Avolitional tics in Tourette syndrome may be worsened by stress and triggered by social cues such as the facial expressions of others. Rae et al. show that during viewing of emotional faces, the insula is hyperactive in Tourette syndrome and displays increased functional connectivity with cortical and subcortical motor regions.
Keywords: basal ganglia, movement disorders, neuropsychiatry, tic disorder, Tourette syndrome
Abstract
Tourette syndrome is a neurodevelopmental disorder, characterized by motor and phonic tics. Tics are typically experienced as avolitional, compulsive, and associated with premonitory urges. They are exacerbated by stress and can be triggered by external stimuli, including social cues like the actions and facial expressions of others. Importantly, emotional social stimuli, with angry facial stimuli potentially the most potent social threat cue, also trigger behavioural reactions in healthy individuals, suggesting that such mechanisms may be particularly sensitive in people with Tourette syndrome. Twenty-one participants with Tourette syndrome and 21 healthy controls underwent functional MRI while viewing faces wearing either neutral or angry expressions to quantify group differences in neural activity associated with processing social information. Simultaneous video recordings of participants during neuroimaging enabled us to model confounding effects of tics on task-related responses to the processing of faces. In both Tourette syndrome and control participants, face stimuli evoked enhanced activation within canonical face perception regions, including the occipital face area and fusiform face area. However, the Tourette syndrome group showed additional responses within the anterior insula to both neutral and angry faces. Functional connectivity during face viewing was then examined in a series of psychophysiological interactions. In participants with Tourette syndrome, the insula showed functional connectivity with a set of cortical regions previously implicated in tic generation: the presupplementary motor area, premotor cortex, primary motor cortex, and the putamen. Furthermore, insula functional connectivity with the globus pallidus and thalamus varied in proportion to tic severity, while supplementary motor area connectivity varied in proportion to premonitory sensations, with insula connectivity to these regions increasing to a greater extent in patients with worse symptom severity. In addition, the occipital face area showed increased functional connectivity in Tourette syndrome participants with posterior cortical regions, including primary somatosensory cortex, and occipital face area connectivity with primary somatosensory and primary motor cortices varied in proportion to tic severity. There were no significant psychophysiological interactions in controls. These findings highlight a potential mechanism in Tourette syndrome through which heightened representation within insular cortex of embodied affective social information may impact the reactivity of subcortical motor pathways, supporting programmed motor actions that are causally implicated in tic generation. Medicinal and psychological therapies that focus on reducing insular hyper-reactivity to social stimuli may have potential benefit for tic reduction in people with Tourette syndrome.
Introduction
Tourette syndrome is a hyperkinetic neurodevelopmental disorder characterized by motor and phonic tics. Tics may be simple or complex. They are experienced as unwanted and compulsive (Cavanna and Nani, 2013), and are frequently preceded by premonitory sensations or urges (Cavanna et al., 2017). While the structural, functional and neurochemical alterations underpinning tics and their accompanying premonitory sensations are yet to be precisely understood, dysfunction within cortico-striato-thalamo-cortical pathways plays a central role (Draganski et al., 2010; Buse et al., 2013; Ganos et al., 2013; Worbe et al., 2013; Jackson et al., 2015).
A striking feature of tic expression in Tourette syndrome is its marked sensitivity to environmental context. For example, specific tics may be induced by the presence of a particular person or object (Eapen et al., 1994). They may also mirror the actions or speech of others within the environment, phenomena known as echopraxia and echolalia, respectively (Ganos et al., 2012). Indeed, echophenomena increase in frequency further when the observed actions or speech form part of a patient’s own tic repertoire (Finis et al., 2012). Emotional states, particularly stress and anxiety, can also increase tic severity (Conelea and Woods, 2008; Godar and Bortolato, 2017). This effect is linked to states of autonomic arousal, which itself may enhance the expression of tics (Hawksley et al., 2015). In addition, focusing attention on tics tends to increase their frequency, while diverting attention away to other tasks or stimuli decreases tic expression (Brandt et al., 2015; Misirlisoy et al., 2015), effects that may well be mediated in part by autonomic arousal. Environmental and physiological factors therefore appear to influence not just tic frequency, but also which particular motor or phonic tic action is performed. These triggering factors have an important impact on everyday functioning and quality of life: the presence of others can exacerbate an individual’s symptoms, while the enhanced ‘public’ visibility carries a negative psychosocial impact through stigma, exclusion and social anxiety (Wadman et al., 2013; Eapen et al., 2016).
Despite the long-recognized ability of environmental cues or autonomic signals to trigger tics and increase their severity, the neural circuitry mediating this phenomenon is yet to be established. Perceptual inputs and their cortical representation likely act as antecedents that facilitate activity within subcortical motor pathways, in line with cortico-striato-thalamo-cortical models of motor control (Ganos et al., 2013; Neuner et al., 2013). Theoretical and empirical data suggest the insula cortex as a likely substrate for premonitory urges, which can trigger tics as mitigating actions through functional pathways to basal ganglia and midline motor regions, notably the supplementary motor area (Jackson et al., 2011; Conceicao et al., 2017; Rae et al., 2018). From a theoretical perspective, mappings from posterior to anterior insula are proposed to provide an interoceptive representation of embodied salience. Furthermore, insula grey matter thickness, and strength of resting state functional connectivity between the insula and supplementary motor area, are associated with the severity of premonitory urges in people with Tourette syndrome (Tinaz et al., 2015; Draper et al., 2016). However, these sites are yet to be confirmed empirically as the key regions driving the neural processes by which external contextual triggers might effect a worsening of tic severity.
The facial identities and expressions of other people represent potent cues that rapidly signal contextual social and emotional information. Face processing feeds in to drive motivated behaviours and actions, including responses to threat (Parkinson et al., 2017). Face stimuli thus permit the investigation of how contextual cues trigger tics. Face stimuli are processed in well characterized pathways incorporating the occipital face area (OFA), fusiform face area (FFA), and amygdala (Haxby et al., 2002; Ishai, 2008; Pitcher et al., 2011). To date, one previous neuroimaging study examined activity during face viewing in people with Tourette syndrome. This study noted amygdala hyperactivity in response to faces wearing both neutral and emotional expressions (Neuner et al., 2010). However, amygdala hyperactivity did not predict severity of motor symptoms, recorded using the Yale Global Tic Severity Scale (YGTSS). This finding suggests that there may be other aspects of neural circuitry that contribute to tic severity, particularly in relation to tic triggers such as emotional social cues. Theoretical and empirical evidence on the contribution of the insula to premonitory sensations, and motor regions to tic expression, predict that these regions will show altered reactivity and connectivity under viewing of emotional social stimuli in people with Tourette syndrome.
Here, we used functional MRI in combination with an emotional face perception task to ascertain the mechanisms in neural activation and functional connectivity by which emotional social cues may trigger tics, and how they relate to symptom experience. We presented neutral and angry faces, with social anger stimuli selected since they pose the potentially most potent social threat cue of the core human expressions, and applied psychophysiological interaction analyses to examine how functional connectivity varies depending on psychological context (Friston et al., 1997).
