Abstract
Deregulation of microRNA (miRNA/miR) expression has been implicated in the development of pancreatic ductal adenocarcinoma (PDAC). However, the role of miR-661 in PDAC remains unknown. In the present study, it was revealed that miR-661 expression was significantly upregulated in PDAC tissues compared with that in adjacent normal tissues by using reverse transcription-quantitative polymerase chain reaction assays. Higher miR-661 expression revealed a positive association with lymph node metastasis, an advanced T stage and a poor prognosis in patients with PDAC. Furthermore, ectopic expression of miR-661 significantly promoted the cell proliferation ability in PDAC cell lines, and simultaneously promoted Wnt signaling pathway-related protein expression of β-catenin, transcription factor 4 and cyclin D1 in vitro. However, the downregulation of miR-661 revealed reverse effects. Thus, the results of the present study indicated that miR-661 may function as a prognostic marker and provide insight for pancreatic cancer treatment.
Keywords: microRNAs, pancreatic ductal adenocarcinoma, microRNA-661, tumor prognosis, Wnt signaling pathway
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related mortality (1). In 2016, there were an estimated 53,000 new cases and 42,000 cases of cancer-related mortality in the United States. The 5-year relative survival rate of PDAC is only 7% (2). Due to the difficulty in diagnosing PDAC at an early stage, >50% of patients present with metastasis upon diagnosis (3). Thus, investigating novel diagnostic markers and therapeutic targets for PDAC is critical.
MircroRNAs (miRNAs/miRs) are small and endogenous single-stranded RNA molecules. Previous studies revealed that miRNAs could bind to the 3′untranslated region of their target genes and regulate their post-transcriptional expression, which led to targeting the mRNA translational repression or degradation of genes (4,5). miRNAs have been shown to function as tumor suppressors or oncogenes in the development and progression of PDAC. Certain miRNAs, including miR-454 (6), miR-183-5p (7), miR-200a (8) and miR-138-5p (9), have been reported to affect tumor cell proliferation, migration, invasion and metastasis.
miR-661 has been revealed to function as an oncogene in certain tumors, including breast cancer (10), ovarian cancer (11) and non-small cell lung cancer (12). However, the role of miR-661 in PDAC expression remains unknown. The present study aimed to reveal the clinical significance and functional effects of miR-661 on PDAC. The aim of the present study was to examine the expression of miR-661 in PDAC tissues and determine the association between miR-661 expression and poor prognosis of patients with PDAC. Furthermore, the effects of ectopic expression of miR-661 on proliferation of PDAC cells in vitro and the Wnt signalling pathway were examined.
Materials and methods
Tissue samples and cell lines
PDAC tissues and corresponding adjacent normal ductal epithelial tissues were obtained following surgery from 59 patients included 39 men and 20 women, aged 34–82 years old, with a mean age of 53.5 years old. following pancreaticoduodenal resection at Zhujiang Hospital of Southern Medical University (Guangzhou, China) between June 2008 and July 2012. The clinical data of the patients is summarized in Table I. The study was approved by the Committees for the Ethical Review of Research at Zhujiang Hospital of Southern Medical University, and written informed consent was obtained from all patients. All patients had not received any treatment prior to surgery. The fresh tissues obtained during surgery were stored at −80°C for further analysis.
Table I.
Association between miR-661 expression and clinical factors.
| miR-661 expression levels | ||||
|---|---|---|---|---|
| Characteristics | Patients (n=59) | Lower (n=27) | Higher (n=32) | P-value |
| Sex | 0.933 | |||
| Male | 39 | 18 | 21 | |
| Female | 20 | 9 | 11 | |
| Age, years | 0.296 | |||
| ≤60 | 37 | 15 | 22 | |
| >60 | 22 | 12 | 10 | |
| Differentiation | 0.465 | |||
| Well and moderate | 40 | 17 | 23 | |
| Poor | 19 | 10 | 9 | |
| Lymph node metastasis | 0.039a | |||
| Negative | 40 | 22 | 18 | |
| Positive | 19 | 5 | 14 | |
| Pathological T stage | 0.034a | |||
| T1, T2 | 20 | 13 | 7 | |
| T3, T4 | 39 | 14 | 25 | |
| Tumor location | 0.942 | |||
| Head | 29 | 12 | 17 | |
| Uncinate process | 16 | 7 | 9 | |
| Body and tail | 14 | 8 | 6 | |
| Tumor size, cm | 0.612 | |||
| ≤4 | 36 | 16 | 20 | |
| >4 | 23 | 11 | 12 | |
P<0.05; miR, microRNA; T, tumor.
