Abstract
Effects of polo-like kinase (PLK1) on proliferation, migration and invasion capacities of gastric cancer cells through epithelial-mesenchymal transition (EMT) were investigated. Small-interfering ribonucleic acid (siRNA) with targeted interference in PLK1 gene was designed and transfected into gastric cancer MGC-803 cells via Lipofectamine to inhibit the expression of PLK1 gene in MGC-803 cells. The proliferation of MGC-803 cells was detected via methyl thiazolyl tetrazolium (MTT) assay. The mRNA and protein expression of PLK1 and EMT-related marker (E-cadherin) was detected via real-time polymerase chain reaction and western blot analysis, respectively. The effects of interference in PLK1 gene on migration and invasion of MGC-803 cells were studied via wound healing assay and Transwell chamber assay, respectively. Results of MTT assay showed that compared with that in control group, the cell proliferation in PLK1 siRNA group was significantly inhibited (p<0.01). Compared with those in control group, the mRNA and protein expression of PLK1 in PLK1 siRNA group was significantly decreased (p<0.01), but the mRNA and protein expression of E-cadherin was obviously upregulated (p<0.01). Results of wound healing assay and invasion assay showed that the capacity of migration and invasion of MGC-803 cells in PLK1 siRNA group was significantly inhibited compared with those in control group (p<0.01). In conclusion, PLK1 enhances the proliferation, migration and invasion of gastric cancer MGC-803 cells through affecting EMT.
Keywords: PLK1, gastric cancer cells, epithelial-mesenchymal transition, proliferation and invasion
Introduction
It was found in epidemiological statistics that the mortality rate of gastric cancer in malignant tumors is only lower than that of lung cancer, and its incidence rate ranks fourth in the world (1,2). The clinical treatment means of early gastric cancer is mainly surgery, but most patients are in the advanced stage when diagnosed, so the chemotherapy-based comprehensive treatment is mainly adopted. Unfortunately, chemotherapy does not significantly improve the survival rate of patients with advanced gastric cancer, and patients have to face the adverse effects brought by chemotherapy (3–5). Gastric cancer does not have typical clinical features in the early stage, so it is often neglected. There are reports showing that 50–60% of patients with gastric cancer in China are in the advanced stage, and the resection rate is only 40% in diagnosed patients. Due to a high malignant degree of tumor, gastric cancer is still vulnerable to recurrence and metastasis even after resection, there is a poor prognosis, and only 20–30% of patients can live for 5 years after surgery. Therefore, the effect of simple surgical treatment is often disappointing for patients with advanced gastric cancer, especially for those who have undergone local tumor spread or metastasis to other tissues (6). Currently, the clinical treatment of gastric cancer has been developed from simple surgical treatment to comprehensive treatment of surgery and chemoradiotherapy, and individualized and targeted therapies have become hot spots in treatment (7).
The process in which epithelial cells differentiate into cells with biological characteristics of mesenchymal phenotype according to a certain procedure is called epithelial-mesenchymal transition (EMT). EMT is a key link in invasion and distant spread of tumor cells (8). Polo-like kinase (PLK) is a kind of serine/threonine protein kinase, which widely exists in eukaryotic cells with certain cycle dependence. Its name is derived from the fact that it can lead to abnormality in the formation of spindle body in Drosophila (9). Νumerous studies have shown that PLK1 plays an important role in chromosome segregation, promotion of centrosome maturation, cytokinesis and other processes. However, the roles of PLK1 in the occurrence and development of cancer have not been elucidated.
The experiments were carried out to investigate the effects of PLK1 on the proliferation, migration and invasion of gastric cancer cells, and to explore the roles of EMT in the proliferation, migration and invasion processes of gastric cancer cells.