Materials and methods
Participants
Twenty-one participants with Tourette syndrome (13 male; age 18–51 years, mean 33) and 21 controls with no history of any major neurological or psychiatric disorder (11 male; age 19–55 years, mean 34) participated. Diagnosis of Tourette syndrome was made by a UK neurologist or psychiatrist experienced in assessment of Tourette syndrome in a suitable specialist clinic, including participants recruited from the UK National Health Service Sussex Partnership Trust Neurobehavioural clinic (run by H.C. and N.H.), and participants recruited via Tourettes Action UK (who specified details of their clinical assessment prior to inclusion in the study). Diagnosis of obsessive compulsive disorder (OCD) and attention deficit hyperactivity disorder (ADHD) from a specialist clinician was also recorded. Patient exclusion criteria were: (i) co-occurring psychopathology (current depression, substance abuse, current or previous history of psychosis); and (ii) contraindications to MRI.
Severity of tics, premonitory sensations, OCD and ADHD were assessed using the YGTSS (including symptom severity: maximum 50, and impairment: maximum 50, global total: 100) (Leckman et al., 1989), Premonitory Urge for Tics Scale (PUTS, Woods et al., 2005), Yale Brown Obsessive Compulsive Scale (YBOCS, Goodman et al., 1989) and Adult ADHD Self-Report Scale (ASRS, Kessler et al., 2005).
One patient was taking dopaminergic medication (pimozide), five were taking serotonergic medication (serotonin reuptake inhibitors), and one patient was taking both dopaminergic and serotonergic medications (pimozide and a serotonin reuptake inhibitor). One patient on sertraline was also taking a benzodiazepine (lorazepam). One patient took melatonin as a sleep aid. The remaining 13 were unmedicated.
Table 1 reports demographic details of participants and summary clinical features of patients (see Supplementary Table 1 for individual patient data). All participants gave written informed consent, and the study was approved by the National Research Ethics Service South East Coast Brighton Research Ethics Committee.
Table 1.
Demographic details of participants and clinical features of patients
| Features/measures | Tourette syndrome (n = 21) | Control (n = 21) | Group difference |
|---|---|---|---|
| Number of males/females | 13/8 | 11/10 | 0.756a |
| Age | 33 (10) | 34 (12) | 0.461b |
| Years of education | 15 (2) | 14 (2) | 0.589b |
| OCD, n | 10 | 0 | - |
| ADHD. n | 6 | 0 | - |
| YGTSS: symptom severity | 27 (8) | - | - |
| YGTSS: impairment | 20 (12) | - | - |
| YGTSS: total (symptom severity and impairment) | 46 (17) | - | - |
| PUTS | 24 (6) | - | - |
| YBOCS | 16 (9) | 6 (6) | <0.001b |
| ASRS | 4 (2) | 1 (1) | <0.001b |
Data are presented as means (SD). Group difference P-values refer to achi-squared or btwo-tailed t-tests. ASRS = Adult ADHD Self-Report Scale; YBOCS = Yale-Brown Obsessive Compulsive Scale.
Face perception task
Participants underwent functional MRI during a face perception task in which male and female faces were presented wearing neutral and angry expressions (Fig. 1). Three male and three female faces from the NIMSTIM database (Tottenham et al., 2009) were presented, with each individual face shown on 20 trials, 10 with neutral and 10 with angry expression, in a randomized order (120 trials total). Hair and peripheral features were removed from the original NIMSTIM images by applying a greyscale circle to leave only the facial expression. Faces were presented on a greyscale background for 800 ms, before a response screen asked participants to indicate with an index or middle finger button press whether the face had been male or female. Participants were therefore not overtly instructed to focus on the expression of the face. However, by requiring a gender judgement we ensured that participants attended to the faces. The response period ended at the participant’s button press indicating a gender discrimination judgement. A white fixation cross on grey background was displayed during intertrial intervals, which were jittered in duration according to the OptSeq functional MRI design tool (http://surfer.nmr.mgh.harvard.edu/optseq) for event-related functional MRI design efficiency (35% 1000 ms, 30% 1130 ms, 20% 1250 ms, 10% 1380 ms, 5% 1500 ms).
Figure 1.
Face perception task. Neutral and angry faces were presented for 800 ms before participants were asked to indicate whether the face had been male or female. Face enlarged for illustrative purposes.
MRI acquisition
Functional MRI data were acquired on a Siemens Avanto 1.5 T with a 32 channel head coil (T2*-weighted echo planar images, repetition time = 2520 ms, echo time = 43 ms, 34 ascending oblique slices 3-mm thick with 0.6 mm slice gap, in-plane resolution 3 × 3 mm). Total number of functional MRI volumes acquired depended on participants’ response times (mean volumes acquired: 125). The first five volumes were discarded to allow for steady state magnetization. A T1 structural was acquired for registration (repetition time = 2730 ms, echo time = 3.57 ms, 1 × 1 × 1 mm resolution). Participants’ heads were tightly cushioned within the head coil to reduce large amplitude head movements.
Tic monitoring
We did not instruct participants to suppress tics during functional MRI, to reduce distress during data acquisition, and to mitigate contamination of task-related blood oxygen level-dependent (BOLD) signal with signal relating to intentional tic suppression. Instead, we undertook tic monitoring time-locked to the functional MRI data then included tic expression as a regressor in general linear modelling to remove BOLD signal relating to tic generation. Specifically, we acquired video using an in-bore MRI compatible camera (MRC Systems, www.mrc-systems.de), mounted on the head coil to view participants’ faces, and an out-of-bore camera to view their limbs and body. Both camera feeds and functional MRI volume markers were simultaneously relayed to Spike2 physiological recording software (version 7.17, Cambridge Electronic Design). Tics were identified during post hoc video assessment and an in-house Spike2 script was used to extract tic onsets and durations, time-locked to the functional MRI acquisition. Phonic tics were often visible in facial movement; however, while all motor tics were captured, it is possible that occasional phonic tics were not, since we did not additionally record auditory signs. During the functional MRI time-locked video recordings, the researcher (C.R.) watched the live video feeds from the control room and noted the functional MRI volume number at which she observed any tics, providing a written tic record alongside the video recordings. Video recordings failed for two participants; in these two cases, the written records were used to identify tic onsets and durations in relation to the functional MRI time series. In addition, head movement parameters were obtained for each participant from the realignment stage of preprocessing and inspected for any volume-to-volume translational displacements >3 mm (as an indicative value close to the voxel size). To reduce large amplitude head movements arising from tics, we tightly cushioned participants within the head coil. In the whole cohort, there were no movements >3 mm, with the exception of a single volume in one Tourette syndrome participant.
During the whole functional MRI acquisition, across participants an average of 36 tics occurred (ranging from 0 to 90 in individual participants, standard deviation: 30). Of the bodily locations at which tics were expressed, on average 38% involved facial movement, 14% the head (e.g. twist or nod), 7% both face and head, and 41% body or limbs.
Functional MRI preprocessing
Functional MRI data were preprocessed and analysed using SPM12 (v6906, www.fil.ion.ucl.ac.uk/spm). Preprocessing was applied with default options, including realignment to the mean image, slice-time correction to the middle slice, co-registration to the T1 structural, normalization to MNI space, and smoothing at 8 mm full-width at half-maximum (FWHM).
