Abstract
Only few systematic and comprehensive studies have focused on osteosarcoma in children and adolescents. In the present study, 3,085 patients with osteosarcoma were identified in the Surveillance, Epidemiology and End Results Program database. The patients were <25 years of age and diagnosed between 1973 to 2012. A retrospective study was performed to investigate the factors associated with tumor incidence, metastasis, treatment and survival. The results indicated that the incidence of osteosarcoma was higher in male patients compared with female patients. In addition, the incidence rate of osteosarcoma was higher among male and female patients between the ages of 10 and 19. Osteosarcoma located in the chest and pelvic bones was associated with metastatic disease; however, metastasis in two histological types, parosteal and periosteal, was infrequent. Survival analysis revealed the following were associated with poor outcomes: Sex, patients diagnosed between 1973 and 1982, distant metastasis, treatment without surgery or with radiation, a tumor with a poorly differentiated or undifferentiated grade, tumor size ≥100 mm, and a tumor in the pelvic bones. Patient's whose histologic type was parosteal osteosarcoma and whose tumor was located in one of the limbs, or who underwent local or radical excision, exhibited a good survival outcome. Survival outcomes were ranked according to the type of surgery, from best to worst, as follows: Local excision, radical excision, amputation and no surgery. In summary, the incidence of osteosarcoma is higher in male patients compared with female patients. Furthermore, individuals between the ages of 10 and 19 have a higher risk of osteosarcoma. Osteosarcoma located in the chest and pelvic bones has a high risk of metastasis. Limb-salvage surgery may be the optimal treatment approach for non-metastatic osteosarcoma.
Keywords: osteosarcoma, incidence, treatment, outcomes
Introduction
Osteosarcoma is frequently diagnosed in children and adolescents (1). Patients <25 years of age exhibit a higher incidence and constituent ratio compared with any other age group (2). Studies suggested patients <25 years of age with osteosarcoma constitute as a separate specific subgroup of the population (3,4). Mirabello et al (2) reported that the epidemiologic features of osteosarcoma were unique among the 0–24; 25–59 and ≥60 years age groups, therefore emphasizing the need to study the aforementioned age groups separately. In the present study, osteosarcoma was examined in the younger age groups by conducting a systematic study of patients <25 years of age.
Single center and nationwide studies on osteosarcoma have been indicated to provide limited sample size (5,6). The Surveillance, Epidemiology and End Results (SEER) program, which currently consists of 17 geographically defined registries and covers ~26% of the U.S. population, is able to provide a large sample size. For osteosarcoma, the SEER program provides information regarding tumor site, histologic type, surgical type and incidence, which are useful parameters for clinical researchers. Therefore, the SEER program may assist clinicians with regard to early diagnosis and optimal treatment of the aforementioned tumor type.
Previous studies have primarily focused on all types of tumors, particularly bone tumors in <25 years of age (7–10) or osteosarcoma cases of all ages (3,4,11). However, further systematic and comprehensive studies focusing on osteosarcoma in children and adolescents are required.
In the present study, the incidence based on year of diagnosis, age, sex, race, region, and metastasis was examined from different sites and histologic types. In addition, the study assessed the risk factors for survival outcomes from 15 factors, performed a pairwise comparison of these factors and elucidated optimal surgical options to provide additional knowledge regarding the characteristics of osteosarcoma in patients <25 years of age. The aim of the present cohort study was to identify useful factors for the prevention and treatment of osteosarcoma.
Materials and methods
Data source
All data were obtained from the SEER program (https://seer.cancer.gov/) and the SEER*Stat application 8.3.4 software (Surveillance Research Program, National Cancer Institute, Bethesda, MD, USA) was used for analysis. Patients between 0 and 24 years of age, who were diagnosed between 1973 and 2012 were selected for the present study. Histologic Type International Classification of Disease (ICD)-O-3 was input as 9180–9187 and 9192–9195, and Primary Site-Labeled was input as C40.0-C41.9 in the software to represent osteosarcoma. A total of 3,085 cases were available. Incidence, frequency and survival outcomes were analyzed according to the following 15 factors.
Study design
A total of 15 factors, including patient- associated factors, tumor-associated factors and treatment-associated factors, were included in the present study. Patient-associated factors consisted of year of diagnosis, sex, age at diagnosis, race, Contract Health Service Delivery Areas (CHSDA) region, and rural or urban. Tumor-associated factors included stage, grade, tumor size, laterality, Histologic Type ICD-O-3 and Primary Site-Labeled. Finally, treatment-associated factors consisted of surgery, surgery type and radiation.
The year of diagnosis was divided into the following 4 groups: 1973–1982; 1983–1992; 1993–2002; and 2003–2012. Age at diagnosis was divided as follows: 0–4 years; 5–9 years; 10–14 years; 15–19 years; and 20–24 years. Individuals were also categorized as Caucasian, African descent or other, which included American Indian/Alaska (AK) Native and Asian/Pacific Islander. CHSDA region was categorized as East, Northern Plains, Pacific Coast and Southwest. Rural or urban: Urban for patients in a metropolitan area and rural for patients not in a metropolitan area. Stage was divided into localized, regional and distant. Grade was divided into well-differentiated, moderately differentiated, poorly differentiated and undifferentiated. Tumor size was divided into the following groups: <50 mm; 50–99 mm; 100–119 mm; and ≥120 mm. Laterality was divided into right and left, and surgery type was divided into no surgery, local excision, radical excision and amputation. Various histologic types, which had small samples in the univariate analysis, were excluded, while osteosarcoma, not otherwise specified, chondroblastic, fibroblastic, telangiectatic and parosteal were included. For Primary Site-Labeled the following were combined: C40.0 and C40.1, upper limbs; C40.2 and C40.3, lower limbs; C41.0 and C41.1, skull and mandible; C41.2 and C41.3, vertebral and chest bones; C41.4, pelvic bones.
Variables that had incomplete data among the 3,085 patients included surgery type, tumor size and grade. Cases for the present study were available through the SEER program, including 1,976 cases for surgery type recorded since 1998, and 1,074 cases for tumor size recorded since 2004. Therefore, in the survival curve, the x-axis for surgery type and tumor size did not correspond to 40 years. For tumor grade data, 1,834 cases were available in total, distributed throughout 1973–2012; however, there were numerous missing data.
Statistical analysis
The SEER*Stat application 8.3.4 software was used for statistical analysis of the data. Rate session was used to calculate incidence, and frequency session was used to calculate frequency. Incidence is indicated as the number per 1,000,000. Case listing session was used to collect the data of each patient and for further survival analysis. The SPSS software 17.0 (SPSS, Inc., Chicago, IL, USA) was used to perform the survival analysis, log-rank testing, pairwise comparisons, five-year survival rate analysis, univariate analysis and multivariate Cox regression analysis. Associations among histological type, tumor site and stage were analyzed using χ2 tests. P<0.05 was considered to indicate a statistically significant difference.
The aforementioned 15 factors were used to plot survival curves, and in log-rank testing, pairwise comparisons, five-year survival rate analysis and univariate analysis. A total of 3 factors, including grade, tumor size and surgery type, had incomplete data, and therefore, only 12 factors were included in the multivariate Cox regression analysis. Model 1 included all 12 factors, whereas Model 2 included 9 factors subsequent to excluding 3 factors, which exhibited no significant difference in the univariate analysis. The association between surgery type and survival was analyzed as a whole, but also for each stage and grade. The numbers of each case, the sequence of survival outcomes ranked from best to worst, and pairwise comparisons were calculated.