The PDAC ASPC-1, PANC-1 and MIA PaCa-2 cell lines, and one human pancreatic duct epithelial HPDE6 cell line, were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a humidified 5% CO2 atmosphere at 37°C.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and all cell lines using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The RNA concentrations were determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA) at 260 and 280 nm (A260/280). The cDNA was reverse transcribed using the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). The RT-qPCR was performed using a TaqMan miRNA assay on an ABI7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The thermocycling conditions were as follows: Denaturation at 95°C for 5 min followed by 35 cycles of denaturation at 95°C for 15 sec and annealing/elongation at 60°C for 30 sec. The relative mRNA expression was determined using the comparative 2−ΔΔCq method (13). The primer sequence for miR-661 was as follows: miR-661 forward: 5′-GTGCCTGGGTCTCTGGCCT-3′. U6 forward: 5′-CTCGCTTCGGCAGCACA-3′ and U6 reverse: 5′-AACGCTTCACGAATTTGCGT-3′. The mRNA expression was normalized to U6.
Cell transfection
ASPC-1 and PANC-1 cells were transfected with 100 nM miRNA-negative control (miR-NC), miR-661 mimic (100 nM) or miR-661 inhibitor (100 nM) (Chang Jing Bio-Tech, Ltd., Changsha, China) using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Following cell transfection for 48 h, the cells were harvested for RT-qPCR or western blot analysis to assess the mRNA and protein expression.
Cell proliferation assay
A cell proliferation assay was performed using Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen, China). Briefly, a 96-well plate was seeded with ASPC-1 and PANC-1 cells (1×103 cells per well) and incubated for 24 h. Cells were transfected with miR-NC, miR-663 mimic or miR-663 inhibitor as aforementioned. Cell counting kit solution (10 µl) was added to each well according to the manufacturer's protocol, and cell proliferation was detected at 0, 1, 2 and 3 days. The cell proliferation was measured using a microplate spectrophotometer (Molecular Devices, LLC, Sunnyvale, CA, USA) at 450 nm.
Cell colony assay
ASPC-1 and PANC-1 cells (500 cells per well) were seeded in 6-well plates and cultured for 7 days. Cell colonies were fixed in 100% methanol at room temperature for 24 h and subsequently stained with 1% crystal violet at room temperature for 1 h under a light microscope and the magnification was ×200. All experiments were performed in triplicate.
Western blot analysis
Transfected cells were lysed in radioimmunoprecipitation assay buffer (Pierce; Thermo Fisher Scientfic, Inc.). The concentration of protein was detected by Bradford assay protein quantitation kit (Abcam, Cambridge, UK). Equal quantities of protein (30 µg) were separated on 10% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked with 5% skimmed milk in TBS/0.1% Tween (TBST) for 1 h at room temperature. The membranes were probed with primary antibodies against cyclin D1 (catalog no. sc-8396; 1:500), β-catenin (catalog no. sc-376841; 1:1,000; Santa Cruz Biotechnology) and transcription factor 4 (TCF4; catalog no. sc-166699, 1:500) and GAPDH (catalog no. sc-69778; 1:1,000) (all Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. The membranes were incubated with horseradish peroxidase (HRP)-conjugated bovine, anti-mouse secondary antibody (catalog no. sc-2380, 1:500, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature. The blots were detected using an enhanced chemiluminescence system (GE Healthcare, Chicago, IL, USA) and analysed with Image Lab software (version 3.0; Bio-Rad Laboratories, Inc.). GAPDH was used as an internal control for protein expression.