General linear modelling
Task events were modelled in a general linear model, with two regressors representing the onset and duration of presentation of neutral and angry faces, respectively. In addition, the general linear model of Tourette syndrome participants contained a further regressor, comprising the onsets and durations of tics identified in the functional MRI time-locked video recordings, to remove variance in BOLD signal relating to generation and expression of tics from task events. In all participants, regressors for the six movement parameters calculated during realignment modelled head movements. Single-regressor T-contrasts were generated for viewing (i) neutral; and (ii) angry faces, with an implicit baseline of the intertrial interval fixation cross. These were entered to a full factorial second-level analysis, with group (Tourette syndrome or control) as an independent (between-subjects) factor, and facial expression (neutral or angry) as a non-independent (repeated measures) factor. In addition, three covariates were entered for (i) medication (1/0 yes/no); (ii) ADHD diagnosis; and (iii) OCD diagnosis to control for any potential effects of medication or comorbidity. F-contrasts were generated testing for all effects (1 1 1 1), main effect of group [Tourette syndrome (TS), controls: (1 1 −1 −1)], and main effect of task [neutral, angry: (1 −1 1 −1)]. Group differences in viewing neutral or angry faces (control neutral > TS neutral; control angry > TS angry; TS neutral > control neutral; TS angry > control angry), and individual group effects for neutral and angry faces (control neutral, control angry, TS neutral, TS angry) were examined using T contrasts.
A series of four further second-level models in Tourette syndrome participants only examined the correlation of symptom severity with task effects. First-level contrasts for (i) neutral; and (ii) angry faces were entered to second-level one-way t-tests, with (i) total YGTSS; or (ii) PUTS scores, entered as a covariate, and a regressor generated for the interaction with task effect. Medication and comorbidities were also entered as covariates. T-contrasts tested for a positive interaction.
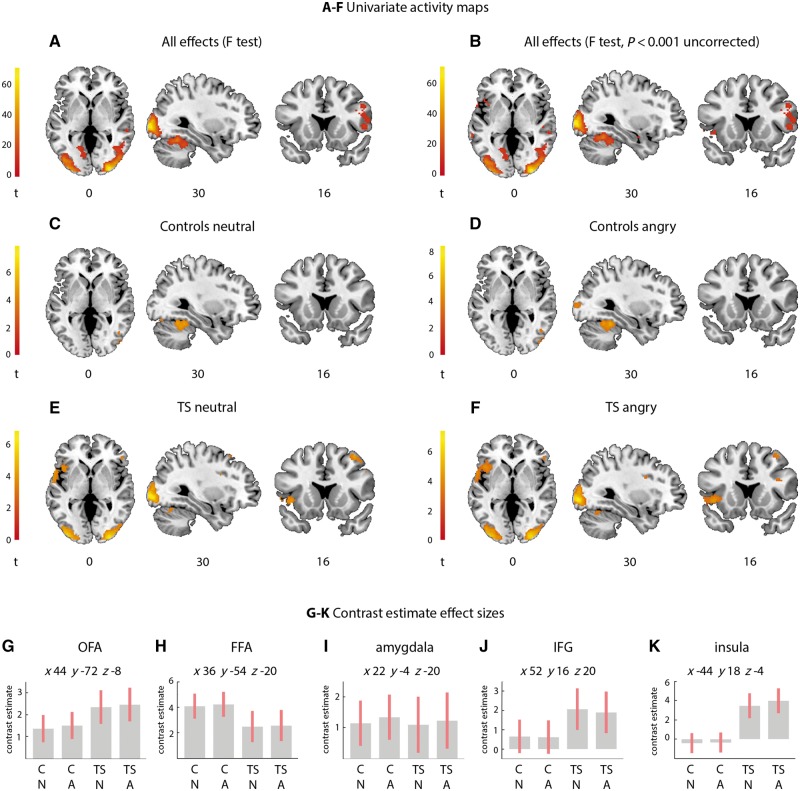
Statistic images were thresholded at a cluster-forming threshold of P < 0.001 for cluster-wise false discovery rate (FDR) correction for multiple comparisons at P < 0.05 (Chumbley et al., 2010; Eklund et al., 2016). Significant clusters were localized according to the Anatomy toolbox (v 2.2b, Eickhoff et al., 2007), and the FSL Harvard-Oxford cortical and subcortical atlases (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) where the Anatomy toolbox did not contain a relevant label. Contrast estimate effect size plots were generated in SPM for neutral and angry faces, in Tourette syndrome participants and in controls, at the peak coordinate of significant regions according to the F-contrast for all effects (Fig. 2).
Figure 2.
Activity during viewing neutral and angry faces in Tourette syndrome participants and controls. The insula is hyperactive in Tourette syndrome for both facial expressions. (A) F-test of all effects, (B) F-test of all effects thresholded at more liberal threshold of P < 0.001 and minimum cluster size of 10 voxels, additionally showing the amygdala and insula, (C) controls neutral, (D) controls angry, (E) Tourette syndrome neutral, (F) Tourette syndrome angry. All images thresholded at P < 0.05 cluster-wise FDR (cluster-forming threshold P < 0.001) unless specified. (G–K) Contrast estimate effect size plots (pink bar represents 90% confidence interval) for the OFA (G), FFA (H), amygdala (I), IFG (J), and insula (K), respectively, for (left-to-right) controls neutral (CN), controls angry (CA), Tourette syndrome neutral (TS N), Tourette syndrome angry (TS A), plotted at co-ordinates given in G-K. Unthresholded statistic images are available at https://neurovault.org/collections/4167/.
Psychophysiological interactions
Functional connectivity can vary between two regions depending on the psychological context (Friston et al., 1997). To investigate how reactivity to faces within early face processing areas and the insula modulated activity elsewhere in the brain, we performed a series of psychophysiological interaction analyses. First, we examined whether reactivity within the OFA, which showed activity in response to both face types in both groups (Fig. 2C–F), was associated with changes in functional connectivity to regions elsewhere. Second, we examined whether activity in the anterior insula was associated with changes in functional connectivity to elsewhere. This region showed above criterion threshold activity in response to presentation of neutral and angry faces in people with Tourette syndrome, but not controls (Fig. 2C–F). Finally, in participants with Tourette syndrome, we examined whether the strength of functional connectivity of either OFA or insula was related to tic severity, according to YGTSS scores (maximum: 100, comprising symptom severity and impairment scores; Table 1), and premonitory sensation severity, according to PUTS scores.
We extracted the first eigenvariate (weighted mean of BOLD time series), for (i) the OFA; and (ii) the insula, thresholding the contrast representing viewing of angry faces (which showed the greatest insula response in Tourette syndrome participants at the second-level) at P < 1 for each individual. A 10 mm sphere was extracted at the group peak from the second-level univariate F-contrast for all effects for the OFA (x 44, y −72, z −8), and insula (x −44, y 18, z −4), adjusting for effects of interest according to a subject-specific F-contrast (‘eye’) of all effects (neutral faces, angry faces, and additionally for Tourette syndrome participants, tics).
