens or epitopes, which leads to a wide range of applications including redirecting T cells or NK cells to tumor cells, blocking two different signaling pathways, dual targeting of different disease mediators, and delivering payloads to targeted sites. Immunotherapy has been demonstrating promising clinical results (26). For simultaneous bi-lineage malignancies, immunotherapy may provide a possible remedy.
In conclusion, simultaneous bi-lineage malignancies of myeloid leukemia and lymphomais rarely seen and there is no statistical difference in survival for different types of bi-lineage malignancy in this study. Simultaneous T-NHL and myeloid leukemia is much more than simultaneous B-NHL andmyeloid leukemia, so it is deserved vigilant to the occurence of myeloid leukemia when diagnosed T-NHL. The pathogenesis in unclear and quickly accurate diagnosis is important. For treatment, allogeneic hematopoietic stem cell transplantation may improve survival. More cases are needed to explore pathogenesis and validate our conclusion.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 81570203), the Health Science and Technology Innovation Talents Project of Henan Province (grant no. 2109901), the Key Science and Technology Research Project of Henan province (grant no. 162102310194), the Medical Key Science and Technology Research Project of Henan province (grant no. 201503044).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
MZZ has made substantial contributions to the conception and design of the study and critically revised the manuscript. YFS collated and analyzed the patient data and wrote the manuscript. XRF was responsible for managing the patients, provided the two cases and revised the manuscript. LZ, LL, XL, XHW and ZCS analyzed and interpreted the data and critically revised the manuscript for important intellectual content. All authors approved the final version of the paper for publication.
Ethics approval and consent to participate
The study was approved by the Ethics Committee for Scientific Research and Clinical Trials of Zhengzhou University and informed consent was obtained from all patients.
Patient consent for publication
All patients provided written informed consent for the publication of their data and associated images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Szumera-Ciećkiewicz A, Gałązka K, Szpor J, Rymkiewicz G, Jesionek-Kupnicka D, Gruchała A, Ziarkiewicz-Wróblewska B, Poniatowska-Broniek G, Demczuk S, Prochorec-Sobieszek M. Distribution of lymphomas in Poland according to World Health Organization classification: Analysis of 11718 cases from National Histopathological Lymphoma Register project-the Polish Lymphoma Research Group study. Int J Clin Exp Pathol. 2014;7:3280–3286. [PMC free article] [PubMed] [Google Scholar]
- 2.Zámecníková A, Vranovský A, Hlavcák P. Coexistence of Philadelphia-positive chronic granulocytic leukemia and diffuse large B-cell lymphoma at initial diagnosis. Leuk Lymphoma. 2002;43:429–431. doi: 10.1080/10428190290006288. [DOI] [PubMed] [Google Scholar]
- 3.Shen ZL, Yin LF, Mao WW, Liang J, Yang L. Philadelphia chromosome-negative non-Hodgkin's lymphoma occurring in Philadelphia chromosome-positive chronic myeloid leukemia: A case report and literature review. Oncol Lett. 2016;11:2909–2912. doi: 10.3892/ol.2016.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichenauer DA, Thielen I, Haverkamp H, Franklin J, Behringer K, Halbsguth T, Klimm B, Diehl V, Sasse S, Rothe A, et al. Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: A report from the German Hodgkin Study Group. Blood. 2014;123:1658–1664. doi: 10.1182/blood-2013-07-512657. [DOI] [PubMed] [Google Scholar]
