Frigerio et al. report that pro-resolving mechanisms of neuroinflammation are dysregulated during epileptogenesis, thereby promoting a persistent neuroinflammatory response that contributes to seizure generation and cognitive deficits. Boosting endogenous resolution responses by administering a specific lipid mediator improves disease outcomes in a murine epilepsy model, suggesting a novel treatment avenue.
Keywords: pro-resolving lipid mediators, epilepsy, comorbidities, ALX/FPR2, ChemR23/ERV1
Abstract
Epilepsy therapy is based on drugs that treat the symptoms rather than the underlying mechanisms of the disease (epileptogenesis). There are no treatments for preventing seizures or improving disease prognosis, including neurological comorbidities. The search of pathogenic mechanisms of epileptogenesis highlighted that neuroinflammatory cytokines [i.e. interleukin-1β (IL-1β), tumour necrosis factor-α (Tnf-α)] are induced in human and experimental epilepsies, and contribute to seizure generation in animal models. A major role in controlling the inflammatory response is played by specialized pro-resolving lipid mediators acting on specific G-protein coupled receptors. Of note, the role that these pathways have in epileptogenic tissue remains largely unexplored. Using a murine model of epilepsy, we show that specialized pro-resolving mechanisms are activated by status epilepticus before the onset of spontaneous seizures, but with a marked delay as compared to the neuroinflammatory response. This was assessed by measuring the time course of mRNA levels of 5-lipoxygenase (Alox5) and 15-lipoxygenase (Alox15), the key biosynthetic enzymes of pro-resolving lipid mediators, versus Il1b and Tnfa transcripts and proteins. In the same hippocampal tissue, we found a similar delayed expression of two main pro-resolving receptors, the lipoxin A4 receptor/formyl peptide receptor 2 and the chemerin receptor. These receptors were also induced in the human hippocampus after status epilepticus and in patients with temporal lobe epilepsy. This evidence supports the hypothesis that the neuroinflammatory response is sustained by a failure to engage pro-resolving mechanisms during epileptogenesis. Lipidomic LC-MS/MS analysis showed that lipid mediator levels apt to resolve the neuroinflammatory response were also significantly altered in the hippocampus during epileptogenesis with a shift in the biosynthesis of several pro-resolving mediator families including the n-3 docosapentaenoic acid (DPA)-derived protectin D1. Of note, intracerebroventricular injection of this mediator during epileptogenesis in mice dose-dependently reduced the hippocampal expression of both Il1b and Tnfa mRNAs. This effect was associated with marked improvement in mouse weight recovery and rescue of cognitive deficit in the novel object recognition test. Notably, the frequency of spontaneous seizures was drastically reduced by 2-fold on average and the average seizure duration was shortened by 40% after treatment discontinuation. As a result, the total time spent in seizures was reduced by 3-fold in mice treated with n-3 DPA-derived protectin D1. Taken together, the present findings demonstrate that epilepsy is characterized by an inadequate engagement of resolution pathways. Boosting endogenous resolution responses significantly improved disease outcomes, providing novel treatment avenues.
Introduction
Epilepsy is a brain disease that affects around 65 million people worldwide (Devinski et al., 2018). Epileptic seizures, the hallmarks of epilepsy, reduce the quality of life, increase the risk of death, and impose socio-economic burdens to affected people and society. Despite the availability of a significant number of anti-seizure drugs, ∼30% of newly diagnosed epilepsy patients are resistant to therapies (Kwan et al., 2011; Loscher et al., 2013). Moreover, there are no drugs able to prevent the onset or progression of epilepsy in patients exposed to epileptogenic injuries, such as status epilepticus, trauma, stroke or CNS infections. Thus, next generation therapies should target the mechanisms of epileptogenesis to either prevent the disease or improve its outcomes (Loscher et al., 2013).