Statistical analysis
Statistical analyses were performed using SPSS statistical software 18.0 (IBM, Corp., Armonk, NY, USA). Data are presented as the mean ± standard deviation from at least three independent experiments. Difference between two groups or multiple groups were evaluated by Student's t-test or one-way analysis of variance with Student-Newman-Keuls (SNK) test as the post hoc test, respectively. To validate the association between miR-661 expression and clinical factors, the χ2 test was performed. Kaplan-Meier survival analysis and a log rank test was used to examine the survival plots for disease-free survival rate. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-661 expression is upregulated in PDAC tissues and is associated with a poor survival rate
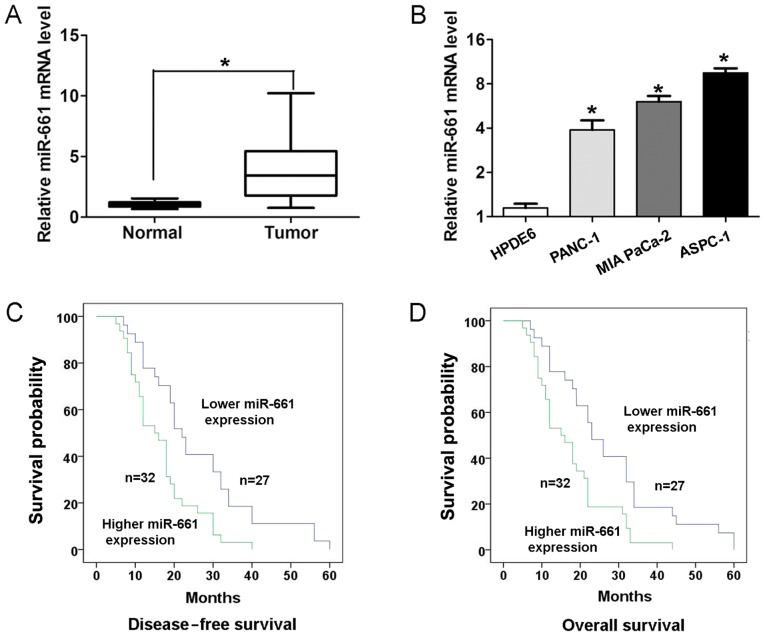
To determine the expression of miR-661 expression in PDAC tissues and adjacent normal tissues, RT-qPCR analyses were performed. As presented in Fig. 1A, PDAC tissues exhibited higher expression of miR-661 compared with adjacent normal tissues (P<0.05). In addition, the expression of miR-661 was higher in three human PDAC ASPC-1, PANC-1 and MIA PaCa-2 cell lines compared with that in the human pancreatic duct epithelial HPDE6 cell line (Fig. 1B). To validate the association between miR-661 expression and clinical factors, the χ2 test was performed. Results revealed that higher miR-661 expression demonstrated a positive association with lymph node metastasis (P<0.05; Table I) and advanced pathological T stage (P<0.05; Table I) in patients with PDAC. Kaplan-Meier survival analysis revealed that the patients with higher miR-661 expression had a significantly worse disease-free survival rate (log-rank, 9.388; P<0.05; Fig. 1C) and overall survival rate (log-rank, 9.629; P<0.05; Fig. 1D) than those with lower miR-661 expression.
Figure 1.
miR-661 expression is upregulated in PDAC tissues and is associated with a poor survival rate. (A) miR-661 expression level in 59 paired PDAC tissues and corresponding adjacent normal tissues was examined with RT-qPCR. U6 was used as an internal control. (B) miR-661 expression level in three human PDAC ASPC-1, PANC-1 and MIA PaCa-2 cell lines and one human pancreatic duct epithelial HPDE6 cell line was examined via RT-qPCR. U6 was used as an internal control. (C) Kapan-Meier survival rate analysis revealed that the patients with higher miR-661 expression had a significantly worse disease-free survival (log-rank, 9.388; P<0.05) and (D) overall survival (log-rank, 9.629; P<0.05) rate than those with lower miR-661 expression. *P<0.05. miRNA/miR, microRNA; PDAC, pancreatic ductal adenocarcinoma; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Upregulation of miR-661 promotes proliferation of PDAC cell lines in vitro