Next, the psychophysiological interaction term was calculated according to the main effect of task (contrast weights: 1 for neutral, and 1 for angry) and the time series of (i) the OFA; and (ii) the insula. The psychophysiological interaction term for (i) the OFA; and (ii) the insula were entered respectively to a first-level model in all participants, alongside a regressor representing the BOLD activity from the region of interest (PPI.Y) and the main effect of task (PPI.P). In addition, six regressors modelled head movement, and for Tourette syndrome participants, a further regressor comprised the onsets and durations of tics identified in the functional MRI time-locked video recordings. Single regressor T-contrasts were generated for the psychophysiological interaction term. These were entered to a series of second-level models. The first two examined the psychophysiological interaction for the OFA, and the insula, in controls, and Tourette syndrome participants (using a two-sample t-test design). Then, in Tourette syndrome participants only (using a one-sample t-test design), two second-level models (1: OFA, 2: insula) included YGTSS scores as a covariate, creating an interaction between the YGTSS scores and psychophysiological interaction term. Finally, two second-level models in Tourette syndrome participants (1: OFA, 2: insula) included PUTS scores as a covariate, creating an interaction between PUTS and the psychophysiological interaction term. In all second-level models, as with the univariate functional MRI analysis, (i) medication (1/0 yes/no); (ii) ADHD diagnosis; and (iii) OCD diagnosis were entered as covariates.
In the two-sample models, an F-contrast on the psychophysiological interaction term examined the group effect [TS, control (1 −1)], and T-contrasts the direction of effect and individual group effects. In the tic and premonitory sensation severity models, T-contrasts tested for correlation with the YGTSS or PUTS interaction covariates. Contrasts were thresholded at a cluster-forming threshold of P < 0.001 for cluster-wise FDR at P < 0.05. Significant clusters were localized according to the SPM Anatomy (v 2.2b, Eickhoff et al., 2007) and FSL Harvard-Oxford cortical and subcortical atlases (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). Plots of the YGTSS and PUTS correlations with the insula psychophysiological interaction in Tourette syndrome participants were generated in SPM for the globus pallidus, thalamus, and supplementary motor area, at each region’s peak coordinates in the psychophysiological interaction contrast, using the adjusted data.
Data availability
The data that support the findings of this study (unthresholded statistic images for every contrast reported) are openly available in Neurovault, at https://neurovault.org/collections/4167/, reference number 4167 (Gorgolewski et al., 2015).
Results
Face perception task: behavioural performance
Control subjects and Tourette syndrome participants showed equivalent reaction times when making face gender ratings [mean control 473 ms, mean Tourette syndrome 488 ms, t(40,2) = −0.299, P = 0.766], and did not significantly differ in face gender rating accuracy [mean control 90%, mean Tourette syndrome 91%, t(40,2) = −0.525, P = 0.603] confirming equivalent task difficulty across groups.
Univariate functional MRI and contrast estimate effect sizes
The F-contrast for all effects (1 1 1 1) showed that neutral and angry faces elicited activity across participants in a canonical set of face perception regions, including the OFA, FFA, and inferior frontal gyrus (IFG) (Fig. 2A and Supplementary Table 2). Thresholded at a more liberal uncorrected threshold of P < 0.001 uncorrected (cluster extent ≥ 10 contiguous voxels), this set extended to include the right amygdala and left anterior insula (Fig. 2B and Supplementary Table 2).
There was a significant main effect of group (F-contrast; Tourette syndrome, controls) in the superior frontal gyrus (Supplementary Table 2). Post hoc T-contrasts confirmed participants with Tourette syndrome showed greater activity in the superior frontal gyrus when viewing neutral faces relative to controls (Supplementary Fig. 1A and Supplementary Table 2). All other T-contrasts for a group effect were not significant.
There was no significant main effect of task (F-contrast; neutral, angry). Examining individual group effects for neutral and angry faces showed that both face types evoked occipital and temporal lobe activity (within the OFA and FFA) in controls and Tourette syndrome participants (Fig. 2C–F and Supplementary Table 2). Furthermore, in Tourette syndrome participants only, right IFG and left insula were active during viewing of both neutral and angry faces (Fig. 2E and F).
Contrast estimate effect size plots, representing activity at the peak co-ordinate of a region identified according to the F-contrast for all effects, demonstrated similar levels of activity in Tourette syndrome participants and controls for viewing neutral and angry faces in the OFA, FFA, and amygdala (Fig. 2G–I). However, Tourette syndrome participants showed slightly elevated activity in IFG (Fig. 2J) and a hyperactive insula in response to viewing both neutral and angry faces, relative to control participants (Fig. 2K), although this did not attain stringent significance in the formal whole-brain contrasts.
In the series of second-level models in Tourette syndrome participants only examining correlations between task effects (1: neutral, 2: angry) and symptom severity (1: total YGTSS, 2: PUTS), none showed significant correlations (P < 0.05 cluster-wise FDR).
Psychophysiological interactions
OFA and insula
Two second-level models examined changes in functional connectivity with (i) the OFA; and (ii) the insula, depending on the psychological context of viewing neutral and angry faces, in Tourette syndrome participants and controls.
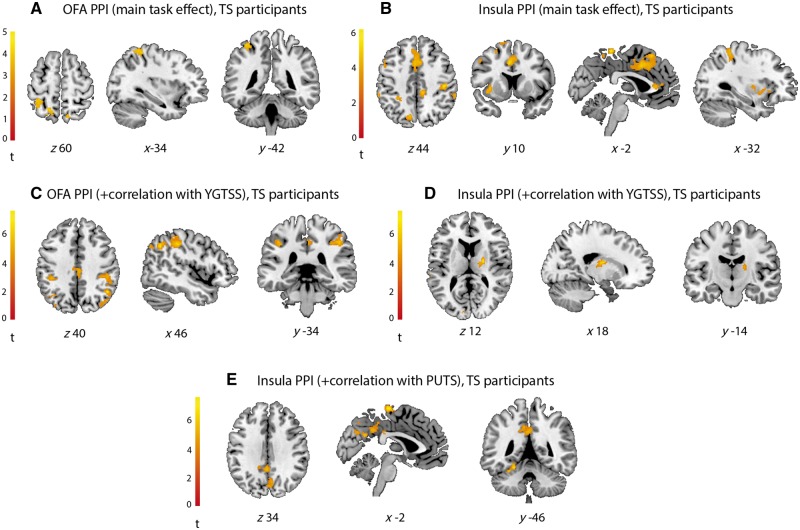
In the OFA psychophysiological interaction, there was no significant effect of group (F-contrast; Tourette syndrome, controls). The contrast testing for individual group effects in control participants was not significant; however, Tourette syndrome participants showed a psychophysiological interaction between the OFA and the primary somatosensory cortex [Brodmann area (BA) 2], the intraparietal sulcus, and the superior parietal lobule (BA 7) (Fig. 3A and Supplementary Table 3).
Figure 3.
Psychophysiological interaction results in Tourette syndrome participants, demonstrating changes in functional connectivity when viewing faces. (A) OFA, (B) insula, (C) OFA, in relation to tic severity (YGTSS), (D) insula, in relation to YGTSS, (E) insula, in relation to premonitory sensations (PUTS). Unthresholded statistic images are available at https://neurovault.org/collections/4167/. PPI = psychophysiological interaction; TS = Tourette syndrome.