Neuroinflammation is a mechanism of epileptogenesis commonly induced in seizure-susceptible brain areas by differing brain injuries (Aronica et al., 2017; Klein et al., 2018). It persists in chronic epilepsy in both experimental animals and patients (Aronica and Crino, 2011; Butler et al., 2016; Klein et al., 2018). Neuroinflammation is characterized by increased cytokines, such as interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), high mobility group box 1 (HMGB1), their cognate receptors and downstream effector molecules in glia, neurons and blood–brain barrier cellular components (Ravizza et al., 2006, 2008, 2011; Boer et al., 2008; Vezzani et al., 2013). These inflammatory molecules significantly contribute to the generation and recurrence of spontaneous seizures (Vezzani et al., 2011a, b; Terrone et al., 2017) and to neurological comorbidities (Mazarati et al., 2017) in animal models. There is evidence that the neuroinflammatory milieu persists in epilepsy because is not efficiently controlled by endogenous anti-inflammatory mechanisms (Ravizza et al., 2006; Aronica et al., 2007; Pernhorst et al., 2013; Sun et al., 2016), thereby contributing to dysfunction of brain cells and neuronal network hyperexcitability (Vezzani et al., 2011b; Vezzani and Viviani, 2015). In support, pharmacological or genetic interventions for increasing the endogenous anti-inflammatory IL-1 receptor antagonist (IL-1Ra, encoded by IL1RN) drastically reduced the frequency of seizures in experimental models (De Simoni et al., 2000; Vezzani et al., 2000, 2002; Librizzi et al., 2012; Bertani et al., 2017; Walker et al., 2017) and in humans (Jyonouchi, 2016; Kenney-Jung et al., 2016; DeSena et al., 2018), afforded neuroprotection (Noé et al., 2013) and rescued neurological deficits (Mazarati et al., 2017).
Specialized pro-resolving mediators (e.g. lipoxins, resolvins, protectins and maresins) are key molecules that mediate the active resolution of inflammation in peripheral tissues and in CNS (Serhan et al., 2008). The biosynthesis of these mediators occurs through the stereoselective conversion of essential fatty acids, including eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and n-3 docosapentaenoic acid (DPA), by lipoxygenase (LOX) and cyclooxygenase enzymes (Serhan et al., 2017). The biological actions of these molecules are mediated by cognate G-protein coupled receptors, including the lipoxin A4 receptor/formyl peptide receptor 2 (ALX/FPR2) and the chemerin receptor (ChemR23/ERV1), to drive active resolution of inflammation. This is attained by reducing the expression of proinflammatory cytokines and chemokines, orchestrating the trafficking of innate and adaptive immune cells to the inflamed tissue, restoring the blood–brain barrier integrity (Cristante et al., 2013) and increasing anti-inflammatory molecules (Serhan et al., 2008).
Pro-resolving mediators exert beneficial actions in a range of preclinical models of peripheral organ dysfunction linked to inflammatory conditions (Romano et al., 2015; Serhan et al., 2017). In the CNS, neuroprotective actions of lipoxins and their synthetic analogues, or neuroprotectin D1 (NPD1) (Zhao et al., 2011) have been reported in models of Alzheimer’s disease, ischaemia-reperfusion injury or neurotrauma where these molecules also alleviate the neurological symptoms (Marcheselli et al., 2003; Wu et al., 2011; Zhao et al., 2011; Luo et al., 2013; Hawkins et al., 2014). In models of neuropathic pain lipoxins (Martini et al., 2016), maresin 1 (Serhan et al., 2012), resolvins (Xu et al., 2010) and NPD1 (Xu et al., 2013) showed analgesic properties (Romano et al., 2015; Keppel Hesselink, 2017).
The ALX/FPR2 receptor and 15-LOX (ALOX15) are expressed in the hippocampus of patients with Alzheimer’s disease suggesting that resolution mechanisms might be active in human CNS diseases with a pathogenic neuroinflammatory component. Of note, reduced levels of lipoxin (LX)A4 in the same specimens suggest a potential alteration of this pathway (Wang et al., 2015).
Recent studies demonstrate that DHA and the DHA-derived NPD1 reduced neuronal hyperexcitability evoked in vivo by electrical hippocampal kindling stimulation (Musto et al., 2011) or injection of a chemoconvulsant in mice (Musto et al., 2015). Moreover, n-3 polyunsaturated fatty acids (such as EPA, DHA) decrease evoked hippocampal neuronal excitability in slice preparations (Xiao and Li, 1999; Taha et al., 2013b) and there is some evidence that they may have anticonvulsive effects in humans and animal models (Taha et al., 2010, 2013a). However, the mechanisms elicited by these n-3 polyunsaturated fatty acids as well as the role of pro-resolving pathways in epilepsy remain to be elucidated.