Results
Osteosarcoma incidence, age at diagnosis and survival for all age groups
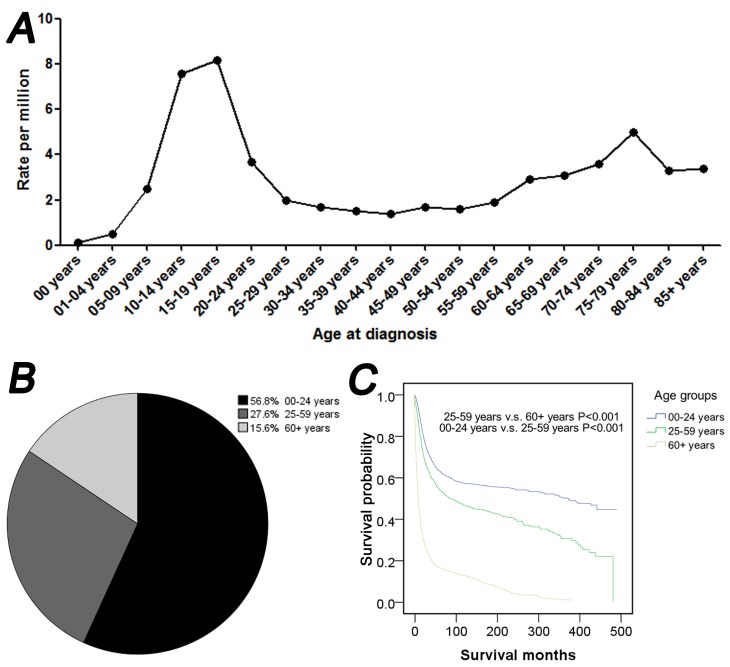
As indicated in Fig. 1, the present study of osteosarcoma was performed in all age groups between 1973 and 2012. In the line chart two peaks for osteosarcoma incidence were indicated. The highest peak corresponded to the 0–24 age group, and the other peak corresponded to the ≥60 age group (Fig. 1A). The majority of osteosarcoma cases were exhibited among patients between 10 and 14 years of age (7.6 per million) and between 15 and 19 years of age (8.2 per million) (Fig. 1A). As indicated in Fig. 1B the ratios of osteosarcoma were 56.8, 27.6 and 15.6% for 0–24, 25–59 and ≥60 years of age, respectively. A survival curve indicated that for the three age groups, patients between 0 and 24 years of age had the best prognosis, while patients ≥60 years of age had the worst prognosis (P<0.001; Fig. 1C).
Figure 1.
Osteosarcoma incidence, age distribution and survival, according to the age of diagnosis between 1973 and 2012. (A) Rate of osteosarcoma, according to the age of diagnosis. (B) Pie chart and (C) survival analysis curve of patients with osteosarcoma in the following age groups: 0–24; 25–59 and ≥60 years of age. (C) Survival analysis curve in the following age groups: 0–24; 25–59 and ≥60 years of age.
Incidence of osteosarcoma in patients <25 years of age
The incidence of osteosarcoma according to generation, sex, race, age group and CHSDA region are demonstrated in Table I. The overall incidence rate of osteosarcoma was 4.5 per million. The time span 1973–1982 had the lowest incidence rate, while the following 3 decades exhibited an increase in incidence rates compared with previous decades. However, differences among the 3 decades were not significant (P>0.05). Male patients had a higher incidence of osteosarcoma compared with female patients within each decade. The incidence rate in male patients with osteosarcoma increased between 1973 and 2003, and decreased between 2003 and 2012. Changes in female patients, according to generation, were not clear. Within each decade, races such as American Indian/Alaska Native and Asian/Pacific Islander, had the highest incidence rate, followed by patients of African descent and Caucasian. As time progressed, the incidence rate of osteosarcoma among Caucasian patients increased. However, the incidence rate of osteosarcoma decreased among other races, and remained unchanged among patients of African descent. No visible trend over time was observed for the 0–24-year-old group, but there were significant differences (P<0.05) within the age group, with the highest incidence rate indicated in patients between 10 and 19 years of age, followed by those of 20 and 24 years of age. No obvious findings for CHSDA regions were identified, except for the East region, which had the lowest incidence of osteosarcoma.
Table I.
Incidence of osteosarcoma in patients <25 years of age between 1973 and 2012 over 10-year intervals, according to sex, age at diagnosis, race and CHSDA region.
| Variables | 1973–1982 | 1983–1992 | 1993–2002 | 2003–2012 | All |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 4.2 | 5.2 | 5.6 | 5.2 | 5.1 |
| Female | 3.8 | 4.0 | 3.9 | 4.0 | 3.9 |
| Age at diagnosis (years) | |||||
| 0–4 | 0.5 | 0.5 | 0.3 | 0.4 | 0.4 |
| 5–9 | 1.5 | 2.9 | 2.7 | 2.7 | 2.5 |
| 10–14 | 7.3 | 7.3 | 7.9 | 7.7 | 7.6 |
| 15–19 | 7.4 | 8.6 | 8.8 | 7.9 | 8.2 |
| 20–24 | 3.0 | 3.6 | 4.0 | 4.1 | 3.7 |
| Race | |||||
| Caucasian | 3.7 | 4.4 | 4.6 | 4.5 | 4.3 |
| African descent | 4.8 | 5.0 | 5.1 | 5.3 | 5.1 |
| Other | 5.6 | 5.6 | 5.3 | 4.0 | 4.9 |
| CHSDA region | |||||
| East | 3.8 | 4.1 | 4.1 | 4.1 | 4.0 |
| Northern plains | 3.7 | 4.5 | 5.1 | 5.4 | 4.6 |
| Pacific coast | 4.3 | 5.0 | 4.5 | 4.4 | 4.6 |
| Southwest | 4.1 | 4.9 | 5.6 | 4.6 | 4.8 |
| All | 4.0 | 4.6 | 4.8 | 4.6 | 4.5 |
CHSDA, Contract Health Service Delivery Areas.
Incidence of osteosarcoma according to sex and age
Table II indicates the incidence rate of osteosarcoma in male and female patients in different age groups. It was demonstrated that female patients had a higher incidence rate of osteosarcoma compared with male patients, between 0–14 years of age, particularly between the ages of 0–4, 5–9 and 10–14 diagnosed between 1983 and 2002, 1993 and 2012, and 1973 and 1992, respectively. When combining the 4 decades, 1973–1982, 1983–1992, 1993–2002 and 2003–2012, female patients had a higher incidence at 5–9 years of age (Table II).
Table II.
Incidence of osteosarcoma in patients <25 years of age between 1973 and 2012, according to age group and sex.
| 1973–1982 | 1983–1992 | 1993–2002 | 2003–2012 | All | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
| 0–4 | 0.6 | 0.3 | 0.3 | 0.6 | 0.1 | 0.4 | 0.5 | 0.3 | 0.4 | 0.4 |
| 5–9 | 1.6 | 1.4 | 2.9 | 2.8 | 2.6 | 2.8 | 2.4 | 3.0 | 2.4 | 2.6 |
| 10–14 | 6.6 | 8.0 | 7.3 | 7.4 | 8.5 | 7.2 | 8.2 | 7.1 | 7.7 | 7.4 |
| 15–19 | 8.6 | 6.2 | 10.9 | 6.2 | 12.5 | 4.8 | 10.1 | 5.7 | 10.5 | 5.7 |
| 20–24 | 3.2 | 2.8 | 4.4 | 2.7 | 3.9 | 4.1 | 4.7 | 3.5 | 4.1 | 3.2 |
Association among histologic type, tumor site and metastasis
Table III indicated the association between histologic type and site with risk of metastatic disease. The ‘distant’ stage was defined as metastasis. The results of the present study indicated that the chest and pelvic bones had a higher prevalence rate of metastatic disease, while the long bone of the upper limbs had a higher prevalence rate of metastatic disease compared with the lower limbs. Parosteal and periosteal osteosarcoma were two histologic types with a low risk of metastasis.
Table III.
Association among histologic type, tumor site and stage in patients <25 years of age with osteosarcoma between 1973 and 2012.