In the insula psychophysiological interaction, there was a significant effect of group (F-contrast; Tourette syndrome, controls) in the temporo-parietal junction (Supplementary Table 3). Post hoc T-contrasts confirmed Tourette syndrome participants showed a greater psychophysiological interaction between the insula and temporo-parietal junction relative to controls (Supplementary Fig. 1B and Supplementary Table 3). The contrast testing for individual group effects in control participants was not significant; however, Tourette syndrome participants showed a psychophysiological interaction between the anterior insula and a set of cortical and subcortical regions, including presupplementary motor area, anterior cingulate cortex, mid-cingulate cortex, middle frontal gyrus, premotor cortex, M1, S1 and S2, mid-insula, temporo-parietal junction, precuneus, the putamen, and cerebellum (Fig. 3B and Supplementary Table 3).
Tic severity (YGTSS)
In Tourette syndrome participants only, two psychophysiological interaction analyses examined whether regional connectivity when viewing faces with (i) the OFA; and (ii) the insula varied in relation to tic severity (according to total YGTSS scores, comprising symptom severity and impairment). Functional connectivity of the OFA varied in proportion to tic severity with premotor cortex, M1, S1, temporo-parietal junction, intraparietal sulcus, supramarginal and angular gyri, posterior cingulate cortex, and visual areas including V1, V2, V3, and V4 (Fig. 3C and Supplementary Table 3); while functional connectivity of the insula varied in proportion to tic severity with the globus pallidus, thalamus, temporo-parietal junction, and early visual cortex (V1, V2) (Fig. 3D and Supplementary Table 3).
Premonitory sensation severity (PUTS)
Two psychophysiological interaction analyses examined whether functional connectivity with (i) the OFA; and (ii) the insula varied in relation to premonitory sensation severity (according to PUTS scores). There were no regions where functional connectivity of the OFA significantly varied in proportion to premonitory sensation severity. However, the insula psychophysiological interaction showed a significant correlation with PUTS in the supplementary motor area, posterior cingulate, precuneus, and fusiform gyrus/cerebellum (Fig. 3E and Supplementary Table 3).
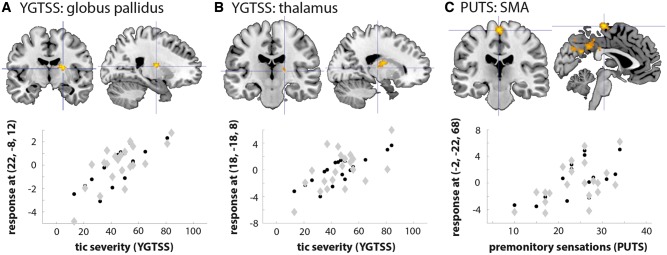
Plots of the correlation between YGTSS and globus pallidus and thalamus functional connectivity, and between PUTS and supplementary motor area functional connectivity, are shown in Fig. 4 at each region’s peak coordinates in the psychophysiological interaction (Supplementary Table 3).
Figure 4.
Regions showing a correlation between tic severity (YGTSS) and premonitory sensations (PUTS) and a psychophysiological interaction with the insula when viewing faces in Tourette syndrome participants. The worse the tic severity and premonitory sensations, the greater the psychophysiological interaction with the insula. Plots show the YGTSS or PUTS correlation with the insula psychophysiological interaction at each region’s peak coordinates in the contrast (Supplementary Table 3). Black circles indicate group mean, grey diamonds indicate individual participants. (A) YGTSS: globus pallidus, (B) YGTSS: thalamus, (C) PUTS: supplementary motor area (SMA).
Discussion
Faces are potent social cues and can act as strong contextual triggers for action particularly in the context of perceived threat (Parkinson et al., 2017). In humans, processing of conspecific faces evokes activity within a well characterized set of neural pathways, incorporating the occipital face area, fusiform face area, and amygdala (Haxby et al., 2002; Ishai, 2008). Here we show that viewing neutral and emotionally threatening (angry) faces does not radically alter activity in canonical face perception regions, such as the fusiform face area, in people with Tourette syndrome. However, when viewing faces individuals with Tourette syndrome do demonstrate additional recruitment of insula cortex (regardless of whether the face appears threatening or not).
Furthermore, face-evoked activity in the insula is associated with increases in functional connectivity to a set of cortical regions that mirrors regions previously implicated in tic generation: presupplementary motor area, premotor cortex, M1, and the putamen. Key basal ganglia regions associated with tic expression—the globus pallidus and thalamus—were seen to vary in functional connectivity with the insula in proportion to tic severity, while connectivity with the supplementary motor area varied in proportion to premonitory sensations. Together, this suggests that when confronted with social stimuli, in people with Tourette syndrome a hyper-reactive insula increases in signalling with a set of cortical and subcortical areas known to have a causal role in generating tics (Ganos et al., 2013; Neuner et al., 2013). This may reveal a neural mechanism underlying a common everyday experience for people with Tourette syndrome in which environmental and autonomic factors can influence the expression of tics, including transient increases in tic severity.
The face perception network in Tourette syndrome
Face perception proceeds in humans via occipito-temporal lobe pathways comprising a characteristic set of regions including the OFA, FFA, and amygdala (Haxby et al., 2002; Ishai, 2008). In addition, the IFG has been proposed to have a role in an extended hierarchy for contextual processing of dynamic aspects of faces, such as valence (Scalaidhe et al., 1997; Ishai, 2008). In our participants with Tourette syndrome, activity within these canonical face perception regions was similar to that of controls, suggesting that cardinal operations underlying face processing do not differ radically in people with tics.
One previous study, also employing a gender judgement task of emotional faces, observed a greater response in the amygdala in participants with Tourette syndrome (Neuner et al., 2010). We did not observe a significant difference in amygdala response between Tourette syndrome participants and controls, although we note the previous result was uncorrected for multiple comparisons, and as such any difference may be subtle.
In addition, it is notable that IFG activity was slightly elevated in Tourette syndrome participants: across numerous cognitive, affective, and motor tasks, the IFG has been identified as a region of altered function in Tourette syndrome (Polyanska et al., 2017). While it may play a key role in tic suppression (Ganos, 2016) and prefrontal regulation of adaptive behaviour (Robbins, 2007), it may be less crucial in the specific context of face perception. In contrast, the insula was notably hyperactive in response to the presentation of faces in participants with Tourette syndrome.
The insula as a tic trigger site in Tourette syndrome
The insula is likely a key site of dysfunction in Tourette syndrome, and in particular has been associated with the generation of uncomfortable premonitory sensations or ‘urges’ that commonly precede tics (Cavanna et al., 2017; Conceicao et al., 2017). In broad terms, the insula functions as an interoceptive hub for processing homeostatically relevant internal physiological signals (Critchley and Harrison, 2013), generating bodily ‘feelings’ that underpin emotional experiences, such as stress and anxiety (Gray and Critchley, 2007). Onward signals to prefrontal and motor systems may then facilitate the production of remedial action (Jackson et al., 2011; Garfinkel and Critchley, 2016). The insula response that we observed in Tourette syndrome participants may therefore suggest an interoceptive experience occurs for participants with Tourette syndrome when viewing faces, in a way that does not for controls. Furthermore, the equivalent response of the anterior insula to both neutral and angry faces suggests that insula signals do not necessarily reflect the specific emotional valence of a face stimulus (at the univariate level), but instead a visceral response when presented with this form of social cue. This may suggest a hypersensitivity to visceral experience in the presence of social cues, in a condition where social anxiety is commonly comorbid (Kurlan et al., 2002; Specht et al., 2011).