In this study, we provide novel evidence of the temporal regulation of specialized pro-resolving mediator expression in the hippocampus during epileptogenesis, and compared them with the kinetics of the neuroinflammatory response in a mouse model of acquired chronic epilepsy (Iori et al., 2017). We also show that lipid mediator levels apt to resolve the neuroinflammatory response are significantly altered during epileptogenesis, and describe the expression of pro-resolving receptors in mouse and human epileptogenic hippocampi versus control brain tissue. Finally, we determined the ability of a specific specialized pro-resolving mediator, n-3 DPA-derived protectin D1 (PD1n-3 DPA), in controlling the neuroinflammatory response during epileptogenesis. This intervention reduced neuroinflammation, improved the post-injury animal’s weight recovery, rescued their cognitive deficit and resulted in a significant decrease in both frequency and duration of epileptic seizures.
Materials and methods
In accordance with the ARRIVE guidelines, procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national (D.L. n.26, G.U. March 4, 2014) and international guidelines and laws (EEC Council Directive 86/609, OJ L 358, 1, December 12, 1987, Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996), and were reviewed and approved by the intramural ethical committee.
Experimental animals
Adult male C57/BL6N mice (30 g; Charles River) were used in all the experiments, except for one experiment where adult male NMRI mice (28–32 g; Charles River) were used (Supplementary material). C57/BL6N mice were housed at constant temperature (23 ± 1°C) and relative humidity (60 ± 5%) with a fixed 12-h light-dark cycle and free access to food and water. Mice were housed four per cage until surgery, and then kept one per cage in an enriched environment with nesting materials (Hutchinson et al., 2005).
Mouse model of epilepsy
Mice were implanted under 1.5% isoflurane anaesthesia with a 23-gauge cannula unilaterally positioned on top of the dura mater for the intra-amygdala injection of kainic acid [from bregma, mm: nose bar 0; anteroposterior (AP) −1.0, lateral (L) −2.8]. A bipolar Teflon-insulated stainless-steel depth electrode (60 µm outer diameter) was implanted in the dorsal hippocampus ipsilateral to the injected amygdala (from bregma, mm: nose bar 0; AP −1.8, L −1.5, −2.0 below dura mater). Additionally, a cortical surface electrode was implanted onto the somatosensory cortex in the contralateral hemisphere. One additional guide cannula was positioned on top of the dura mater (from bregma, mm: nose bar 0; AP 0, L −0.9) ipsilateral to the injected amygdala for intracerebroventricular injections (Franklin and Paxinos, 2008). Finally, two screw electrodes were positioned over the nasal sinus and the cerebellum, and used as ground and reference electrodes, respectively. Electrodes were connected to a multipin socket and secured to the skull by acrylic dental cement (Iori et al., 2017).
One week after surgery, mice were connected to the EEG setup the day before beginning the experiment in order to record an EEG baseline for at least 24 h. Kainic acid (0.3 μg in 0.2 μl; Sigma-Aldrich, #K0250) was dissolved in 0.1 M phosphate-buffered saline (PBS, pH 7.4) and injected into the right basolateral amygdala in freely moving mice using a needle protruding of 4.1 mm below the implanted cannula in order to evoke status epilepticus (Iori et al., 2017).