| Variables | Localized, n (%) | Regional, n (%) | Distant, n (%) | P-value |
|---|---|---|---|---|
| Histologic type ICD-O-3 | aP<0.001 | |||
| Osteosarcoma, NOS | 724 (35.0) | 905 (43.8) | 438 (21.2) | |
| Chondroblastic osteosarcoma | 117 (29.7) | 208 (52.8) | 69 (17.5) | |
| Fibroblastic osteosarcoma | 42 (35.9) | 56 (47.9) | 19 (16.2) | |
| Telangiectatic osteosarcoma | 39 (36.4) | 52 (48.6) | 16 (15.0) | |
| Osteosarcoma in Paget disease of bone | 1 (100.0) | 0 (0.0) | 0 (0.0) | |
| Small cell osteosarcoma | 9 (39.1) | 10 (43.5) | 4 (17.4) | |
| Central osteosarcoma | 16 (32.0) | 28 (56.0) | 6 (12.0) | |
| Intraosseous well-differentiated osteosarcoma | 1 (50.0) | 1 (50.0) | 0 (0.0) | |
| Parosteal osteosarcoma | 69 (65.7) | 31 (29.5) | 5 (4.8) | |
| Periosteal osteosarcoma | 16 (57.1) | 10 (35.7) | 2 (7.1) | |
| High-grade surface osteosarcoma | 1 (12.5) | 4 (50.0) | 3 (37.5) | |
| Primary site-labeled | aP<0.001 | |||
| C40.0-Long bones: Upper limb, scapula, and associated joints | 118 (34.3) | 149 (43.3) | 77 (22.4) | |
| C40.1-Short bones of upper limb and associated joints | 5 (50.0) | 5 (50.0) | 0 (0.0) | |
| C40.2-Long bones of lower limb and associated joints | 796 (36.8) | 973 (45.0) | 394 (18.2) | |
| C40.3-Short bones of lower limb and associated joints | 13 (36.1) | 17 (47.2) | 6 (16.7) | |
| C41.0-Bones of skull and face and associated joints | 32 (36.8) | 39 (44.8) | 16 (18.4) | |
| C41.1 Mandible | 23 (39.7) | 30 (51.7) | 5 (8.6) | |
| C41.2 Vertebral column | 13 (41.9) | 13 (41.9) | 5 (16.1) | |
| C41.3 Rib, Sternum, Clavicle and associated joints | 12 (27.3) | 18 (40.9) | 14 (31.8) | |
| C41.4-Pelvic bones, sacrum, coccyx and associated joints | 22 (17.9) | 59 (48.0) | 42 (34.1) | |
| C41.9-Bone, NOS | 1 (16.7) | 2 (33.3) | 3 (50.0) |
Statistically significant. P<0.05 was considered to indicate statistically significant difference. P-values were calculated by χ2 tests. NOS, not otherwise specified; n, number; ICD, International Classification of Disease.
The five-year survival rate, univariate analysis and pairwise comparisons
Table IV summarized the five-year survival rates and univariate analyses for 15 factors. Survival curves and the results of pairwise comparisons are presented in Fig. 2 for patient-associated factors and Fig. 3 for tumor-associated factors and treatment-associated factors. Survival outcome was worst between 1973–1982, and the following 3 decades exhibited an improved survival outcome. When each of the 3 decades was compared with 1973–1982, all results exhibited significant differences (P<0.05), but comparisons within the 3 decades indicated no differences (P>0.05). Female patients had relatively good survival outcomes compared with male patients (P<0.001). The survival outcome from best to worst among the different age groups was as follows: 10–14; >5-9; >20-24; >15–19 and >0–4 years of age, but there were no significant differences in pairwise comparisons among the groups (P>0.05).
Table IV.
Five-year survival rate and univariate analysis in patients with osteosarcoma <25 years of age between 1973 and 2012.
| Univariate analysis | ||||
|---|---|---|---|---|
| Variables | n (%) | Survival (95% CI) | HR (95% CI) | P-value |
| Year of diagnosis | aP<0.001 | |||
| 1973–1982 | 357 (11.6) | 50.1 (45.0–55.2) | Reference | |
| 1983–1992 | 404 (13.1) | 62.7 (58.0–67.4) | 0.701 (0.576–0.852) | aP<0.001 |
| 1993–2002 | 851 (27.6) | 63.5 (60.2–66.8) | 0.661 (0.557–0.784) | aP<0.001 |
| 2003–2012 | 1,473 (47.7) | 66.3 (63.6–69.0) | 0.582 (0.493–0.687) | aP<0.001 |
| Sex | aP<0.001 | |||
| Male | 1,757 (57.0) | 60.1 (57.7–62.5) | Reference | |
| Female | 1,328 (43.0) | 67.3 (64.8–69.8) | 0.757 (0.675–0.849) | aP<0.001 |
| Age at diagnosis (years) | a0.014 | |||
| 0–4 | 49 (1.6) | 54.1 (39.2–69.0) | Reference | |
| 5–9 | 368 (11.9) | 64.2 (59.1–69.3) | 0.728 (0.468–1.131) | 0.158 |
| 10–14 | 1,064 (34.5) | 66.4 (63.5–69.3) | 0.666 (0.437–1.014) | 0.058 |
| 15–19 | 1,102 (35.7) | 60.0 (57.1–62.9) | 0.828 (0.545–1.258) | 0.376 |
| 20–24 | 502 (16.3) | 63.8 (59.5–68.1) | 0.776 (0.504–1.194) | 0.249 |
| Race | 0.939 | |||
| Caucasian | 2,289 (74.2) | 63.2 (61.0–65.4) | Reference | |
| African descent | 476 (15.4) | 62.1 (57.6–66.6) | 1.023 (0.877–1.194) | 0.771 |
| Other | 296 (9.6) | 62.6 (56.9–68.3) | 0.985 (0.814–1.191) | 0.872 |
| CHSDA region | a0.011 | |||
| East | 843 (27.3) | 65.8 (62.5–69.1) | Reference | |
| Northern Plains | 479 (15.5) | 61.8 (57.3–66.3) | 1.169 (0.981–1.394) | 0.081 |
| Pacific Coast | 1,465 (47.5) | 63.4 (60.9–65.9) | 1.114 (0.970–1.280) | 0.127 |
| Southwest | 295 (9.6) | 56.5 (50.6–62.4) | 1.394 (1.142–1.701) | a0.001 |
| Rural or urban | 0.098 | |||
| Rural | 264 (8.6) | 60.8 (54.7–66.9) | Reference | |
| Urban | 2,758 (89.4) | 63.5 (61.5–65.5) | 0.852 (0.704–1.031) | 0.098 |
| Stage | aP<0.001 | |||
| Localized | 1,034 (33.5) | 77.5 (74.8–80.2) | Reference | |
| Regional | 1,305 (42.3) | 64.7 (62.0–67.4) | 1.640 (1.416–1.900) | aP<0.001 |
| Distant | 562 (18.2) | 31.1 (27.0–35.2) | 4.442 (3.798–5.196) | aP<0.001 |
| Grade | aP<0.001 | |||
| Well | 86 (2.8) | 81.1 (72.5–89.7) | Reference | |
| Moderately | 125 (4.1) | 78.0 (70.4–85.6) | 1.142 (0.653–1.997) | 0.641 |
| Poorly | 524 (17.0) | 61.4 (56.9–65.9) | 2.073 (1.307–3.287) | a0.002 |
| Undifferentiated | 1,100 (35.7) | 64.5 (61.6–67.4) | 2.015 (1.286–3.163) | a0.002 |
| Tumor size (mm) | aP<0.001 | |||
| <50 | 127 (4.1) | 79.5 (71.7–87.3) | Reference | |
| 50–99 | 458 (14.8) | 73.2 (68.7–77.7) | 1.250 (0.809–1.930) | 0.315 |
| 100–119 | 149 (4.8) | 64.3 (55.9–72.7) | 1.751 (1.082–2.836) | a0.023 |
| ≥120 | 340 (11.0) | 56.4 (49.9–62.9) | 2.119 (1.376–3.263) | a0.001 |
| Laterality | 0.371 | |||
| Right | 1,364 (44.2) | 50.0 (47.5–52.5) | Reference | |
| Left | 1,367 (44.3) | 63.3 (60.6–66.0) | 1.056 (0.937–1.191) | 0.371 |
| Histologic Type ICD-O-3 | aP<0.001 | |||
| Osteosarcoma, NOS | 2,223 (72.1) | 60.8 (58.6–63.0) | Reference | |
| Chondroblastic | 407 (13.2) | 62.4 (57.5–67.3) | 0.912 (0.770–1.080) | 0.286 |
| Fibroblastic | 122 (4.0) | 69.9 (61.7–78.1) | 0.732 (0.539–0.994) | a0.045 |
| Telangiectatic | 109 (3.5) | 70.2 (61.0–79.4) | 0.774 (0.557–1.075) | 0.127 |
| Parosteal | 111 (3.6) | 89.0 (82.9–95.1) | 0.287 (0.180–0.457) | aP<0.001 |
| Primary site-labeled | aP<0.001 | |||
| Upper limb | 378 (12.3) | 56.8 (51.5–62.1) | Reference | |
| Lower limb | 2,321 (75.2) | 66.4 (64.4–68.3) | 0.737 (0.627–0.867) | aP<0.001 |
| Skull and mandible | 155 (5.0) | 67.6 (60.0–75.2) | 0.694 (0.511–0.944) | a0.02 |
| Vertebral and chest bones | 79 (2.6) | 48.2 (37.0–59.4) | 1.301 (0.943–1.795) | 0.109 |
| Pelvic bones | 135 (4.4) | 31.1 (22.9–39.3) | 2.339 (1.827–2.993) | aP<0.001 |
| Surgery | aP<0.001 | |||
| Yes | 2,613 (84.7) | 66.3 (64.3–68.3) | Reference | |
| No | 399 (12.9) | 44.6 (39.5–49.7) | 2.113 (1.831–2.438) | aP<0.001 |
| Surgery type | aP<0.001 | |||
| No surgery | 244 (7.9) | 42.4 (35.9–48.9) | Reference | |
| Local excision | 208 (6.7) | 75.3 (69.2–81.4) | 0.310 (0.226–0.426) | aP<0.001 |
| Radical excision | 1,076 (34.9) | 71.1 (68.2–74.0) | 0.344 (0.281–0.422) | aP<0.001 |
| Amputation | 389 (12.6) | 61.7 (56.6–66.8) | 0.494 (0.391–0.624) | aP<0.001 |
| Radiation | aP<0.001 | |||
| Yes | 163 (5.3) | 35.3 (27.7–42.9) | Reference | |
| No | 2,881 (93.4) | 64.8 (63.0–66.6) | 0.373 (0.308–0.450) | aP<0.001 |
Statistically significant. P<0.05 was considered to indicate statistically significant difference. P-values calculated by univariate analysis. HR, hazard ratio; CI, confidence interval; n, number; ICD, International Classification of Disease; NOS, not otherwise specified; CHSDA, Contract Health Service Delivery Areas.