We explored the implications of this insular hyper-reactivity in greater depth, through a series of psychophysiological interaction analyses. In participants with Tourette syndrome, the insula showed a change in functional connectivity when viewing faces, with a number of cortical and subcortical regions, including regions that appear to have key roles in tic generation and expression: the presupplementary motor area, premotor cortex, M1, and putamen (Ganos et al., 2013; Neuner et al., 2013). We also examined functional connectivity with the OFA as a contrasting region of the face processing hierarchy. This region showed changes in functional connectivity in Tourette syndrome during face viewing with primarily posterior cortical regions, notably including primary somatosensory cortex. These observations point towards the insula as a hub of hyper-reactive dysfunction, which interacts with a wider cortical and subcortical network, including critical motor regions associated with tic generation, while the OFA displays a more circumspect pattern of functional connectivity constrained to more posterior (sensory) areas.
Particularly striking were the observed changes in insula functional connectivity that occurred in proportion to tic severity (YGTSS) and premonitory sensations (PUTS). There was greater functional connectivity of the insula with the globus pallidus and thalamus in patients with worse tic severity. These two subcortical nuclei are the primary targets for deep brain stimulation in refractory Tourette syndrome (Akbarian-Tefaghi et al., 2016). Furthermore, functional connectivity of the insula with the supplementary motor area was greater in those with worse premonitory sensations. Our data thus suggest the insula may function as a ‘tic trigger’ site in response to social stimuli, perhaps mediated in part through autonomic arousal responses (Nagai et al., 2009; Hawksley et al., 2015; Godar and Bortolato, 2017), generating a cascade of onward signals to cortico-striato-thalamo-cortical circuitry in proportion to an individual’s experience of sensory and motor symptoms. It is notable that effects on symptom severity were observed only in connectivity analyses: univariate models showed no significant correlations with YGTSS or PUTS scores.
Our evidence for the insula as a ‘tic trigger’ site in Tourette syndrome extends observations that insula grey matter thickness predicts severity of premonitory sensations (Draper et al., 2016), as does resting state functional connectivity between the insula and supplementary motor area (Tinaz et al., 2015). Furthermore, this anatomical focus is consistent with the notion that the expression of tics in Tourette syndrome is likely influenced by neural processes that support experience of bodily urges, which elicit mitigating action via signals to motor regions (Jackson et al., 2011; Conceicao et al., 2017; Rae et al., 2018). Tourette syndrome is a highly heterogeneous condition, with substantial variability between individuals in the severity of premonitory urges and tics (Robertson et al., 2017), and the extent to which such premonitory sensations are consciously perceived as triggers for tics (Leckman et al., 1993). Here, we identify that these individual differences are likely underpinned by the strength of insula signalling with the basal ganglia nuclei and cortical motor regions that are associated with the generation of tics.
In contrast, the OFA showed changes in functional connectivity both overall, and in relation to tic severity, in more posterior cortical regions (highlighting the specificity of the insula effects). These regions included early visual areas, parietal association cortices, and perhaps most notably, primary somatosensory and primary motor cortices. Thus, while the dysfunctional interactions of the insula are likely critical to the generation of premonitory sensations and tics, and their increasing expression under environmental cues, there is nevertheless a probable role for activity in other sensory areas also influencing symptom expression, for example via disrupted perception-action binding processes mediated by parietal cortex (Beste et al., 2016; Polyanska et al., 2017; Petruo et al., 2018).
Impact of environmental and autonomic tic triggers on quality of life
In examining the strength of functional connectivity changes in relation to tic severity, we used the total YGTSS scores that comprise symptom severity and impairment. The symptom scores relate to the number, frequency, and complexity of an individual’s tics, while impairment scores indicate how much impact an individual’s tics have on their everyday life. Those scoring higher on symptom severity tend to also report greater social problems and impact on quality of life (Eapen et al., 2016). In the context of presenting face stimuli that can act as social cues, we therefore applied the total composite score of the YGTSS. Indicative of the extent of tic triggers on quality of life, social impairment may extend to a comorbid diagnosis of social phobia in people with Tourette syndrome (Kurlan et al., 2002; Specht et al., 2011). Identification of the insula as a hub of hyper-reactive dysfunction in social contexts interacting with wider regions outside the canonical face perception network highlights this region as a target for therapeutic interventions that aim to reduce impacts of environmental and autonomic triggers on tic severity.
One way in which this might be achieved is via interoceptive training regimes in which participants practise detection of bodily sensations, such as heartbeats. Interoceptive accuracy, as assessed by a heartbeat counting task, is reduced in people with Tourette Syndrome (Ganos et al., 2015), suggesting that such sensory signals may be noisier. These interoceptive signals are known to be processed by the insula (Critchley et al., 2004). Furthermore, interoceptive accuracy is malleable under practises in which attention is directed to bodily sensations (Bornemann and Singer, 2017): elements of such an approach bear similarity to existing behavioural therapies for Tourette syndrome such as Habit Reversal Therapy, of which awareness training to premonitory sensations is a core component (Woods et al., 2008).
There is also interest in the application of non-invasive brain stimulation techniques (e.g. transcranial magnetic stimulation) for the therapeutic treatment of tics (Grados et al., 2018); however, these have typically been applied to cortical regions—most commonly the supplementary motor area—that are more easily accessible than the insula, which lying ~2 cm under the frontal operculum does not present a practical target.
The development of alternative therapeutic approaches to managing tics is particularly important in a condition in which the typically prescribed frontline dopaminergic medications do not always have therapeutic efficacy in all individuals (Hartmann and Worbe, 2013). Moreover, therapeutic interventions that can aid reductions in tic expression driven by stressful social cues will have important benefits on quality of life (Eapen et al., 2016), and it is in this domain that interoceptive training regimes impacting insula reactivity may have the highest potential.
Methodological considerations of impact of tics
Acquiring task-based functional MRI data from hyperkinetic movement disorder populations presents challenges both for data quality, and for the separation of concurrent BOLD signals relating to tic generation and expression from BOLD signals related to the task. Tic monitoring using video recording has been proposed (Neuner et al., 2007), but other than in a minority of studies (Thomalla et al., 2014), this has not been extensively taken up. Nevertheless, we followed such an approach, monitoring tics time-locked to the functional MRI data, and regressing tic timelines in first level models. As such, we can be confident that the influence of co-occurring tics on our results is as far as practically possible removed.
Tic monitoring deals with the issue of separating BOLD signal relating to tics from BOLD signal relating to the task. However, it does not necessarily deal with the impact on data quality of head movements. Therefore, we also included realignment parameters in first level models, as is common practice (Hodgson et al., 2017). An alternative approach to head motion can include a multi-echo functional MRI acquisition, to remove non-BOLD components of the signal that do not scale with T2* (Kundu et al., 2017). However, this approach would ultimately still not address the contaminating effects of neural activity underpinning tic expression on BOLD signal relating to task performance.