Materials and methods
Materials and reagents
Human gastric cancer cell lines MGC-803 (Cell Bank of Chinese Academy of Sciences, Shanghai, China), methyl thiazolyl tetrazolium (MTT) (Sigma, St. Louis, MO, USA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), PLK1 and E-cadherin human primary antibodies and horseradish peroxidase (HRP)-labeled second antibodies (all from Wuhan Sanying Biotechnology, Wuhan, China), primer synthesis (Takara Biotechnology Co., Ltd., Dalian, China), Dulbecco's modified Eagle's medium (DMEM) (Gibco Life Technologies, Carlsbad, CA, USA), ribonucleic acid (RNA) extraction kits, reverse transcription kits and reverse transcription-polymerase chain reaction (RT-PCR) kits (all from from Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA), bicinchoninic acid (BCA) protein quantification kits and cell lysis solution (all from Beyotime Institute of Biotechnology, Nantong, China). The study was approved by the Ethics Committee of The Second Affiliated Hospital of Zhengzhou University (Zhengzhou, China) and written informed consents were signed by the patients and/or guardians.
Design and construction of PLK1 expression vectors
PLK1 and negative control plasmids (siNC) were designed and synthesized by Shanghai GenePharma Co., Ltd., (Shanghai, China). The sense strand of PLK1 small-interfering ribonucleic acid (siRNA) is 5′-GCAACCUGCAGUGUAAUAATT-3′, and its antisense strand is 5′-UUAUUACACUGCAGGUUGCTT-3′. The sense strand of siNC is 5′-GCCTCAACATCCCCTACAAGA-3′, and its antisense strand is: 5′-CCACGAAGAACAGAAGCACAAA-3′.
Cell culture
MGC-803 cells were cultured routinely using DMEM containing 10% fetal bovine serum (FBS) in an incubator with 5% CO2 at 37°C. When cells were in the logarithmic growth phase, they were digested by trypsin, prepared into single-cell suspension and inoculated into a culture dish at an appropriate density. Cells in this experiment were divided into 3 groups: Blank control (no transfection), negative control (transfected with siNC) and experimental group (transfected with siPLK1).
Detection of cell proliferation inhibition rate
After transfection, cells were inoculated into a 96-well plate at a density of 1×105/ml (100 µl per well). After 48 h, 10 µl MTT (5 mg/ml) was added into each well and cells were cultured for another 4 h. The optical density (OD) value of each well at a wavelength of 570 nm was detected using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The cell proliferation inhibition rate was calculated as follows: Inhibition rate (%) = (OD valueblank control group - OD valueexperimental group/OD valueblank control group) ×100%.
Detection of PLK1 and E-cadherin mRNA expression via RT-qPCR
After transfection, cells were inoculated into a 6-well plate (104/well), the supernatant was discarded after 48 h, and cells in each group were collected. The total RNA was extracted from tissues according to the instructions of RNA extraction kit, and the concentration and purity of total RNA were determined using an ultraviolet-visible spectrophotometer (Hitachi, Ltd., Tokyo, Japan) (qualified if A260/A280>1.8). The complementary deoxyribonucleic acid (cDNA) was obtained via reverse transcription according to the instructions of reverse transcription kit, and then the cDNA was used as a template to detect the mRNA expression of PLK1 and E-cadherin according to the instructions of RT-PCR kit. The primer sequences are shown in Table I, and the reaction conditions are as follows: 95°C for 10 min, 95°C for 15 sec, 60°C for 1 min, and amplification for 40 cycles. The cycle threshold (Cq) value was the output from the instrument software, and the relative expression level was calculated using 2−ΔΔCq method according to the following formula: ΔΔCq (target gene) = Cq (target gene) - Cq (control gene) (10).
Table I.
RT-PCR primer sequences.
| Genes | Primer sequence |
|---|---|
| E-cadherin | F: 5′-GCTTGGAATGAGACTGCTGA-3′ |
| R: 5′-CTGGCCATATCCACCAGAGT-3′ | |
| PLK1 | F: 5′-CGAGGGTGATGAGAACCTGC-3′ |
| R: 5′-CCCATGTGATTCGATGCGT-3′ | |
| GAPDH | F: 5′-CAAGGTCATCCATGACAACTTTG-3′ |
| R: 5′-GTCCACCACCCTGTTGCTGTAG-3′ |
F, forward; R, reverse.