Detection and quantification of status epilepticus and spontaneous seizures
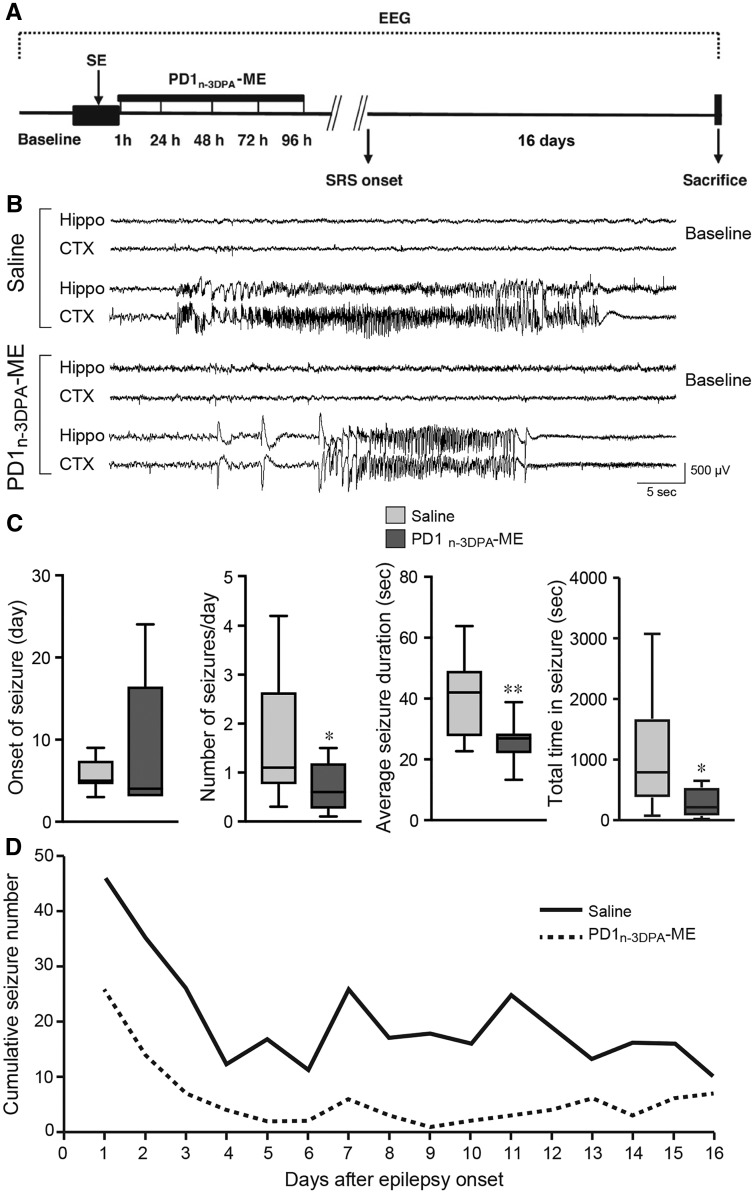
Status epilepticus was defined by the appearance of continuous spike activity with a frequency >1 Hz intermixed with high amplitude and frequency discharges lasting for at least 5 s, with a frequency of >8 Hz. Spikes were defined as sharp waves with amplitude at least 2.5-fold higher than the standard deviation of baseline signal and duration <100 ms, or as a spike-and-wave with duration <200 ms (Pitkanen et al., 2005). Status epilepticus developed after ∼10 min from kainic acid injection, as previously described (Iori et al., 2017). After 40 min from status epilepticus onset, mice were injected with diazepam (10 mg/kg, intraperitoneally) to improve their survival, although EEG status epilepticus was not interrupted. After status epilepticus induction mice were recorded continuously (24/7) until the onset of spontaneous seizures was detected in each mouse (Fig. 6C) and for 16 days thereafter using the Twin EEG Recording System connected with a Comet AS-40 32/8 Amplifier (sampling rate 400 Hz, high-pass filter 0.3 Hz, low-pass filter 70 Hz, sensitivity 2000 mV/cm; Grass-Telefactor). Digitized EEG data were processed using the Twin record and review software. Status epilepticus duration and spiking activity were quantified using Clampfit 9.0 program (Axon Instruments). The end of status epilepticus was defined by the occurrence of interspike intervals longer than 1 s. Spontaneous motor seizures developed 5.4 ± 0.5 days after status epilepticus (Fig. 6C) as described previously (Jimenez-Mateos et al., 2012; Iori et al., 2017). We calculated the total number and total duration of seizures during a 16-day recording period (24/7) starting after the onset of the first spontaneous seizure in each mouse to estimate the frequency of seizures and their average duration. Although we did not record mice for a longer time, we showed previously in this model that all mice exposed to status epilepticus lasting for about 7 h develop spontaneous seizures that recur for more than 2 months, and seizure frequency may increase from 1.5 months onwards in a proportion of mice (Iori et al., 2017).
Figure 6.