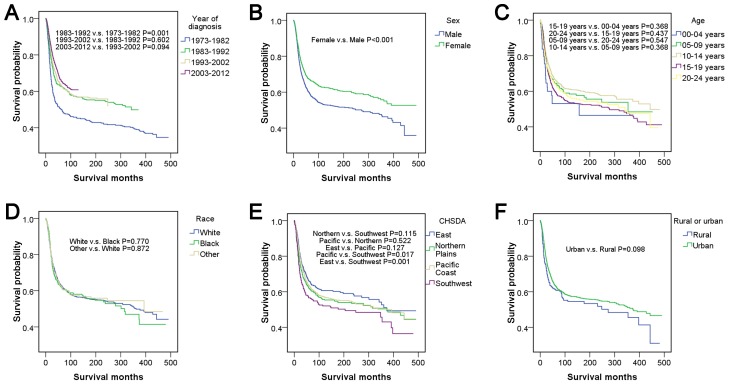
Figure 2.
Survival analyses, according to (A) year of diagnosis, (B) sex, (C) age, (D) race, (E) CHSDA region and (F) rural or urban in patients <25 years of age with osteosarcoma between 1973 and 2012. CHSDA, Contract Health Service Delivery Areas.
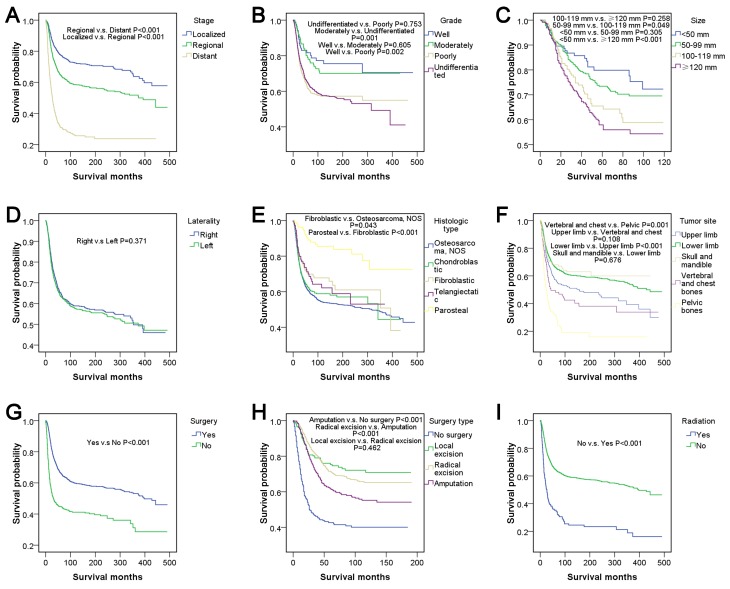
Figure 3.
Survival analyses, according to (A) stage, (B) grade, (C) tumor size, (D) laterality, (E) histologic type, (F) tumor site, (G) surgery, (H) surgery type and (I) radiation in patients <25 years of age with osteosarcoma between 1973 and 2012. NOS, not otherwise specified.
There were no significant differences among races (P>0.05). In addition, no significant differences were observed among CHSDA regions (P>0.05), except that the region with the best outcome, East, was significantly different compared with the region with the worst outcome, Southwest (P<0.05). Patients from rural and urban areas had no significant difference in survival outcome (P>0.05). There were significant differences in stage according to pairwise comparisons and the entire comparison (P<0.001). Well- and moderately differentiated subtypes of grade corresponded to relatively good survival outcomes compared with the poorly differentiated and undifferentiated subtypes (P<0.05), but no significant differences were demonstrated within well- and moderately differentiated or within poorly differentiated and undifferentiated subtypes (P>0.05). The survival curve indicated that a large tumor size was associated with relatively poor survival outcomes, and there was a significant difference in survival outcome between patients with a tumor size <50 mm and patients with a tumor size >100 mm (P<0.05). There was no significant difference between tumors located on the left and right lateral (P>0.05). Parosteal osteosarcoma had the best survival outcome with a five-year survival rate of 89.0% and was significantly different from all other histological types of osteosarcoma (P<0.001). The tumor sites ranked from best to worst survival outcome were as follows: Skull and mandible; lower limb; upper limb; vertebral and chest bones, and pelvic bones. In pairwise comparisons, there were significant differences between chest bones and pelvic bones (P=0.001) and between lower limbs and upper limbs (P<0.001). Patients who underwent surgery had relatively good survival outcomes compared with those who did not (P<0.001). The surgery types, which were ranked from best to worst for survival outcome were local excision, radical excision, amputation and no surgery. There was no significant difference between local excision and radical excision, according to pairwise comparisons (P>0.05), however, all other types of surgery were associated with significant differences (P<0.001). Patients who underwent radiation had relatively poor survival outcomes compared with patients who did not receive radiation (P<0.001).
Association between surgery type and survival outcome
As the association between surgery type and survival outcome may be confounded by other factors, including stage and grade of osteosarcoma, survival curves were plotted and log-rank tests were performed for the same stage or grade (Table V). The frequency of amputation as a treatment for osteosarcoma was higher among patients with ‘localized’ stage of osteosarcoma, while patients with ‘distant’ stage of osteosarcoma were not surgically treated. In addition, amputation was indicated to be higher among patients with ‘undifferentiated’ grade of osteosarcoma. In the comparison of surgery types among patients with the same stage or grade of osteosarcoma, results of survival outcome based on surgery type were almost identical. The results of the present study indicated that for types of surgery the best to worst survival outcomes were as follows: Local excision, radical excision, amputation and no surgery. The aforementioned result was also indicated for the total number of patients. Therefore, local excision may be the optimal choice for patients with any type of osteosarcoma, conflicting with the previous notion that amputation is the optimal choice of treatment. In addition, as indicated in the results of Table V, radical excision may be an optimal choice for patients with metastatic disease.
Table V.