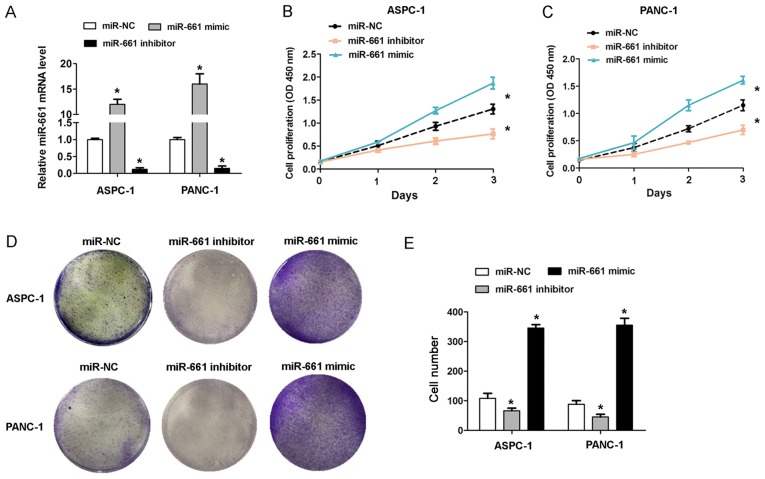
To investigate the effect of miR-661 expression on PDAC cell growth, gain of function and loss of function assays were performed in ASPC-1 and PANC-1 cells. The RT-qPCR analysis revealed that the miR-661 mimic caused an increase in miR-661 expression, while the miR-661 inhibitor caused a decrease in miR-661 expression in the ASPC-1 and PANC-1 cells (Fig. 2A). The CCK-8 cell proliferation assays revealed that transfection of miR-661 mimic promoted cell proliferation in ASPC-1 and PANC-1 cells, whereas miR-661 inhibitor inhibited cell proliferation (Fig. 2B and C). The results of cell colony formation assays revealed that transfection of miR-661 mimic in ASPC-1 and PANC-1 cells caused an increased cell colony number, whereas miR-661 inhibitor reduced the cell colony number (Fig. 2D and E). These results indicated that upregulation of miR-661 promoted the cell proliferation capacity in vitro.
Figure 2.
Upregulation of miR-661 promotes proliferation of PDAC cells in vitro. (A) miR-661 expression in ASPC-1 and PANC-1 cells upon transfection with miR-661 mimic or inhibitor for 48 h. Cell proliferation measured with cell counting kit-8 assay in (B) ASPC-1 and (C) PANC-1 cells upon transfection with miR-661 mimic or inhibitor for 48 h. (D) Representative images and (E) cumulative data from cell colony formation assays in ASPC-1 and PANC-1 cells upon transfection with miR-661 mimic or inhibitor for 48 h. Data are reported as the mean ± standard deviation of 3 independent experiments. *P<0.05. miRNA/miR, microRNA; PDAC, pancreatic ductal adenocarcinoma; NC, negative control.
Upregulation of miR-661 activates the Wnt signaling pathway in vitro
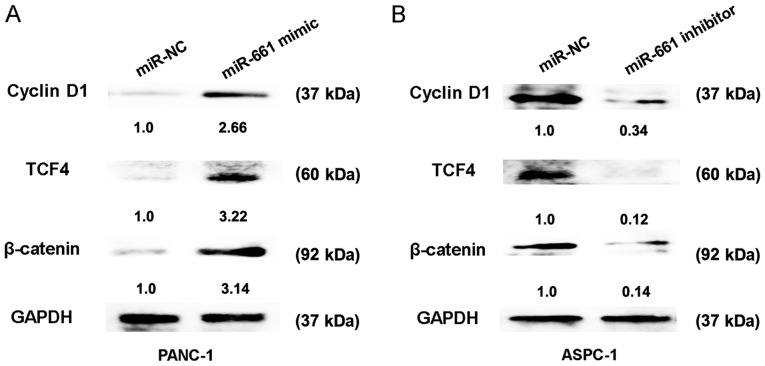
The Wnt signaling pathway has been identified as an important regulating signalling pathway in PDAC (14). To further confirm the regulatory association between miR-661 expression and the Wnt signaling pathway, the relative protein expressions of cyclin D1, TCF4 and β-catenin were detected. The results of the present study indicated that transfection of miR-661 mimic in PANC-1 cells caused an increase in protein expression of cyclin D1, TCF4 and β-catenin, whereas miR-661 inhibitor reduced the protein expression of cyclin D1, TCF4 and β-catenin in ASPC-1 cells (Fig. 3A and B). Thus, these results indicated that upregulation of miR-661 could activate the Wnt signaling pathway in vitro.
Figure 3.