PD1n-3 DPA reduced spontaneous seizures. (A) Experimental design in status epilepticus-exposed mice to study the effect of PD1n-3 DPA-ME on spontaneous seizures. One hour after status epilepticus induction, animals were randomized into treatment (PD1n-3DPAME, n = 9) and saline groups (n = 12). PD1n-3 DPA-ME (200 ng/μl) or saline was injected intracerebroventricularly for four consecutive days starting 1 h after status epilepticus onset. EEG recording was done continuously from status epilepticus induction until the onset of the first two spontaneous seizures (SRS) and for 16 days thereafter. At the end of EEG recording mice were sacrificed for brain histological analysis. Representative EEG tracings depict baseline activity and spontaneous seizures recorded in the right hippocampus (Hippo) and in the left parietal cortex (CTX) of mice treated with saline or PD1n-3 DPA-ME (B). Note the shorter duration of a typical EEG seizure in a drug-treated mouse versus saline-injected mouse. (C) The onset of spontaneous seizures, the number of spontaneous seizures in each day of EEG recording, the average seizure duration and the cumulative time spent in seizures in saline- (n = 12) versus drug-treated mice (n = 9). Data are presented as box-and-whisker plots depicting median, interquartile interval, minimum and maximum (n = number of mice). (D) The cumulative number of seizures per day in each experimental group during 16 days after epilepsy onset. *P < 0.05; **P < 0.01 versus saline injected mice by one-tailed t-test. Friedman’s two-way non-parametric ANOVA was used to detect the treatment effect on number of seizures and duration and their interaction with days. Treatment effect on number of seizures, P < 0.01 and interaction with days, P = 0.51; treatment effect on duration of seizures, P < 0.01 and interaction with days, P = 0.82.
Lipid mediator profiling
Mice were deeply anaesthetized with intraperitoneal injections of ketamine (75 mg/kg) and medetomidine (0.5 mg/kg), then perfused via ascending aorta with 50 mM ice-cold PBS (pH 7.4) for 1 min to remove blood, and decapitated at 72 h post-status epilepticus. The hippocampus ipsilateral to the stimulated amygdala was rapidly dissected out at 4°C, immediately frozen in liquid nitrogen and stored at −80°C. To have enough tissue for the analysis, we pooled two hippocampi ipsilateral to the stimulated amygdala from two different mice (n = 16 mice; eight samples). Sham mice (n = 8) were injected with vehicle instead of kainic acid; for each mouse the hippocampi from both hemispheres were pooled together.
Tissues were placed in 1 ml of ice-cold methanol containing 500 pg each of d5-lipoxin A4 (LXA4), d5-resolvin D2 (RvD2), d4-prostaglandin E2 (PGE2), d4-leukotriene B4 (LTB4) and d8-5S-hydroexeicosatetraneoic acid (diHETE). The tissues were then gently homogenized using a glass dounce. These were then kept at −20°C for 45 min, to allow protein precipitation, then centrifuged at 1500 g for 10 min, 4°C. All samples for LC-MS/MS-based profiling were extracted using solid-phase extraction columns (Dalli et al., 2013; Rathod et al., 2017). Briefly, supernatants were subjected to solid phase extraction, methyl formate fraction collected, brought to dryness and suspended in phase (methanol/water, 1:4, vol/vol) for injection on a Shimadzu LC-20AD HPLC and a Shimadzu SIL-20AC autoinjector, paired with a QTRAP® 6500 plus (Sciex). An Agilent Poroshell 120 EC-C18 column (100 mm × 4.6 mm × 2.7 μm) was kept at 50°C and mediators eluted using a mobile phase consisting of methanol-water-acetic acid of 20:80:0.01 (vol/vol/vol) that was ramped to 50:50:0.01 (vol/vol/vol) over 0.5 min and then to 80:20:0.01 (vol/vol/vol) from 2 min to 11 min, maintained till 14.5 min and then rapidly ramped to 98:2:0.01 (vol/vol/vol) for the next 0.1 min. This was subsequently maintained at 98:2:0.01 (vol/vol/vol) for 5.4 min, and the flow rate was maintained at 0.5 ml/min. The QTRAP® 6500 plus was operated using a multiple reaction monitoring method (Dalli et al., 2013; Rathod et al., 2017). Each lipid mediator was identified using established criteria including matching retention time to synthetic materials and authentic material and at least six diagnostic ions (Dalli et al., 2013; Rathod et al., 2017). Calibration curves were obtained for each mediator using synthetic and authentic standard mixtures at 0.78, 1.56, 3.12, 6.25, 12.5, 25, 50, 100, and 200 pg that gave linear calibration curves with a r2-values of 0.98–0.99.