Association between surgery type and survival outcome in patients <25 years of age with osteosarcoma between 1973 and 2012, according to stage and grade.
| Stage (n=1,915) | Grade (n=1,439) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Localized | Regional | Distant | Well | Moderately | Poorly | Undifferentiated | All (n=1977) |
| Number | ||||||||
| No surgery | 53 | 53 | 107 | 2 | 4 | 42 | 88 | 244 |
| Local excision | 98 | 75 | 29 | 16 | 13 | 48 | 78 | 208 |
| Radical excision | 396 | 504 | 163 | 25 | 53 | 229 | 492 | 1076 |
| Amputation | 101 | 235 | 101 | 8 | 12 | 91 | 238 | 449 |
| Order of outcome | ||||||||
| Best | 2 | 2 | 3 | 1 | 3 | 2 | 2 | 2 |
| Relatively good | 3 | 3 | 2 | 3 | 2 | 3 | 3 | 3 |
| Relatively poor | 4 | 4 | 4 | 2 | 1 | 4 | 4 | 4 |
| Worst | 1 | 1 | 1 | 4 | 4 | 1 | 1 | 1 |
| Significance | ||||||||
| No surgery vs. local excision | a0.031 | aP<0.001 | a0.011 | 0.622 | 0.523 | a0.005 | aP<0.001 | aP<0.001 |
| No surgery vs. radical excision | a0.046 | aP<0.001 | aP<0.001 | 0.724 | 0.113 | aP<0.001 | aP<0.001 | aP<0.001 |
| No surgery vs. amputation | 0.478 | aP<0.001 | a0.001 | 0.385 | 0.994 | a0.033 | aP<0.001 | aP<0.001 |
| Local excision vs. radical excision | 0.469 | 0.557 | 0.202 | 0.505 | 0.393 | 0.682 | 0.576 | 0.462 |
| Local excision vs. amputation | 0.092 | 0.243 | 0.589 | a0.037 | 0.358 | 0.165 | 0.087 | a0.001 |
| Radical excision vs. amputation | 0.139 | 0.263 | a0.002 | a0.006 | a0.014 | 0.081 | a0.042 | aP<0.001 |
Statistically significant. P<0.05 was considered to indicate statistically significant difference. P-values were calculated by log-rank tests. n, number.
Multivariate Cox regression analysis
In the univariate analysis, factors with significant differences included year of diagnosis, sex, age at diagnosis, CHSDA region, stage, grade, tumor size, histologic type, tumor site, surgery, surgery type and radiation (P<0.05). The results of the multivariate Cox regression analysis are indicated in Table VI. Year of diagnosis, sex, age at diagnosis, CHSDA region, stage, histologic type, tumor site, surgery and radiation were independent risk factors in Model 1 of the multivariate Cox regression analysis (P<0.05). In Model 2 of the multivariate Cox regression analysis, independent risk factors included year of diagnosis, sex, CHSDA region, stage, histologic type, tumor site, surgery and radiation (P<0.05).
Table VI.
Multivariate Cox regression analysis in patients <25 years of age with osteosarcoma between 1973 and 2012.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95%CI) | P-value |
| Year of diagnosis | P<0.001 | aP<0.001 | ||
| 1973–1982 | Reference | Reference | ||
| 1983–1992 | 0.649 (0.500–0.841) | 0.001 | 0.657 (0.529–0.816) | aP<0.001 |
| 1993–2002 | 0.611 (0.484–0.771) | P<0.001 | 0.594 (0.491–0.719) | aP<0.001 |
| 2003–2012 | 0.528 (0.418–0.666) | P<0.001 | 0.507 (0.419–0.613) | aP<0.001 |
| Sex | 0.042 | aP<0.001 | ||
| Male | Reference | Reference | ||
| Female | 0.866 (0.754–0.995) | 0.042 | 0.805 (0.710–0.912) | aP<0.001 |
| Age at diagnosis (years) | 0.042 | 0.081 | ||
| 0–4 | Reference | Reference | ||
| 5–9 | 0.859 (0.518–1.423) | 0.554 | 0.837 (0.52–1.349) | 0.466 |
| 10–14 | 0.726 (0.448–1.176) | 0.193 | 0.716 (0.454–1.129) | 0.151 |
| 15–19 | 0.909 (0.561–1.471) | 0.697 | 0.832 (0.528–1.311) | 0.428 |
| 20–24 | 0.920 (0.558–1.516) | 0.743 | 0.902 (0.566–1.440) | 0.667 |
| Race | 0.452 | |||
| Caucasian | Reference | |||
| African descent | 1.123 (0.935–1.348) | 0.214 | ||
| Other | 1.043 (0.818–1.332) | 0.733 | ||
| CHSDA region | 0.003 | a0.035 | ||
| East | Reference | Reference | ||
| Northern Plains | 1.372 (1.101–1.711) | 0.005 | 1.190 (0.978–1.448) | 0.082 |
| Pacific Coast | 1.164 (0.978–1.385) | 0.088 | 1.133 (0.971–1.323) | 0.114 |
| Southwest | 1.515 (1.187–1.934) | 0.001 | 1.378 (1.108–1.715) | a0.004 |
| Rural or urban | 0.71 | |||
| Rural | Reference | |||
| Urban | 1.046 (0.824–1.328) | 0.71 | ||
| Stage | P<0.001 | aP<0.001 | ||
| Localized | Reference | Reference | ||
| Regional | 1.612 (1.360–1.911) | P<0.001 | 1.585 (1.360–1.848) | aP<0.001 |
| Distant | 4.036 (3.357–4.853) | P<0.001 | 3.899 (3.292–4.616) | aP<0.001 |
| Laterality | 0.944 | |||
| Right | Reference | |||
| Left | 0.995 (0.873–1.135) | 0.944 | ||
| Histologic type ICD-O-3 | P<0.001 | aP<0.001 | ||
| Osteosarcoma, NOS | Reference | Reference | ||
| Chondroblastic | 0.821 (0.669–1.008) | 0.060 | 0.869 (0.721–1.046) | 0.137 |
| Fibroblastic | 0.669 (0.464–0.965) | 0.032 | 0.779 (0.567–1.070) | 0.124 |
| Telangiectatic | 0.770 (0.538–1.102) | 0.154 | 0.782 (0.553–1.107) | 0.166 |
| Parosteal | 0.365 (0.218–0.614) | P<0.001 | 0.354 (0.218–0.576) | aP<0.001 |
| Primary site-labeled | P<0.001 | aP<0.001 | ||
| Upper limb | Reference | Reference | ||
| Lower limb | 0.737 (0.617–0.881) | 0.001 | 0.777 (0.654–0.923) | a0.004 |
| Skull and mandible | 0.668 (0.316–1.495) | 0.344 | 0.689 (0.494–0.960) | a0.028 |
| Vertebral and chest bones | 0.834 (0.487–1.429) | 0.509 | 0.982 (0.691–1.395) | 0.917 |
| Pelvic bones | 1.625 (1.157–2.281) | 0.005 | 1.612 (1.210–2.147) | a0.001 |
| Surgery | P<0.001 | aP<0.001 | ||
| Yes | Reference | Reference | ||
| No | 1.726 (1.434–2.077) | P<0.001 | 1.645 (1.391–1.946) | aP<0.001 |
| Radiation | P<0.001 | aP<0.001 | ||
| Yes | Reference | Reference | ||
| No | 0.600 (0.461–0.781) | P<0.001 | 0.548 (0.442–0.679) | aP<0.001 |
Statistically significant. P<0.05 was considered to indicate statistically significant difference. P-values were calculated by multivariate Cox regression analysis. Model 1 excluded grade, tumor size and surgery type, which had incomplete data in the univariate analysis. Model 2 further excluded race, rural or urban, and laterality, which had no significant difference in the univariate analysis. HR, hazard ratio; CI, confidence interval; n, number; ICD, International Classification of Disease; NOS, not otherwise specified; CHSDA, Contract Health Service Delivery Areas.
Discussion
At the start of the present study, the distribution characteristics for the incidence of osteosarcoma was indicated according to age. The results demonstrated that patients <25 years of age with osteosarcoma had relatively good survival outcomes, but also had the highest ratio (56.8%) and incidence (8.2 per million) among all age groups. The aforementioned result may be due to certain characteristics of this age group, which remain unknown. Therefore, this specific age group requires further study in terms of incidence, metastasis, survival prognosis and treatment options for osteosarcoma, according to the aforementioned 15 factors.
The lowest incidence rate of osteosarcoma and worst survival outcomes were observed between 1973 and 1982, while the subsequent 3 decades had the highest incidence rate and best outcomes. Within these 3 decades, incidences and survival outcomes minimally changed. The five-year survival rate was 50.1% prior to 1982 and >60% subsequent to this year. The increased incidence of osteosarcoma, following 1982, may be due to the diagnostic improvements for osteosarcoma. Numerous studies have also observed that there was improvement in survival for patients diagnosed with osteosarcoma subsequent to 1982, which according to the studies may be due to the introduction of chemotherapeutic regimens (12,13). Duffaud et al (14) reported that localized high-grade osteosarcoma had a long-term disease-free survival rate of <20% prior to the administration of intensive chemotherapy and 55–75% subsequent to the introduction of the aforementioned treatment. The patient-derived orthotopic xenograft model, developed over the past 30 years, is a promising research method for effective individualized therapy, which has been applied to various types of cancer, including breast, ovarian, lung, cervical, colon, stomach, pancreatic, melanoma, sarcoma, and osteosarcoma (15). Using the aforementioned model, Murakami et al (16) and Igarashi et al (17) indicated that the tumor-targeting Salmonella typhimurium A1-R is a powerful treatment option and they reported that it was able to regress osteosarcoma. The authors of the aforementioned studies also used this model to screen drugs and identify effective treatment drugs or drug combinations for osteosarcoma (18,19).