Upregulation of miR-661 activates the Wnt signaling pathway in vitro. (A) Relative expression of cyclin D1, TCF4 and β-catenin was detected using western blotting following cell transfection with miR-NC or miR-661 mimic in PANC-1 cells at 48 h. (B) Relative expression of cyclin D1, TCF4 and β-catenin was detected using western blotting following cell transfection with miR-NC or miR-661 inhibitor in ASPC-1 cells at 48 h. Data are reported as the mean ± standard deviation of 3 independent experiments. miRNA/miR, microRNA; TCF4, transcription factor; NC, negative control.
Discussion
Understanding the molecular mechanisms underlying PDAC progression is important for investigating novel diagnostic markers and treatment targets (15). miRNAs have emerged as a class of gene regulators involved in PDAC. For example, miR-545 inhibits PDAC growth by targeting retinoic acid-inducible gene-I (16). miR-337 regulates proliferation and invasion in PDAC by targeting homeobox protein B7 (HOXB7) (17), and miR-429 exhibited a poor outcome in patients with PDAC and inhibits PDAC growth by targeting TANK-binding kinase 1 (18). However, the role of miR-661 in PDAC progression remains unknown.
In the present study, it was revealed that miR-661 expression was significantly upregulated in PDAC tissues and cells. Higher miR-661 expression revealed a positive association with lymph node metastasis, an advanced T stage and a poor prognosis of patients with PDAC. Furthermore, ectopic expression of miR-661 significantly promoted cell proliferation in PDAC cells in vitro. These findings indicated that miR-661 may serve a tumor-promoting role in PDAC. Lower expression of miR-661 has been revealed in certain cancers in previous studies. For example, miR-661 contributed to the proliferation of human ovarian cancer cells by repressing inositol polyphosphate-5-phosphatase J expression (11). miR-661 expression in SNAI1-induced epithelial-to-mesenchymal transition contributes to breast cancer cell invasion via targeting of nectin-1 and starD10 messengers (10). miR-661 also promotes tumor cell invasion and metastasis by directly inhibiting retinoblastoma protein in non-small cell lung cancer (12). Thus, the results of the present study indicated that miR-661 exhibited tumor proliferation promoting effects in PDAC.
Furthermore, the present study also demonstrated that transfection of the miR-661 mimic caused increased expression of TCF4 and β-catenin, whereas miR-661 inhibitor reduced the expression of TCF4 and β-catenin in PDAC cells. These results indicated that upregulation of miR-661 activated the Wnt signaling pathway in vitro. In a previous study, Wnt signaling was shown to serve a crucial role in pancreatic cancer progression; activation of Wnt/β-Catenin signaling enhanced pancreatic cancer development and the malignant potential via upregulation of cysteine-rich angiogenic inducer 61 (19). The long non-coding RNA HOX transcript antisense RNA affects the radiosensitivity of PDAC by regulating the expression of Wnt inhibitory factor 1 (20). The present study revealed that upregulation of miR-661 could activate the Wnt signaling pathway in vitro, which revealed the importance of miR-661 in PDAC progression.
In conclusion, the results of the present study revealed that miR-661 expression was higher in PDAC tissues and associated with prognosis. In vitro, miR-661 promoted cell proliferation and activated Wnt signaling. These results indicated that miR-661 may function as a prognostic biomaker and provide insight for the treatment of PDAC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
FL, KZ and JY conceived and designed the study, and drafted the manuscript. FL, KZ, JY and ZH and collected, analysed and interpreted the experiment data, and revised the manuscript critically for important intellectual content. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Committees for the Ethical Review of Research at Zhujiang Hospital of Southern Medical University, and written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Verbeke C, Löhr M, Karlsson JS, Del Chiaro M. Pathology reporting of pancreatic cancer following neoadjuvant therapy: Challenges and uncertainties. Cancer Treat Rev. 2015;41:17–26. doi: 10.1016/j.ctrv.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Verbeke C. Morphological heterogeneity in ductal adenocarcinoma of the pancreas-Does it matter? Pancreatology. 