Future directions
People with tics often take medications influencing monoaminergic transmission, including treatments for associated conditions such as ADHD, OCD, and anxiety (Roessner et al., 2011). Medications influencing serotonergic transmission, for example, can modulate cortical influences over canonical face perception regions such as the amygdala during face viewing (Passamonti et al., 2012). Our present sample was heterogenous in comorbidity and medication status, which is representative of the ‘TS+’ spectrum (Cavanna et al., 2009). We therefore included covariates for medication and ADHD and OCD comorbidity in second-level models to account for this heterogeneity. A larger sample, containing subgroups of individuals on different medications, would enable a more detailed investigation of the impact of these features of the condition, and permit stratification of participants according to medication or comorbidity status and neural response to emotional social cues.
Psychophysiological interaction analyses are a useful approach to identify functional influences within neural systems under experimental manipulations (Rowe, 2010). To more concretely dissect the causative role of the insula, it would be useful to apply generative models of effective connectivity using dynamic causal modelling (Friston et al., 2017). Historically, event-related designs have been suboptimal when applying such analyses, but forthcoming advances in neural mass models will permit the inversion of models with event-related datasets previously limited by their relative poverty of signal-to-noise for experimental events (Friston et al., 2017).
Conclusion
Tics can be triggered in people with Tourette syndrome by environmental and autonomic factors. When viewing faces, which are potent social cues, people with Tourette syndrome show a hyperactive insula. Furthermore, functional connectivity increases between the insula and key regions associated with tic generation and expression. This suggests people with Tourette syndrome may experience a hypersensitivity to embodied experiences associated with the presence of social cues, with insula signals influencing the expression of tics via signals to subcortical regions within cortico-striato-thalamo-cortical circuits. These results highlight the potential neural mechanisms by which tic trigger factors such as social cues can transiently increase tic severity, and suggest that treatment strategies focused on reducing insula hyper-reactivity may have therapeutic potential.
Supplementary Material
Glossary
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- FFA
fusiform face area
- IFG
inferior frontal gyrus
- OCD
obsessive compulsive disorder
- OFA
occipital face area
- PUTS
Premonitory Urge for Tics Scale
- YGTSS
Yale Global Tic Severity Scale
Funding
This work was funded by a donation from the Dr Mortimer and Theresa Sackler Foundation.
Competing interests
The authors report no competing interests.
References
- Akbarian-Tefaghi L, Zrinzo L, Foltynie T. The use of deep brain stimulation in Tourette syndrome. Brain Sci 2016; 6, pii: E35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Tubing J, Seeliger H, Baumer T, Brandt V, Stock AK, et al. Altered perceptual binding in Gilles de la Tourette syndrome. Cortex 2016; 83: 160–6. [DOI] [PubMed] [Google Scholar]
- Bornemann B, Singer T. Taking time to feel our body: steady increases in heartbeat perception accuracy and decreases in alexithymia over 9 months of contemplative mental training. Psychophysiology 2017; 54: 469–82. [DOI] [PubMed] [Google Scholar]
- Brandt VC, Lynn MT, Obst M, Brass M, Munchau A. Visual feedback of own tics increases tic frequency in patients with Tourette's syndrome. Cogn Neurosci 2015; 6: 1–7. [DOI] [PubMed] [Google Scholar]
- Buse J, Schoenefeld K, Munchau A, Roessner V. Neuromodulation in Tourette syndrome: dopamine and beyond. Neurosci Biobehav Rev 2013; 37: 1069–84. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Black KJ, Hallett M, Voon V. Neurobiology of the premonitory urge in Tourette's syndrome: pathophysiology and treatment implications. J Neuropsychiatry Clin Neurosci 2017; 29: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Nani A. Tourette syndrome and consciousness of action. Tremor Other Hyperkinet Mov 2013; 3, pii: tre-03-181-4368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Servo S, Monaco F, Robertson MM. The behavioral spectrum of Gilles de la Tourette syndrome. J Neuropsychiatry Clin Neurosci 2009; 21: 13–23. [DOI] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K. Topological FDR for neuroimaging. Neuroimage 2010; 49: 3057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conceicao VA, Dias A, Farinha AC, Maia TV. Premonitory urges and tics in Tourette syndrome: computational mechanisms and neural correlates. Curr Opin Neurobiol 2017; 46: 187–99. [DOI] [PubMed] [Google Scholar]
- Conelea CA, Woods DW. The influence of contextual factors on tic expression in Tourette's syndrome: a review. J Psychosom Res 2008; 65: 487–96. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron 2013; 77: 624–38. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7: 189–95. [DOI] [PubMed] [Google Scholar]
- Draganski B, Martino D, Cavanna AE, Hutton C, Orth M, Robertson MM, et al. Multispectral brain morphometry in Tourette syndrome persisting into adulthood. Brain 2010; 133 (Pt 12): 3661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper A, Jackson GM, Morgan PS, Jackson SR. Premonitory urges are associated with decreased grey matter thickness within the insula and sensorimotor cortex in young people with Tourette syndrome. J Neuropsychol 2016; 10: 143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen V, Cavanna AE, Robertson MM. Comorbidities, social impact, and quality of life in Tourette syndrome. Front Psychiatry 2016; 7: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen V, Moriarty J, Robertson MM. Stimulus induced behaviours in Tourette's syndrome. J Neurol Neurosurg Psychiatry 1994; 57: 853–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 2007; 36: 511–21. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016; 113: 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finis J, Moczydlowski A, Pollok B, Biermann-Ruben K, Thomalla G, Heil M, et al. Echoes from childhood–imitation in Gilles de la Tourette syndrome. Mov Disord 2012; 27: 562–5. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997; 6: 218–29. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Preller KH, Mathys C, Cagnan H, Heinzle J, Razi A, et al. Dynamic causal modelling revisited. Neuroimage 2017, doi: 10.1016/j.neuroimage.2017.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganos C. Tics and Tourette's: update on pathophysiology and tic control. Curr Opin Neurol 2016; 29: 513–18. [DOI] [PubMed] [Google Scholar]
- Ganos C, Garrido A, Navalpotro-Gomez I, Ricciardi L, Martino D, Edwards MJ, et al. Premonitory urge to tic in Tourette's is associated with interoceptive awareness. Mov Disord 2015; 30: 1198–202. [DOI] [PubMed] [Google Scholar]
- Ganos C, Ogrzal T, Schnitzler A, Munchau A. The pathophysiology of echopraxia/echolalia: relevance to Gilles de la Tourette syndrome. Mov Disord 2012; 27: 1222–9. [DOI] [PubMed] [Google Scholar]
- Ganos C, Roessner V, Munchau A. The functional anatomy of Gilles de la Tourette syndrome. Neurosci Biobehav Rev 2013; 37: 1050–62. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Critchley HD. Threat and the body: how the heart supports fear processing. Trends Cogn Sci 2016; 20: 34–46. [DOI] [PubMed] [Google Scholar]