Detection of protein expression of PLK1 and E-cadherin via western blot analysis
After transfection, cells were inoculated into the 6-well plate (104/well), and the supernatant was discarded after 48 h. Then cells in each group were collected, lysed with the cell lysis solution, and centrifuged at 8,000 × g at 4°C for 15 min, and the supernatant was collected. The protein concentration was determined using the BCA kit, followed by degeneration, separation via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and membrane transfer using the wet method. Then the membrane was sealed via bull serum albumin (BSA) for 2 h, and Rabbit anti-human GAPDH, PLK1 and E-cadherin polyclonal antibodies (cat. nos. 10494-1-AP, 10305-1-AP and 20874-1-AP; 1:1,000; Wuhan Sanying Biotechnology) was added for incubation at 4°C overnight. After the membrane was washed, goat anti-rabbit polyclonal secondary antibody (cat. no. SA00001-2; 1:2,000; Wuhan Sanying Biotechnology) was added for incubation at room temperature for 2 h. After the membrane was washed again, the image was developed in a darkroom via enhanced chemiluminescence (ECL), scanned and recorded using a gel imager (Bio-Rad Laboratories, Richmond, CA, USA). The gray scale was analyzed and compared with GADPH as internal reference.
Detection of migration capacity of human gastric cancer MGC-803 cells via wound healing assay
After transfection, cells were inoculated into the 6-well plate (104/well). After about 80% cells were fused, a 100 µl spearhead was used to scratch at the marker line. The loose cells were gently rinsed off with phosphate buffered saline (PBS), and the remaining cells continued to be cultured using FBS-free DMEM culture solution. After 48 h, the distance between scratches were observed and photographed.
Detection of invasion capacity of human gastric cancer MGC-803 cells via Transwell assay
The upper Transwell chamber was evenly added with 100 µl single-cell suspension in a concentration of 4×105/ml and 100 µl serum-free culture solution, while the lower chamber was added with 500 µl FBS-free culture solution. Cells were treated according to the above experimental method. After 48 h, cells were fixed with 4% paraformaldehyde, stained via crystal violet for 15 min, photographed and analyzed.
Statistical analysis
Data were presented as mean ± standard deviation (SD) and Statistical Product and Service Solutions (SPSS) 17.0 software (SPSS, Inc., Chicago, IL, USA) was used for data processing. One-way analysis of variance was used for the statistical analysis of data obtained and the post hoc test was Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of siPLK1 on MGC-803 cell proliferation
Results of MTT assay showed that at 48 h after transfection into MGC-803 cells, the cell inhibition rate in PLK1 siRNA group was significantly increased compared with that in blank control group, and the difference was statistically significant (p<0.01) (Table II).
Table II.
Inhibitory effect of transfection with PLK1 siRNA for 48 h on MGC-803 cell proliferation (mean ± SD).
| Proliferation inhibition rate (%) | |||
|---|---|---|---|
| Groups | 24 h | 48 h | 72 h |
| Blank control | 0 | 0 | 0 |
| Negative control | 0 | 0 | 0 |
| PLK1 siRNA | 11.3±0.32a | 23.32±2.13a | 37.33±2.06a |
Compared with control group
p<0.01. PLK1, polo-like kinase; siRNA, small-interfering ribonucleic acid.
Effects of siPLK1 transfection on mRNA expression of PLK1 and E-cadherin
Results showed that compared with those in blank control group, PLK1 siRNA could significantly decrease the mRNA expression of PLK1 (p<0.01), but significantly upregulate the mRNA expression of E-cadherin (p<0.01) at 48 h after transfection into MGC-803 cells, and the differences were statistically significant (Fig. 1).
Figure 1.