- 5.Roberts E, III, Oncale M, Safah H, Schmieg J. Therapy-related T/myeloid mixed phenotype acute leukemia in a patient treated with chemotherapy for cutaneous diffuse large B cell lymphoma. J La State Med Soc. 2016;168:16–20. [PubMed] [Google Scholar]
- 6.Lam CJ, Curtis RE, Dores GM, Engels EA, Caporaso NE, Polliack A, Warren JL, Young HA, Levine PH, Elmi AF, et al. Risk factors for second acute myeloid leukemia/myelodysplastic syndrome among survivors of non-Hodgkin lymphoma. Leukemia. 2016;30:1187–1190. doi: 10.1038/leu.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt VR, Giri S, Verma V, Dahal S, Shah BK, Pathak R, Bociek RG, Vose JM, Armitage JO. Secondary acute myeloid leukemia in survivors of Hodgkin lymphoma. Future Oncol. 2016;12:1565–1575. doi: 10.2217/fon-2016-0048. [DOI] [PubMed] [Google Scholar]
- 8.International Non-Hodgkin's Lymphoma Prognostic Factors Project, corp-author. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 9.Abe Y, Takatsuki H, Okada Y, Saito A, Kimura T, Nishimura J. Mucosa-associated lymphoid tissue type lymphoma of the gallbladder associated with acute myeloid leukemia. Intern Med. 1999;38:442–444. doi: 10.2169/internalmedicine.38.442. [DOI] [PubMed] [Google Scholar]
- 10.Van Crombrugge L, De Vos G, Vanclooster C, Lemmerling M, Kerre T. The simultaneous appearance of a nasal natural killer-cell lymphoma and acute myelogenous leukemia. B-ENT. 2012;8:49–52. [PubMed] [Google Scholar]
- 11.Tsukasaki K, Koba T, Iwanaga M, Murata K, Maeda T, Atogami S, Nakamura H, Yamada Y, Kamihira S, Tomonaga M. Possible association between adult T-cell leukemia/lymphoma and acute myeloid leukemia. Cancer. 1998;82:488–494. doi: 10.1002/(SICI)1097-0142(19980201)82:3<488::AID-CNCR10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Metzgeroth G, Walz C, Score J, Siebert R, Schnittger S, Haferlach C, Popp H, Haferlach T, Erben P, Mix J, et al. Recurrent finding of the FIP1L1-PDGFRA fusion gene in eosinophilia-associated acute myeloid leukemia and lymphoblastic T-cell lymphoma. Leukemia. 2007;21:1183–1188. doi: 10.1038/sj.leu.2404662. [DOI] [PubMed] [Google Scholar]
- 13.Chang H, Chuang WY, Sun CF, Barnard MR. Concurrent acute myeloid leukemia and T lymphoblastic lymphoma in a patient with rearranged PDGFRB genes. Diagn Pathol. 2012;7:19. doi: 10.1186/1746-1596-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holroyd A, Cross NC, Macdonald DH. The two faces of myeloproliferative neoplasms: Molecular events underlying lymphoid transformation. Leuk Res. 2011;35:1279–1285. doi: 10.1016/j.leukres.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Melo JV, Deininger MW. Biology of chronic myelogenous leukemia-signaling pathways of initiation and transformation. Hematol Oncol Clin North Am. 2004;18(545–568):vii–viii. doi: 10.1016/j.hoc.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Tsukasaki K, Fujimoto T, Hata T, Yamada Y, Kamihira S, Tomonaga M. Concomitant complete remission of APL and smoldering ATL following ATRA therapy in a patient with the two diseases simultaneously. Leukemia. 1995;9:1797–1798. [PubMed] [Google Scholar]