Sex-associated differences revealed that male patients had a higher incidence rate of osteosarcoma compared with female patients, which was consistent even within the same race and region (data not shown). The only exception was that female patients 5–9 years of age had a higher incidence rate of osteosarcoma compared with male patients. The aforementioned results may be due to the active bone growth reported in males and females (20). It has been reported that males undergo more rapid bone growth compared with females (21). However, females between the ages of 11 and 13 have been reported to be taller and undergo rapid bone growth compared with age-matched males (22). Numerous studies have reported that as the height of an individual increases so does the risk of osteosarcoma (20,23–25). A higher incidence rate of osteosarcoma was additionally observed in female patients between 0 and 14 years of age in a study by Mirabello et al (2) and in female patients between 10 and 14 years of age in a study by Homa et al (26). Regarding sex-associated differences in survival, numerous studies (27,28) have reported that females have a longer life span compared with males; results which confirm the present study's findings. Researchers have attributed the aforementioned survival difference to the relatively poor response reported in male patients to chemotherapy, and their high recurrence rate (29,30). In the present study it was additionally proposed that males may exhibit symptoms in the long-term and therefore, do not participate actively in treatment.
Regarding age, the highest incidence of osteosarcoma was among those 10–19 years of age, followed by those between 20 and 24 years of age, which may be due to the rapid bone growth of the aforementioned age groups. The optimal survival rate was observed in patients between 10 and 14 years of age and between 5 and 9 years of age, whereas the worst survival rate was observed in patients between 1 and 4 years of age. The survival results of the present study were verified by other single-center studies (31–33). Guillon et al (31) analyzed 15 patients <5 years of age with osteosarcoma and reported a mortality rate of 45% (7 patients) within 5 years of follow-up, suggesting that osteosarcoma is highly invasive in patients <5 years of age. Worch et al (32) reported that the five-year survival rate of children ≤5 years of age and >5 years of age was 51.9 and 67.3%, respectively. Sugalski et al (33) reported that the five-year survival rate of children <12 years of age and >12 years of age was 11 and 57%, respectively. However, Hagleitner et al (34) reported an opposite trend, where the 5-year overall survival rate was 70.6±0.8, 52.5±1.1, 33.3±0.9% in patients ≤14, 15–19 and 20–40 years of age, respectively. The reason for the decrease in survival rate in young patients remains unclear, however, the aforementioned findings suggest that tumors in patients with different ages have different biological characteristics. Furthermore, limb salvage surgery poses surgical challenges for skeletally immature patients, as it may cause leg-length inequality in the long-term (35). It has been reported that patients <5 years of age undergo amputation at a higher rate, whereas only a number of patients receive chemotherapy (32).
Miller et al (36) defined patients at the stage of ‘distant’ in the SEER database as metastatic, whereas patients at the stage of ‘localized’ or ‘regional’ were defined as non-metastatic. Miller et al (36), including other researchers, have reported that patients with metastatic osteosarcoma had a relatively poor prognosis compared with patients with localized osteosarcoma (37,38). The present study compared the aforementioned three subtypes of osteosarcoma and the differences were reported as significant for all 15 factors in pairwise comparisons and the overall comparison. This may be due to the fact that the predominant factor associated to survival was metastasis vs. non-metastasis. Lee (39) reported that the event-free survival (EFS) rate of Korean children and adolescents with osteosarcoma at 5 years following diagnosis was 27.0 and 65.3% with and without metastasis, respectively. Kantar et al (40) reported that the 5-year EFS rates were 67 and 25% in patients with non-metastatic and metastatic disease, respectively. The present study demonstrated that the five-year survival rates in localized, regional and distant stages were 77.5, 64.7 and 31.1%, respectively.
Two factors, grade and tumor size, had incomplete data and have been rarely analyzed in other studies. In the present study, tumors with grades of well and moderate differentiation had relatively good outcomes compared with those that were poorly differentiated and undifferentiated in the univariate analysis, possibly due to the fact that tumor differentiation reflects tumor malignancy. Larger tumors were reported to have relatively poor prognoses (41), which was confirmed in the present study, where tumors >100 mm in size had relatively poor outcomes compared with tumors <50 mm. Therefore, larger tumor sizes may reflect a more advanced stage of tumor development.
Miller et al (36) performed a histological analysis for patients with osteosarcoma of all ages, among which Paget diseases were reported to be more common in the elderly group (≥60 years of age). However, in the present study only one case of Paget disease presented in the younger group (<25 years of age). In addition, small cell osteosarcoma was commonly associated with metastatic disease in the aforementioned study, in contrast to the present study. Nakajima et al (41) reviewed 72 cases with small cell osteosarcoma, concluding that this subtype of osteosarcoma is highly aggressive and less responsive to chemotherapy. The present study revealed two histologic types, parosteal and periosteal osteosarcoma, which were not associated with metastatic diseases. Parosteal osteosarcoma had relatively good survival outcomes compared with any other type of tumor, in accordance with the study of Mankin et al (42). Bacci et al (43) reported that fibroblastic and telangiectatic tumors had significantly higher 5-year overall survival rates, whereas chondroblastic and osteoblastic tumors had significantly lower 5-year overall survival rates. The effect of histologic type on metastasis and survival may be determined by biological characteristics of the tumors, however, further investigation is required.
The results of the present study indicated that the long bones of the four limbs were predilection sites for osteosarcoma. Lee (39) further reported that the most frequently affected site in children and adolescents was the distal femur (52.3%). In the present study, extremity osteosarcoma had low metastasis and relatively good outcome, while axial skeletal osteosarcoma had the highest metastasis and worst outcome, confirming previous observations by Janinis et al (44), who reported that extremity tumors had a 2- and 3-year survival rate of 50 and 21%, respectively, and axial skeletal tumors had a 2- and 3-year survival rate of 19 and 13%, respectively. Meazza et al (45) studied 20 patients between the ages of 3 and 19 with axial skeletal osteosarcoma and reported a 5-year overall survival rate as low as 40%. Among 129 cases of osteosarcomas, Akyuz et al (46) observed 6 cases of axial skeletal osteosarcomas, in which mortality occurred in 5 cases between three and sixteen months subsequent to diagnosis, indicating a poor prognosis. In the present study, the recorded sites were divided into 5 types to provide detailed information of tumor site and prognosis. According to the analysis, the tumor types ranked from best to worst survival outcome followed skull and mandible, lower limb, upper limb, vertebral and chest bones, and pelvic bones. An explanation for the aforementioned results is that axial skeletal osteosarcomas in vertebral, chest and pelvic bones may in close proximity to important organs, vessels or nerves, therefore, making complete resection difficult and possibly contributing to poor survival.
The type of surgery was analyzed in detail in the present study. Previous meta-analyses (47,48) have reported that limb-salvage surgery confers better survival compared with amputation. Schrager et al (7) additionally reported the same trend in children and adolescents. Once modern prosthetics became available in the 1970s, there was an increase in limb-salvage surgeries performed, which corresponded to survival outcomes in comparison to amputation (7). It has been reported that limb-salvage surgery is the optimal choice in 85% of children patients with osteosarcoma (49,50). Limb-salvage surgery may provide better outcomes compared with amputation, as amputation can cause psychological and functional impairment in young patients (51,52). Furthermore, the present study compared survival outcomes among different types of surgery of the same stage or grade to exclude confounding factors and provide reliable results, as favorable outcomes may be due to mild disease rather than type of surgery. According to the order of best to worst survival outcome reported in the present study, surgery provided better outcomes compared with non-surgical treatment approaches, while excision provided better outcomes compared with amputation. Therefore, although removing the entire tumor may be the ideal choice for the treatment of osteosarcoma, excessive excision negatively affects body recovery. An interesting finding in the present study was that patients with metastatic diseases had a better survival rate when radical excision was chosen as a treatment option for osteosarcoma, possibly due to the fact that metastatic tumors tend to be larger and require to be thoroughly removed. Therefore, radical excision should be recommended for patients with metastatic diseases. Picci et al (53) identified inadequate margins in surgery as a risk factor for poor prognosis, confirming to an extent the results of the present study.