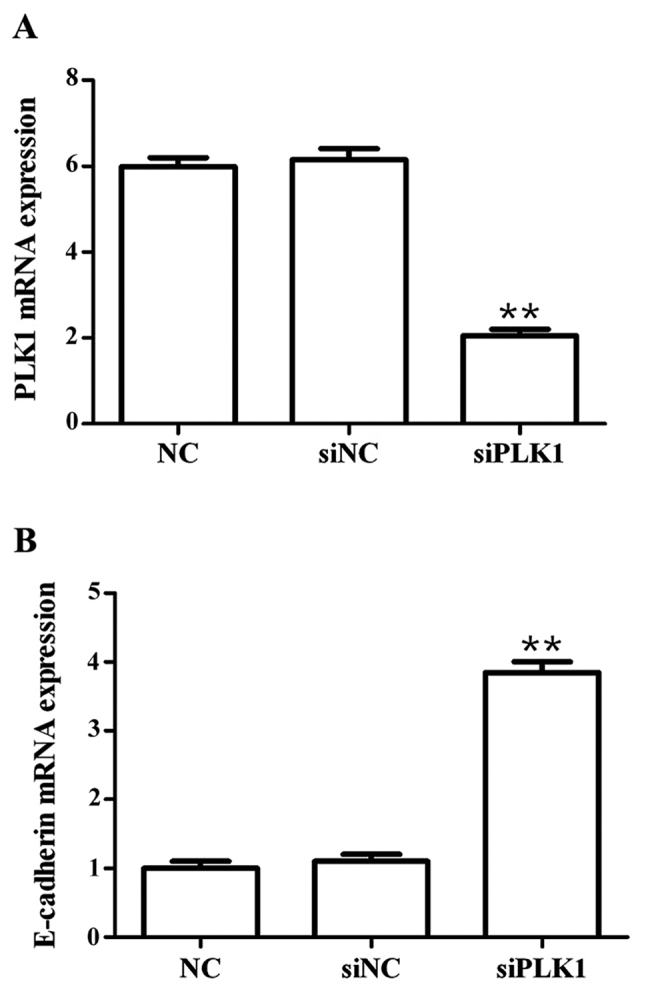
Detection of effects of siPLK1 transfection on mRNA expression of PLK1 and E-cadherin via RT-PCR. (A) PLK1 mRNA expression level. (B) E-cadherin mRNA expression level. Compared with those in blank control group, the PLK1 mRNA expression level is significantly inhibited, but the E-cadherin mRNA expression level is significantly upregulated **p<0.01. PLK1, polo-like kinase.
Effects of siPLK1 transfection on protein expression of PLK1 and E-cadherin
Results showed that compared with those in control group, PLK1 siRNA could obviously decrease the protein expression of PLK1 (p<0.01), but obviously upregulate the protein expression of E-cadherin (p<0.01) at 48 h after transfection into MGC-803 cells, and the differences were statistically significant (Fig. 2).
Figure 2.
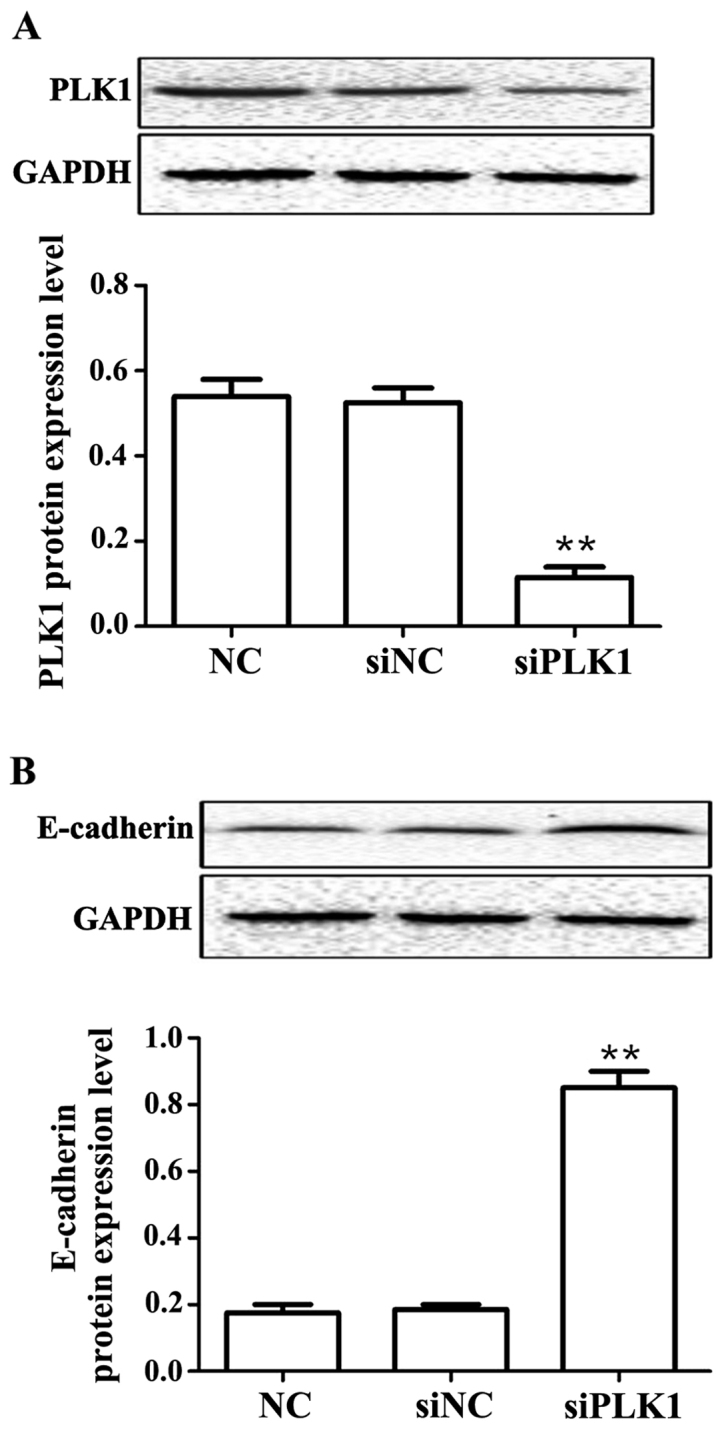
Detection of effects of siPLK1 transfection on protein expression of PLK1 and E-cadherin via western blot analysis. (A) PLK1 protein expression level. (B) E-cadherin protein expression level. Compared with those in blank control group, the PLK1 protein expression level is significantly inhibited, but the E-cadherin protein expression level is significantly upregulated. **p<0.01. PLK1, polo-like kinase.