- 17.Bhaker P, Das A, Rajwanshi A, Gautam U, Trehan A, Bansal D, Varma N, Srinivasan R. Precursor T-lymphoblastic lymphoma: Speedy diagnosis in FNA and effusion cytology by morphology, immunochemistry, and flow cytometry. Cancer Cytopathol. 2015;123:557–565. doi: 10.1002/cncy.21584. [DOI] [PubMed] [Google Scholar]
- 18.Jain N, Lamb AV, O'Brien S, Ravandi F, Konopleva M, Jabbour E, Zuo Z, Jorgensen J, Lin P, Pierce S, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: A high-risk subtype. Blood. 2016;127:1863–1869. doi: 10.1182/blood-2015-08-661702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goradia A, Bayerl M, Cornfield D. The 8p11 myeloproliferative syndrome: Review of literature and an illustrative case report. Int J Clin Exp Pathol. 2008;1:448–456. [PMC free article] [PubMed] [Google Scholar]
- 20.Vellenga E, van Putten W, Ossenkoppele GJ, Verdonck LF, Theobald M, Cornelissen JJ, Huijgens PC, Maertens J, Gratwohl A, Schaafsma R, et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood. 2011;118:6037–6042. doi: 10.1182/blood-2011-07-370247. [DOI] [PubMed] [Google Scholar]
- 21.Gorin NC, Labopin M, Reiffers J, Milpied N, Blaise D, Witz F, de Witte T, Meloni G, Attal M, Bernal T, et al. Higher incidence of relapse in patients with acute myelocytic leukemia infused with higher doses of CD34+ cells from leukapheresis products autografted during the first remission. Blood. 2010;116:3157–3162. doi: 10.1182/blood-2009-11-252197. [DOI] [PubMed] [Google Scholar]
- 22.Gorin NC, Labopin M, Blaise D, Reiffers J, Meloni G, Michallet M, de Witte T, Attal M, Rio B, Witz F, et al. Higher incidence of relapse with peripheral blood rather than marrow as a source of stem cells in adults with acute myelocytic leukemia autografted during the first remission. J Clin Oncol. 2009;27:3987–3993. doi: 10.1200/JCO.2008.20.1400. [DOI] [PubMed] [Google Scholar]
- 23.Toze CL, Shepherd JD, Connors JM, Voss NJ, Gascoyne RD, Hogge DE, Klingemann HG, Nantel SH, Nevill TJ, Phillips GL, et al. Allogeneic bone marrow transplantation for low-grade lymphoma and chronic lymphocytic leukemia. Bone Marrow Transplant. 2000;25:605–612. doi: 10.1038/sj.bmt.1702191. [DOI] [PubMed] [Google Scholar]
- 24.Haioun C, Mounier N, Emile JF, Ranta D, Coiffier B, Tilly H, Récher C, Fermé C, Gabarre J, Herbrecht R, et al. Rituximab versus observation after high-dose consolidative first-line chemotherapy with autologous stem-cell transplantation in patients with poor-risk diffuse large B-cell lymphoma. Ann Oncol. 2009;20:1985–1992. doi: 10.1093/annonc/mdp237. [DOI] [PubMed] [Google Scholar]
- 25.Wei G, Ding L, Wang J, Hu Y, Huang H. Advances of CD19-directed chimeric antigen receptor-modified T cells in refractory/relapsed acute lymphoblastic leukemia. Exp Hematol Oncol. 2017;6:10. doi: 10.1186/s40164-017-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei G, Wang J, Huang H, Zhao Y. Novel immunotherapies for adult patients with B-lineage acute lymphoblastic leukemia. J Hematol Oncol. 2017;10:150. doi: 10.1186/s13045-017-0516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapadia SB, Kaplan SS. Simultaneous occurrence of non-Hodgkin's lymphoma and acute myelomonocytic leukemia. Cancer. 1976;38:2557–2560. doi: 10.1002/1097-0142(197612)38:6<2557::AID-CNCR2820380646>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 28.Youness E, Ahearn MJ, Drewinko B. Simultaneous occurrence of non-Hodgkin's lymphoma and spontaneous acute granulocytic leukemia. Am J Clin Pathol. 1978;70:415–420. doi: 10.1093/ajcp/70.3.415. [DOI] [PubMed] [Google Scholar]