- Godar SC, Bortolato M. What makes you tic? Translational approaches to study the role of stress and contextual triggers in Tourette syndrome. Neurosci Biobehav Rev 2017; 76 (Pt A): 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The yale-brown obsessive compulsive scale. I. development, use, and reliability. Arch Gen Psychiatry 1989; 46: 1006–11. [DOI] [PubMed] [Google Scholar]
- Gorgolewski KJ, Varoquaux G, Rivera G, Schwartz Y, Ghosh SS, Maumet C, et al. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the brain. Front Neuroinform 2015; 9: 8. doi: 10.3389/fninf.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grados M, Huselid R, Duque-Serrano L. Transcranial magnetic stimulation in Tourette syndrome: a historical perspective, its current use and the influence of comorbidities in treatment response. Brain Sci 2018; 8, pii: E129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron 2007; 54: 183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Worbe Y. Pharmacological treatment of Gilles de la Tourette syndrome. Neurosci Biobehav Rev 2013; 37: 1157–61. [DOI] [PubMed] [Google Scholar]
- Hawksley J, Cavanna AE, Nagai Y. The role of the autonomic nervous system in Tourette syndrome. Front Neurosci 2015; 9: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry 2002; 51: 59–67. [DOI] [PubMed] [Google Scholar]
- Hodgson K, Poldrack RA, Curran JE, Knowles EE, Mathias S, Goring HHH, et al. Shared genetic factors influence head motion during MRI and body mass index. Cereb Cortex 2017; 27: 5539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A. Let's face it: it's a cortical network. Neuroimage 2008; 40: 415–19. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Draper A, Dyke K, Pepes SE, Jackson SR. Inhibition, disinhibition, and the control of action in Tourette syndrome. Trends Cogn Sci 2015; 19: 655–65. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Parkinson A, Kim SY, Schuermann M, Eickhoff SB. On the functional anatomy of the urge-for-action. Cogn Neurosci 2011; 2: 227–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. The world health organization adult ADHD self-report scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005; 35: 245–56. [DOI] [PubMed] [Google Scholar]
- Kundu P, Voon V, Balchandani P, Lombardo MV, Poser BA, Bandettini PA. Multi-echo fMRI: a review of applications in fMRI denoising and analysis of BOLD signals. Neuroimage 2017; 154: 59–80. [DOI] [PubMed] [Google Scholar]
- Kurlan R, Como PG, Miller B, Palumbo D, Deeley C, Andresen EM, et al. The behavioral spectrum of tic disorders: a community-based study. Neurology 2002; 59: 414–20. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The yale global tic severity scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989; 28: 566–73. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome. Am J Psychiatry 1993; 150: 98–102. [DOI] [PubMed] [Google Scholar]
- Misirlisoy E, Brandt V, Ganos C, Tubing J, Munchau A, Haggard P. The relation between attention and tic generation in Tourette syndrome. Neuropsychology 2015; 29: 658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Cavanna A, Critchley HD. Influence of sympathetic autonomic arousal on tics: implications for a therapeutic behavioral intervention for Tourette syndrome. J Psychosom Res 2009; 67: 599–605. [DOI] [PubMed] [Google Scholar]
- Neuner I, Kellermann T, Stocker T, Kircher T, Habel U, Shah JN, et al. Amygdala hypersensitivity in response to emotional faces in Tourette's patients. World J Biol Psychiatry 2010; 11: 858–72. [DOI] [PubMed] [Google Scholar]
- Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol 2013; 112: 35–71. [DOI] [PubMed] [Google Scholar]
- Neuner I, Wegener P, Stoecker T, Kircher T, Schneider F, Shah NJ. Development and implementation of an MR-compatible whole body video system. Neurosci Lett 2007; 420: 122–7. [DOI] [PubMed] [Google Scholar]
- Parkinson J, Garfinkel S, Critchley H, Dienes Z, Seth AK. Don't make me angry, you wouldn't like me when I'm angry: volitional choices to act or inhibit are modulated by subliminal perception of emotional faces. Cogn Affect Behav Neurosci 2017; 17: 252–68. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Crockett MJ, Apergis-Schoute AM, Clark L, Rowe JB, Calder AJ, et al. Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol Psychiatry 2012; 71: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruo V, Bodmer B, Brandt VC, Baumung L, Roessner V, Munchau A, et al. Altered perception-action binding modulates inhibitory control in Gilles de la Tourette syndrome. J Child Psychol Psychiatry 2018, doi: 10.1111/jcpp.12938. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Exp Brain Res 2011; 209: 481–93. [DOI] [PubMed] [Google Scholar]
- Polyanska L, Critchley HD, Rae CL. Centrality of prefrontal and motor preparation cortices to Tourette syndrome revealed by meta-analysis of task-based neuroimaging studies. Neuroimage Clin 2017; 16: 257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Critchley HD, Seth AK. A bayesian account of tics. Open Science Framework; 2018. Available at: https://osf.io/xcbgz/. [Google Scholar]
- Robbins T. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci 2007; 362: 917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, et al. Gilles de la Tourette syndrome. Nat Revi Dis Primers 2017; 3: 16097. [DOI] [PubMed] [Google Scholar]
- Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry 2011; 20: 173–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB. Connectivity analysis is essential to understand neurological disorders. Front Syst Neurosci 2010; 4, pii: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalaidhe OSP, Wilson FA, Goldman-Rakic PS. Areal segregation of face-processing neurons in prefrontal cortex. Science 1997; 278: 1135–8. [DOI] [PubMed] [Google Scholar]
- Specht MW, Woods DW, Piacentini J, Scahill L, Wilhelm S, Peterson AL, et al. Clinical characteristics of children and adolescents with a primary tic disorder. J Dev Phys Disabil 2011; 23: 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomalla G, Jonas M, Baumer T, Siebner HR, Biermann-Ruben K, Ganos C, et al. Costs of control: decreased motor cortex engagement during a Go/NoGo task in Tourette's syndrome. Brain 2014; 137 (Pt 1): 122–36. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Malone P, Hallett M, Horovitz SG. Role of the right dorsal anterior insula in the urge to tic in Tourette syndrome. Mov Disord 2015; 30: 1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009; 168: 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadman R, Tischler V, Jackson GM. 'Everybody just thinks I'm weird': a qualitative exploration of the psychosocial experiences of adolescents with Tourette syndrome. Child Care Health Dev 2013; 39: 880–6. [DOI] [PubMed] [Google Scholar]
- Woods DW, Piacentini J, Himle MB, Chang S. Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. J Dev Behav Pediatr 2005; 26: 397–403. [DOI] [PubMed] [Google Scholar]
- Woods DW, Piacentini JC, Chang SW, Deckersbach T, Ginsburg GS, Peterson AL, et al. Managing Tourette syndrome: a behavioural intervention for children and adults. New York, NY: Oxford University Press; 2008. [Google Scholar]
- Worbe Y, Sgambato-Faure V, Epinat J, Chaigneau M, Tande D, Francois C, et al. Towards a primate model of Gilles de la Tourette syndrome: anatomo-behavioural correlation of disorders induced by striatal dysfunction. Cortex 2013; 49: 1126–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study (unthresholded statistic images for every contrast reported) are openly available in Neurovault, at https://neurovault.org/collections/4167/, reference number 4167 (Gorgolewski et al., 2015).