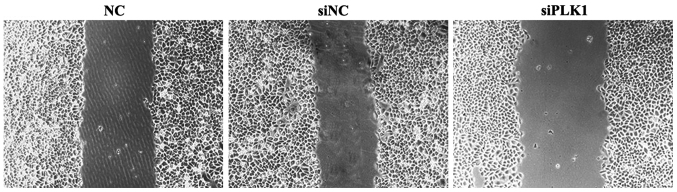
Effect of siPLK1 transfection on migration capacity of MGC-803 cells
Results of wound healing assay showed that compared with those in blank control and negative control groups, the space between wounds in experimental group was wider, the healing was obviously inhibited, and the cell migration capacitywas weakened. The difference in the wound space was not significant between blank control and negative control groups (Fig. 3).
Figure 3.
Detection of effect of siPLK1 on migration capacity of MGC-803 cells via wound healing assay. PLK1, polo-like kinase.
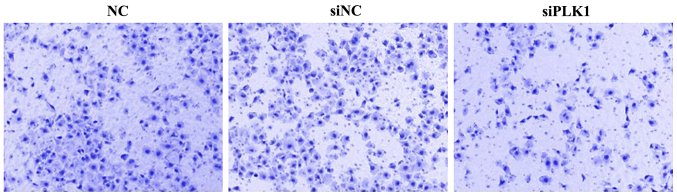
Effect of siPLK1 transfection on invasion capacity of MGC-803 cells
At 48 h after transfection into MGC-803 cells, cells were inoculated into the upper Transwell chamber for culture for 48 h. The number of MGC-803 cells passing through Matrigel and reaching the filter membrane in PLK1 siRNA group was significantly smaller than those in blank control and negative control groups, indicating that PLK1 siRNA can inhibit the invasion of MGC-803 cells. The difference was not significant between blank control and negative control groups (Fig. 4).