2016;16:295–301. doi: 10.1016/j.pan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Baranwal S, Alahari SK. miRNA control of tumor cell invasion and metastasis. Int J Cancer. 2010;126:1283–1290. doi: 10.1002/ijc.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Y, Xu LL, Shi CY, Wei W, Wang DS, Cai DF. MicroRNA-454 regulates stromal cell derived factor-1 in the control of the growth of pancreatic ductal adenocarcinoma. Sci Rep. 2016;6:22793. doi: 10.1038/srep22793. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Miao F, Zhu J, Chen Y, Tang N, Wang X, Li X. MicroRNA-183-5p promotes the proliferation, invasion and metastasis of human pancreatic adenocarcinoma cells. Oncol Lett. 2016;11:134–140. doi: 10.3892/ol.2015.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Wu G, Wu Z, Yao X, Li G. MiR-200a suppresses the proliferation and metastasis in pancreatic ductal adenocarcinoma through downregulation of DEK gene. Transl Oncol. 2016;9:25–31. doi: 10.1016/j.tranon.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X, Deng Y, Jiang J, Sun C. MicroRNA-138-5p regulates pancreatic cancer cell growth through targeting FOXC1. Cell Oncol (Dordr) 2015;38:173–181. doi: 10.1007/s13402-014-0200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vetter G, Saumet A, Moes M, Vallar L, Le Béchec A, Laurini C, Sabbah M, Arar K, Theillet C, Lecellier CH, Friederich E. miR-661 expression in SNAI1-induced epithelial to mesenchymal transition contributes to breast cancer cell invasion by targeting Nectin-1 and StarD10 messengers. Oncogene. 2010;29:4436–4448. doi: 10.1038/onc.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Zhu T, Yuan J, Wang Y, Gong C, Xie Y, Li H. MiR-661 contributed to cell proliferation of human ovarian cancer cells by repressing INPP5J expression. Biomed Pharmacother. 2015;75:123–128. doi: 10.1016/j.biopha.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Cai Y, Rong X, Chen J, Zheng D, Chen L, Zhang J, Luo R, Zhao P, Ruan J. MiR-661 promotes tumor invasion and metastasis by directly inhibiting RB1 in non small cell lung cancer. Mol Cancer. 2017;16:122. doi: 10.1186/s12943-017-0698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Arensman MD, Kovochich AN, Kulikauskas RM, Lay AR, Yang PT, Li X, Donahue T, Major MB, Moon RT, Chien AJ, Dawson DW. WNT7B mediates autocrine Wnt/β-catenin signaling and anchorage-independent growth in pancreatic adenocarcinoma. Oncogene. 2014;33:899–908. doi: 10.1038/onc.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wald P, Liu XS, Pettit C, Dillhoff M, Manilchuk A, Schmidt C, Wuthrick E, Chen W, Williams TM. Prognostic value of microRNA expression levels in pancreatic adenocarcinoma: A review of the literature. Oncotarget. 2017;8:73345–73361. doi: 10.18632/oncotarget.20277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song B, Ji W, Guo S, Liu A, Jing W, Shao C, Li G, Jin G. miR-545 inhibited pancreatic ductal adenocarcinoma growth by targeting RIG-I. FEBS Lett. 2014;588:4375–4381. doi: 10.1016/j.febslet.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Leng H, Huang J, Du Y, Wang Y, Zang W, Chen X, Zhao G. miR-337 regulates the proliferation and invasion in pancreatic ductal adenocarcinoma by targeting HOXB7. Diagn Pathol. 2014;9:171. doi: 10.1186/1746-1596-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song B, Zheng K, Ma H, Liu A, Jing W, Shao C, Li G, Jin G. miR-429 determines poor outcome and inhibits pancreatic ductal adenocarcinoma growth by targeting TBK1. Cell Physiol Biochem. 2015;35:1846–1856. doi: 10.1159/000373995. [DOI] [PubMed] [Google Scholar]
- 19.Sano M, Driscoll DR, DeJesus-Monge WE, Quattrochi B, Appleman VA, Ou J, Zhu LJ, Yoshida N, Yamazaki S, Takayama T, et al. Activation of WNT/β-catenin signaling enhances pancreatic cancer development and the malignant potential via Up-regulation of Cyr61. Neoplasia. 2016;18:785–794. doi: 10.1016/j.neo.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Li Z, Zheng S, Chen H, Zhao X, Gao W, Bi Z, You K, Wang Y, Li W, et al. The long non-coding RNA HOTAIR affects the radiosensitivity of pancreatic ductal adenocarcinoma by regulating the expression of Wnt inhibitory factor 1. Tumour Biol. 2016;37:3957–3967. doi: 10.1007/s13277-015-4234-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.