Pharmacological treatment with PD1n-3 DPA-ME
PD1n-3 DPA-ME, the methylester (ME) pro-drug form of PD1n-3 DPA, which is rapidly converted by cellular esterases to the free acid form, was prepared as previously described (Aursnes et al., 2014). This was aliquoted in ethanol and stored at −80°C in the dark until use. Immediately prior to administration in mice, ethanol was evaporated using a gentle stream of nitrogen. Then, the mediator was suspended in 50 mM PBS (pH 7.4), placed in a water bath sonicator for <10 s and vortexed for no more than 30 s.
Mice were exposed to status epilepticus, then they were assigned randomly to treatment or vehicle (50 mM PBS) groups. PD1n-3 DPA-ME (20 or 200 ng/μl in 50 mM PBS, pH 7.4) or vehicle was injected intracerebroventricularly (1 μl/site) twice daily for four consecutive days starting 1 h after status epilepticus onset. This schedule was designed to encompass the epileptogenesis phase preceding disease onset, then the treatment was stopped.
Supplementary methods
The Supplementary material provides further details for: real-time quantitative polymerase chain reaction analysis (RT-qPCR); intraperitoneal pilocarpine injection in mice; novel object recognition test; immunohistochemistry and double-immunostaining; human subjects; ALXR/FPR2 and ChemR23/ERV1 immunohistochemistry in human tissue; and histological analysis and quantification of neuronal cell loss, neurogenesis and glia activation.
Statistical analysis
In each animal experiment no ad interim analysis was done. Sample size was a priori determined based on previous experience with the animal model. All efforts were made to minimize the number of animals used and their suffering according to the principles of the three Rs (Replacement, Reduction and Refinement; https://www.nc3rs.org.uk/the-3rs). Endpoints (outcome measures) and statistical tests were prospectively selected. A simple random allocation using a website randomization program (www.randomization.com) was applied to assign a subject to a particular experimental group. Data acquisition and analysis were done blindly.
Statistical analysis was performed by GraphPad Prism 6 (GraphPad Software, USA) for Windows using absolute values. Data are presented as box-and-whisker plots depicting median, interquartile interval, minimum and maximum (n = number of individual samples). Mann Whitney U-test for two independent groups and Kruskal-Wallis test with Dunn’s post hoc correction for more than two independent groups were used for statistical analysis of data. The temporal distribution of spikes during status epilepticus was analysed by two-way ANOVA followed by Bonferroni’s multiple comparisons test. Based on normality test’s results, one-way ANOVA with Tukey’s post hoc test and t-test were used for behavioural data. One tailed t-test was used for analysis of the effect of treatment with PD1n-3DPA-ME on spontaneous seizures. Friedman’s two-way non-parametric ANOVA was used to detect the treatment effect on number of seizures and duration and their interaction with days. Differences were considered significant with a P < 0.05.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Distinct temporal regulation of pro-resolving mediators versus neuroinflammatory mediators during epileptogenesis
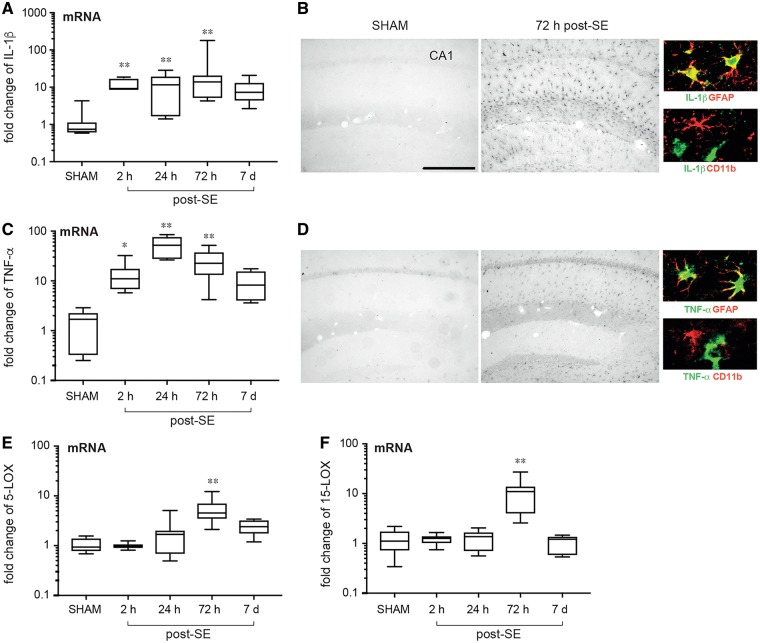