Figure 4.
Detection of effect of siPLK1 transfection on cell invasion capacity via Transwell assay. PLK1, polo-like kinase.
Discussion
Gastric cancer is a common malignant tumor of digestive tract, and its incidence rate is increasing year by year, seriously affecting human health (11). At present, clinical treatment means have not only side effects, but also slow progression, and the mechanisms of the occurrence and development of gastric cancer are not clear. Therefore, exploring new mechanisms is of great significance to find a new breakthrough for gastric cancer treatment.
EMT is a key link in the metastasis of tumor cells, involving the activation of multiple factors and different signaling pathways (12). The adhesion between epithelial cells with EMT is significantly decreased, but the cell motility is significantly enhanced, thus cells further migrate. During the occurrence of EMT in cells, relevant markers are also changed, such as the deletion of epithelial phenotype marker E-cadherin. It has been reported that the typical sign of EMT is the deletion of E-cadherin expression, and this process involves another important factor, namely the zinc-finger transcriptional factor Snail that plays a key role in the EMT process. It is found that Snail can promote the mesenchymal transition of a variety of tumor cells, and the invasion and metastasis of tumor cells (13,14). Zinc-finger transcriptional factor directly binds to the promoter of E-cadherin, thus leading to the downregulation of E-cadherin protein expression, further reducing the adhesion between cells and destroying the physiological tissue structure, thereby promoting the invasion and metastasis of tumor cells (15). In addition, PLK1, as a kinase, is a key factor in mitosis, which is closely associated with multiple processes of mitosis, such as formation of spindle body, centrosome replication and chromosome segregation (16). A large number of research reports (17–19) have shown that the PLK1 expression is increased in various solid tumor tissues (20), such as esophageal cancer, melanoma, breast cancer, colon cancer and renal cell carcinoma, suggesting that there is a close relationship between PLK1 and tumor occurrence. Smith et al transfected the exogenously-recombinant PLK1 genes into normal fibroblasts, and results showed that malignant transformation of cells could be induced, and the tumor could be implanted into nude mice successfully, indicating that the PLK1 gene can directly cause malignant transformation of cells (21). In addition, recent reports have indicated that the high expression of PLK1 gene in liver and pancreatic cancers occurs mainly in the early stage of tumor cell progression (22).
In this experiment, siRNA with targeted interference in PLK1 gene was designed and transfected into gastric cancer MGC-803 cells via Lipofectamine (Thermo Fisher Scientific, Inc., Waltham, MA, USA) to inhibit the expression of PLK1 gene in MGC-803 cells. Results of MTT assay showed that compared with that in control group, the cell proliferation in PLK1 siRNA group was significantly inhibited. Results of real-time PCR and western blot analysis showed that compared with those in control group, the mRNA and protein expression of PLK1 in PLK1 siRNA group were significantly decreased, but the mRNA and protein expression of E-cadherin was obviously upregulated. Results of wound healing assay and invasion assay showed that the migration and invasion of MGC-803 cells in PLK1 siRNA group were significantly inhibited compared with those in control group. It is reported (23) that epithelial cancerous cells will have stronger migration and invasion capacities after EMT. In addition, it has been reported (24) that the upregulation of PLK1 expression will lead to the accumulation of oncogenes in tumor cells and formation of tumors with migration and invasion capacities in nude mice successfully. Clinically, Tokumitsu et al (25) studied the PLK1 mRNA expression level in patients with gastric cancer, and results showed that PLK1 mRNA was highly expressed in 73% of patients, and the difference was significant compared with that in normal and atypical hyperplastic tissues. Besides, the expression level of PLK1 was positively correlated with the clinical staging and depth of tumor infiltration. All of these reports indicate that PLK1 plays an extremely important role in the occurrence and development of human tumors. Therefore, exploring the mechanism of action of PLK1 in tumors will provide a new direction for further treatment of tumors.