- 29.Ramji S, Rusia U, Basu TK. Simultaneous occurrence of a chronic myeloid leukemia and a malignant T-cell lymphoma. Indian Pediatr. 1988;25:566–568. [PubMed] [Google Scholar]
- 30.Ohtsu T, Tobinai K, Minato K, Mukai K, Kagami Y, Miwa M, Arai C, Shimoyama M. Concurrent adult T-cell leukemia and acute myeloblastic leukemia. Jpn J Clin Oncol. 1988;18:33–41. doi: 10.1093/jjco/18.1.33. [DOI] [PubMed] [Google Scholar]
- 31.Morales E, Bancalari G, Fahrenkrog AM, Rossle A. Chronic myeloid leukemia and non Hodgkin lymphoma in the same patient. Clinical case. Rev Med Chil. 1999;127:1105–1107. (In Spanish) [PubMed] [Google Scholar]
- 32.Montefusco E, Fazi F, Cordone I, Ariola C, Nanni M, Spadea A, Spiriti MA, Fenu S, Mandelli F, Petti MC. Molecular remission following high-dose hydroxyurea and fludarabine plus cytarabine in a patient with simultaneous acute myeloid leukemia and low-grade lymphoma. Leuk Lymphoma. 2001;40:671–674. doi: 10.3109/10428190109097666. [DOI] [PubMed] [Google Scholar]
- 33.Au WY, Ma SK, Wan TS, Wang EP, Lau TC, Kwong YL. Concurrent mediastinal B cell lymphoma and chronic myeloid leukemia with an unusually favorable response to chemotherapy. Leuk Lymphoma. 2003;44:535–538. doi: 10.1080/1042819021000038029. [DOI] [PubMed] [Google Scholar]
- 34.Lamb LS, Jr, Neuberg R, Welsh J, Best R, Stetler-Stevenson M, Sorrell A. T-cell lymphoblastic leukemia/lymphoma syndrome with eosinophilia and acute myeloid leukemia. Cytometry B Clin Cytom. 2005;65:37–41. doi: 10.1002/cyto.b.20033. [DOI] [PubMed] [Google Scholar]
- 35.Capovilla M, Cayuela JM, Bilhou-Nabera C, Gardin C, Letestu R, Baran-Marzak F, Fenaux P, Martin A. Synchronous FIP1L1-PDGFRA-positive chronic eosinophilic leukemia and T-cell lymphoblastic lymphoma: A bilineal clonal malignancy. Eur J Haematol. 2008;80:81–86. doi: 10.1111/j.1600-0609.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 36.Li YH, Xiao Y, Jiang ZJ. Childhood lymphoblastic lymphoma with acute myeloid leukemia: A case report and literature review. Zhonghua Xue Ye Xue Za Zhi. 2011;32:127–128. (In Chinese) [PubMed] [Google Scholar]
- 37.Sharkunov NN, Moiseyeva TN, Zybunova EE, Vinogradova OY, Kravchenko SK. Successful treatment for Hodgkin's lymphoma in a female patient with Ph+ chronic myeloid leukemia. Ter Arkh. 2012;84:71–74. (In Russian) [PubMed] [Google Scholar]
- 38.Wan DM, Zhang SP, Zhang C. Haploidentical hematopoietic stem cell transplantation for treatment of T-lymphoblastic lymphoma with chronic myeloid leukemia: A case report and literature review. Zhonghua Xue Ye Xue Za Zhi. 2012;33:227–228. (In Chinese) [PubMed] [Google Scholar]
- 39.Kunitomi A, Kotani S, Ukyo N, Ono K, Nakamine H, Nohgawa M. Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly complicated by the onset of acute myeloid leukemia. Intern Med. 2014;53:51–56. doi: 10.2169/internalmedicine.53.0219. [DOI] [PubMed] [Google Scholar]
- 40.Dong HJ, Wu W, Wang JH, Zhu HF, Gao S, Hou LP, Bai QX. Acute myeloid leukemia complicated with complex karyotypes and T-lymphoblastic lymphoma: A case report. Zhonghua Xue Ye Xue Za Zhi. 2016;37:237. doi: 10.3760/cma.j.issn.0253-2727.2016.03.012. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai Y, Shuai X, Kuang P, Wang L, Liu T, Niu T. Philadelphia chromosome with acute myeloid leukemia and concurrent large B cell lymphoma of different origins: A case report. Oncol Lett. 2017;13:1189–1193. doi: 10.3892/ol.2017.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.