The results of the present study initially indicated poor outcomes in patients who underwent radiation, however, further analysis indicated that the aforementioned result was not entirely valid. A total of 11.2% patients with metastatic diseases had undergone radiotherapy, whereas 3.9% of patients with non-metastatic diseases had undergone radiotherapy (data not shown). Therefore, a higher number of patients with metastatic diseases had received radiotherapy, indicating that the observed negative effect of radiotherapy was because patients with metastatic diseases had relatively poor overall prognosis.
The systematic analysis of the present study may provide useful information for guiding clinical work. Males patients between 10 and 19 years of age had a high incidence rate of osteosarcoma, suggesting the requirement for an early screening of this aforementioned high-risk population for osteosarcoma. Chest and pelvic bones were at high risk of metastasis, therefore, metastatic lesions should be checked among high-risk patients. Year of diagnosis, sex, CHSDA region, stage, histologic type, tumor site, surgery and radiation were demonstrated in the present study to be independent risk factors in the multivariate Cox regression analysis. In order to improve survival rate, local excision can be used in the majority of patients with osteosarcoma, and radical excision is suggested for patients with metastatic diseases.
However, the present study presents certain limitations. Firstly, information regarding chemotherapy treatment is not available in the SEER database, therefore extracting information associated with the type of drugs used for chemotherapy treatments was not possible. Secondly, no detailed record of the type of surgery was available, therefore, determining for example if patients had undergone joint replacement was not possible. Thirdly, the present study was retrospective rather than a randomized controlled trial, therefore, whether to perform a limb-salvage surgery or an amputation depended on the doctors' suggestion and the patient's requirement rather than random assignment. Finally, there were no therapy records of biologic markers and no records of tumor recurrence. Despite the aforementioned limitations, the present study incorporated a relatively large number of osteosarcoma cases from the SEER database, increasing the accuracy of present study's results.
Acknowledgements
The authors thank the National Natural Science Foundation of China (Beijing, China). The authors also thank the National Cancer Institute (Bethesda, USA), who provided access to the public SEER database.
Funding
The present study was supported by the National Natural Science Foundation of China (grant, no., 81672154; Beijing, China).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) repository, https://seer.cancer.gov/.
Authors' contributions
ZN acquired data, analyzed data and wrote the manuscript. HP acquired data, designed the study, revised the manuscript and gave final approval of the version to be published. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Ethics approval and consent to participate are not needed as this study is based on already existing data from the National Cancer Institute. The present study was approved by the National Cancer Institute.
Patient's consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and ewing's sarcoma: National cancer data base report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anfinsen KP, Devesa SS, Bray F, Troisi R, Jonasdottir TJ, Bruland OS, Grotmol T. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976–2005) Cancer Epidemiol Biomarkers Prev. 2011;20:1770–1777. doi: 10.1158/1055-9965.EPI-11-0136. [DOI] [PubMed] [Google Scholar]
- 4.Duong LM, Richardson LC. Descriptive epidemiology of malignant primary osteosarcoma using population-based registries, United States, 1999–2008. J Registry Manag. 2013;40:59–64. [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuda Y, Ogura K, Shinoda Y, Kobayashi H, Tanaka S, Kawai A. The outcomes and prognostic factors in patients with osteosarcoma according to age: A Japanese nationwide study with focusing on the age differences. BMC Cancer. 2018;18:614. doi: 10.1186/s12885-018-4487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Yang J, Wang Y, Wang D, Han G, Jia J, Xu M, Bi W. Survival and prognostic factors in Chinese patients with osteosarcoma: 13-year experience in 365 patients treated at a single institution. Pathol Res Pract. 2017;213:119–125. doi: 10.1016/j.prp.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Schrager J, Patzer RE, Mink PJ, Ward KC, Goodman M. Survival outcomes of pediatric osteosarcoma and Ewing's sarcoma: A comparison of surgery type within the SEER database, 1988–2007. J Registry Manag. 2011;38:153–161. [PubMed] [Google Scholar]
- 8.Perkins SM, Shinohara ET, DeWees T, Frangoul H. Outcome for children with metastatic solid tumors over the last four decades. PLoS One. 2014;9:e100396. doi: 10.1371/journal.pone.0100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatta G, Capocaccia R, Coleman MP, Ries LA, Berrino F. Childhood cancer survival in europe and the united states. Cancer. 2002;95:1767–1772. doi: 10.1002/cncr.10833. [DOI] [PubMed] [Google Scholar]
- 10.Novakovic B. U.S. childhood cancer survival, 1973–1987. Med Pediatr Oncol. 1994;23:480–486. doi: 10.1002/mpo.2950230606. [DOI] [PubMed] [Google Scholar]
- 11.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39:593–599. doi: 10.1016/j.canep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Foster L, Dall GF, Reid R, Wallace WH, Porter DE. Twentieth-century survival from osteosarcoma in childhood. Trends from 1933 to 2004. J Bone Joint Surg Br. 2007;89:1234–1238. doi: 10.1302/0301-620X.89B9.19255. [DOI] [PubMed] [Google Scholar]
- 13.Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat Rev. 2006;32:423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Duffaud F, Digue L, Mercier C, Dales JP, Baciuchka-Palmaro M, Volot F, Thomas P, Favre R. Recurrences following primary osteosarcoma in adolescents and adults previously treated with chemotherapy. Eur J Cancer. 2003;39:2050–2057. doi: 10.1016/S0959-8049(03)00435-0. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi K, Igarashi K, Li S, Han Q, Tan Y, Miyake K, Kiyuna T, Miyake M, Murakami T, Chmielowski B, et al. Recombinant methioninase (rMETase) is an effective therapeutic for BRAF-V600E-negative as well as-positive melanoma in patient-derived orthotopic xenograft (PDOX) mouse models. Oncotarget. 2018;9:915–923. doi: 10.18632/oncotarget.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami T, Igarashi K, Kawaguchi K, Kiyuna T, Zhang Y, Zhao M, Hiroshima Y, Nelson SD, Dry SM, Li Y, et al. Tumor-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograft model resistant to a molecular-targeting drug. Oncotarget. 2017;8:8035–8042. doi: 10.18632/oncotarget.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi K, Kawaguchi K, Murakami T, Kiyuna T, Miyake K, Nelson SD, Dry SM, Li Y, Yanagawa J, Russell TA, et al. Intra-arterial administration of tumor-targeting Salmonella typhimurium A1-R regresses a cisplatin-resistant relapsed osteosarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Cell Cycle. 2017;16:1164–1170. doi: 10.1080/15384101.2017.1317417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igarashi K, Murakami T, Kawaguchi K, Kiyuna T, Miyake K, Zhang Y, Nelson SD, Dry SM, Li Y, Yanagawa J, et al. A patient-derived orthotopic xenograft (PDOX) mouse model of a cisplatinum-resistant osteosarcoma lung metastasis that was sensitive to temozolomide and trabectedin: Implications for precision oncology. Oncotarget. 2017;8:62111–62119. doi: 10.18632/oncotarget.19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igarashi K, Kawaguchi K, Kiyuna T, Miyake K, Miyake M, Li Y, Nelson SD, Dry SM, Singh AS, Elliott IA, et al. Temozolomide combined with irinotecan regresses a cisplatinum-resistant relapsed osteosarcoma in a patient-derived orthotopic xenograft (PDOX) precision-oncology mouse model. Oncotarget. 2017;9:7774–7781. doi: 10.18632/oncotarget.22892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirabello L, Pfeiffer R, Murphy G, Daw NC, Patino-Garcia A, Troisi RJ, Hoover RN, Douglass C, Schuz J, Craft AW, Savage SA. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control. 2011;22:899–908. doi: 10.1007/s10552-011-9763-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora RS, Alston RD, Eden TO, Geraci M, Birch JM. The contrasting age-incidence patterns of bone tumours in teenagers and young adults: Implications for aetiology. Int J Cancer. 2012;131:1678–1685. doi: 10.1002/ijc.27402. [DOI] [PubMed] [Google Scholar]