In conclusion, this study proved that PLK1 can enhance the proliferation, migration and invasion of gastric cancer MGC-803 cells through affecting EMT.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
RS and GH designed the study, JUY and JIY collected and analysed the data, CW helped with Transwell assay. TC and ZL were responsible for PCR and western blot analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The Second Affiliated Hospital of Zhengzhou University (Zhengzhou, China) and written informed consents were signed by the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Ryu MH, Kang YK. ML17032 trial: Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in advanced gastric cancer. Expert Rev Anticancer Ther. 2009;9:1745–1751. doi: 10.1586/era.09.149. [DOI] [PubMed] [Google Scholar]
- 4.De Vita F, Vecchione L, Galizia G, Di Martino N, Fabozzi T, Catalano G, Ciardiello F, Orditura M. Perspectives in adjuvant therapy of gastric cancer. Oncology. 2009;77(Suppl 1):38–42. doi: 10.1159/000258494. [DOI] [PubMed] [Google Scholar]
- 5.Mlkvý P. Multimodal therapy of gastric cancer. Dig Dis. 2010;28:615–618. doi: 10.1159/000320063. [DOI] [PubMed] [Google Scholar]
- 6.Mello BS, Lucena AF, Echer IC, Luzia MF. Patients with gastric cancer submitted to gastrectomy: An integrative review. Rev Gaúcha Enferm. 2010;31:803–811. doi: 10.1590/S1983-14472010000400026. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 7.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. ToGA Trial Investigators: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Cárcer G, Escobar B, Higuero AM, García L, Ansón A, Pérez G, Mollejo M, Manning G, Meléndez B, Abad-Rodríguez J, et al. Plk5, a polo box domain-only protein with specific roles in neuron differentiation and glioblastoma suppression. Mol Cell Biol. 2011;31:1225–1239. doi: 10.1128/MCB.00607-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livak and Schmittgen: Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCq method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450–464. doi: 10.1053/j.seminoncol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 13.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 15.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 16.Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, Fukuzawa M, Nakagawara A. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem. 2004;279:25549–25561. doi: 10.1074/jbc.M314182200. [DOI] [PubMed] [Google Scholar]
- 17.Steinhauser I, Langer K, Strebhardt K, Spänkuch B. Uptake of plasmid-loaded nanoparticles in breast cancer cells and effect on Plk1 expression. J Drug Target. 2009;17:627–637. doi: 10.1080/10611860903118823. [DOI] [PubMed] [Google Scholar]
- 18.Kim SA, Kwon SM, Yoon JH, Ahn SG. The antitumor effect of PLK1 and HSF1 double knockdown on human oral carcinoma cells. Int J Oncol. 2010;36:867–872. doi: 10.3892/ijo_00000564. [DOI] [PubMed] [Google Scholar]
- 19.Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24:287–291. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Zhang Z, Liu Z. Polo-like kinase 1 is overexpressed in renal cancer and participates in the proliferation and invasion of renal cancer cells. Tumour Biol. 2013;34:1887–1894. doi: 10.1007/s13277-013-0732-0. [DOI] [PubMed] [Google Scholar]
- 21.Smith MR, Wilson ML, Hamanaka R, Chase D, Kung H, Longo DL, Ferris DK. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem Biophys Res Commun. 1997;234:397–405. doi: 10.1006/bbrc.1997.6633. [DOI] [PubMed] [Google Scholar]
- 22.Petrelli A, Perra A, Schernhuber K, Cargnelutti M, Salvi A, Migliore C, Ghiso E, Benetti A, Barlati S, Ledda-Columbano GM, et al. Sequential analysis of multistage hepatocarcinogenesis reveals that miR-100 and PLK1 dysregulation is an early event maintained along tumor progression. Oncogene. 2012;31:4517–4526. doi: 10.1038/onc.2011.631. [DOI] [PubMed] [Google Scholar]
- 23.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: Mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage check point. Nat Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- 25.Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol. 1999;15:687–692. doi: 10.3892/ijo.15.4.687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.