- 22.Greil H, Kahl H. Assessment of developmental age: Cross-sectional analysis of secondary sexual characteristics. Anthropol Anz. 2005;63:63–75. [PubMed] [Google Scholar]
- 23.Longhi A, Pasini A, Cicognani A, Baronio F, Pellacani A, Baldini N, Bacci G. Height as a risk factor for osteosarcoma. J Pediatr Hematol Oncol. 2005;27:314–318. doi: 10.1097/01.mph.0000169251.57611.8e. [DOI] [PubMed] [Google Scholar]
- 24.Gelberg KH, Fitzgerald EF, Hwang S, Dubrow R. Growth and development and other risk factors for osteosarcoma in children and young adults. Int J Epidemiol. 1997;26:272–278. doi: 10.1093/ije/26.2.272. [DOI] [PubMed] [Google Scholar]
- 25.Endicott AA, Morimoto LM, Kline CN, Wiemels JL, Metayer C, Walsh KM. Perinatal factors associated with clinical presentation of osteosarcoma in children and adolescents. Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26349. [DOI] [PubMed] [Google Scholar]
- 26.Homa DM, Sowers MR, Schwartz AG. Incidence and survival rates of children and young adults with osteogenic sarcoma. Cancer. 1991;67:2219–2223. doi: 10.1002/1097-0142(19910415)67:8<2219::AID-CNCR2820670837>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Petrilli AS, Gentil FC, Epelman S, Lopes LF, Bianchi A, Lopes A, Figueiredo MT, Marques E, De Bellis N, Consentino E. Increased survival, limb preservation, and prognostic factors for osteosarcoma. Cancer. 1991;68:733–737. doi: 10.1002/1097-0142(19910815)68:4<733::AID-CNCR2820680412>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Smeland S, Muller C, Alvegard TA, Wiklund T, Wiebe T, Bjork O, Stenwig AE, Willen H, Holmstrom T, Folleras G, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39:488–494. doi: 10.1016/j.ejca.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 30.Saeter G, Elomaa I, Wahlqvist Y, Alvegard TA, Wiebe T, Monge O, Forrestier E, Solheim OP. Prognostic factors in bone sarcomas. Acta Orthop Scand Suppl. 1997;273:156–160. doi: 10.1080/17453674.1997.11744723. [DOI] [PubMed] [Google Scholar]
- 31.Guillon MA, Mary PM, Brugiere L, Marec-Berard P, Pacquement HD, Schmitt C, Guinebretiere JM, Tabone MD. Clinical characteristics and prognosis of osteosarcoma in young children: A retrospective series of 15 cases. BMC Cancer. 2011;11:407. doi: 10.1186/1471-2407-11-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Osteosarcoma in children 5 years of age or younger at initial diagnosis. Pediatr Blood Cancer. 2010;55:285–289. doi: 10.1002/pbc.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugalski AJ, Jiwani A, Ketchum NS, Cornell J, Williams R, Heim-Hall J, Hung JY, Langevin AM. Characterization of localized osteosarcoma of the extremity in children, adolescents, and young adults from a single institution in south texas. J Pediatr Hematol Oncol. 2014;36:e353–e358. doi: 10.1097/MPH.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagleitner MM, Hoogerbrugge PM, van der Graaf WT, Flucke U, Schreuder HW, te Loo DM. Age as prognostic factor in patients with osteosarcoma. Bone. 2011;49:1173–1177. doi: 10.1016/j.bone.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Neel MD, Wilkins RM, Rao BN, Kelly CM. Early multicenter experience with a noninvasive expandable prosthesis. Clin Orthop Relat Res. 2003:1–81. doi: 10.1097/01.blo.0000093899.12372.25. [DOI] [PubMed] [Google Scholar]
- 36.Miller BJ, Cram P, Lynch CF, Buckwalter JA. Risk factors for metastatic disease at presentation with osteosarcoma: An analysis of the SEER database. J Bone Joint Surg Am. 2013;95:e89. doi: 10.2106/JBJS.L.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jawad MU, Cheung MC, Clarke J, Koniaris LG, Scully SP. Osteosarcoma: Improvement in survival limited to high-grade patients only. J Cancer Res Clin Oncol. 2011;137:597–607. doi: 10.1007/s00432-010-0923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008;134:281–297. doi: 10.1007/s00432-007-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JA. Osteosarcoma in Korean children and adolescents. Korean J Pediatr. 2015;58:123–128. doi: 10.3345/kjp.2015.58.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantar M, Cetingul N, Azarsiz S, Kansoy S, Sabah D, Memis A, Basdemir G, Burak Z. Treatment results of osteosarcoma of the extremity in children and adolescents at ege university hospital. Pediatr Hematol Oncol. 2002;19:475–482. doi: 10.1080/08880010290097297. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima H, Sim FH, Bond JR, Unni KK. Small cell osteosarcoma of bone. Review of 72 cases. Cancer. 1997;79:2095–2106. doi: 10.1002/(SICI)1097-0142(19970601)79:11<2095::AID-CNCR6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 42.Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004:1–291. doi: 10.1097/01.blo.0000145991.65770.e6. [DOI] [PubMed] [Google Scholar]
- 43.Bacci G, Bertoni F, Longhi A, Ferrari S, Forni C, Biagini R, Bacchini P, Donati D, Manfrini M, Bernini G, Lari S. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer. 2003;97:3068–3075. doi: 10.1002/cncr.11456. [DOI] [PubMed] [Google Scholar]
- 44.Janinis J, McTiernan A, Driver D, Mitchell C, Cassoni AM, Pringle J, Kilby A, Whelan JS. London Bone and Soft Tissue Tumour Service: A pilot study of short-course intensive multiagent chemotherapy in metastatic and axial skeletal osteosarcoma. Ann Oncol. 2002;13:1935–1944. doi: 10.1093/annonc/mdf338. [DOI] [PubMed] [Google Scholar]
- 45.Meazza C, Luksch R, Daolio P, Podda M, Luzzati A, Gronchi A, Parafioriti A, Gandola L, Collini P, Ferrari A, et al. Axial skeletal osteosarcoma: A 25-year monoinstitutional experience in children and adolescents. Med Oncol. 2014;31:875. doi: 10.1007/s12032-014-0875-x. [DOI] [PubMed] [Google Scholar]
- 46.Akyuz C, Ilhan I, Kutluk T, Buyukpamukcu M. Primary osteosarcoma presenting in axial bones in childhood. Turk J Pediatr. 1995;37:375–381. [PubMed] [Google Scholar]
- 47.Han G, Bi WZ, Xu M, Jia JP, Wang Y. Amputation versus limb-salvage surgery in patients with osteosarcoma: A meta-analysis. World J Surg. 2016;40:2016–2027. doi: 10.1007/s00268-016-3500-7. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Zhang Y, Wan S, Li H, Li D, Xia J, Yuan Z, Ren M, Yu S, Li S, et al. A comparative study between limb-salvage and amputation for treating osteosarcoma. J Bone Oncol. 2016;5:15–21. doi: 10.1016/j.jbo.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grimer RJ. Surgical options for children with osteosarcoma. Lancet Oncol. 2005;6:85–92. doi: 10.1016/S1470-2045(05)01734-1. [DOI] [PubMed] [Google Scholar]
- 50.Wafa H, Grimer RJ. Surgical options and outcomes in bone sarcoma. Expert Rev Anticancer Ther. 2006;6:239–248. doi: 10.1586/14737140.6.2.239. [DOI] [PubMed] [Google Scholar]
- 51.Postma A, Kingma A, De Ruiter JH, Koops Schraffordt H, Veth RP, Goeken LN, Kamps WA. Quality of life in bone tumor patients comparing limb salvage and amputation of the lower extremity. J Surg Oncol. 1992;51:47–51. doi: 10.1002/jso.2930510113. [DOI] [PubMed] [Google Scholar]
- 52.Aksnes LH, Bauer HC, Jebsen NL, Folleras G, Allert C, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation: A Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br. 2008;90:786–794. doi: 10.1302/0301-620X.90B6.19805. [DOI] [PubMed] [Google Scholar]
- 53.Picci P, Sangiorgi L, Bahamonde L, Aluigi P, Bibiloni J, Zavatta M, Mercuri M, Briccoli A, Campanacci M. Risk factors for local recurrences after limb-salvage surgery for high-grade osteosarcoma of the extremities. Ann Oncol. 1997;8:899–903. doi: 10.1023/A:1008230801849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) repository, https://seer.cancer.gov/.