As an index of neuroinflammation, we measured Il1b and Tnfa mRNA levels by RT-qPCR in the mouse hippocampus from 2 h until 7 days post-status epilepticus to encompass the epileptogenesis phase preceding the onset of spontaneous seizures. Figure 1A and C shows a significant increase in Il1b and Tnfa transcripts between 2 h and 72 h (n = 7–10) compared to sham mice (n = 7), declining to basal values thereafter. The increase in cytokines was confirmed by immunohistochemistry (Fig. 1B and D) showing that both IL-1β and Tnf-α staining was induced in activated GFAP-positive astrocytes 72 h post-status epilepticus (n = 8). No CD3-positive lymphocytes were observed in brain tissue (not shown). Cytokines were undetectable in control hippocampi (sham, n = 8) or in CD11b-positive microglia 72 h after status epilepticus in mice (Fig. 1B and D, colour micrographs), which is in line with previous findings (De Simoni et al., 2000; Maroso et al., 2011).
Figure 1.
Temporal regulation of pro-resolving pathways in mouse hippocampus during epileptogenesis. Messenger RNA (A and C) and protein expression (B and D) of IL1B (IL-1β) and TNFA (TNF-α) and mRNA expression of Alox5 (5-LOX, B) and Alox15 (15-LOX, F) from 2 h until 7 days post-status epilepticus. Reference genes were Mfsd5, Brap, Bcl2l13. Data are presented on a log10 scale as box-and-whisker plots depicting median, interquartile interval, minimum and maximum (n = 7–10). *P < 0.05, **P < 0.01 versus sham by Kruskal-Wallis test with Dunn’s post hoc correction. In the high magnification panels in B and D, we identified the cell types expressing IL-1β and TNF-α (green) by double-immunostaining using specific astrocytic (GFAP, red) and microglial (CD11b, red) cell markers. Co-localization signal is depicted in yellow. Scale bar = 50 μm.
To assess the potential impact of specialized pro-resolving mediators in these settings we determined the mRNA expression of Alox5 (5-LOX) and Alox15 (15-LOX), two key enzymes in the biosynthesis of pro-resolving mediators. In tissues from epileptogenic mice we found a significant upregulation of both enzymes at the 72 h interval (Fig. 1E and F; n = 7–10 mice). To establish whether these results were also reproduced in a distinct model of epilepsy, we administered pilocarpine to mice and assessed the expression of both inflammatory and pro-resolving molecules in the hippocampus. We found a significant upregulation of both inflammatory and pro-resolving mediators tested at the 72 h interval (Supplementary Fig. 1). These results support the hypothesis that neuroinflammation in epileptogenesis may arise from a failure to engage pro-resolving mechanisms.
We also investigated the expression of two main pro-resolving receptors ALX/FPR2 and ChemR23/ERV1 in mouse (Supplementary Fig. 2A–D) and human (Supplementary Fig. 2E and F) epileptogenic tissues. In the mouse hippocampus the mRNA level of either receptor was increased above control value at 72 h (n = 7) but not at 2 h and 7 days after status epilepticus (n = 7–10). ALXR/FPR2 induction was measured at 24 h post-status epilepticus therefore anticipating ChemR23/ERV1 increase (Supplementary Fig. 2A and C). Both ALX/FPR2 and ChemR23/ERV1 immunostaining was increased in GFAP-positive astrocytes but not in CD11b-positive microglia 72 h post-status epilepticus (n = 8) (Supplementary Fig. 2B and D, colour micrographs). ALX/FPR2 and IL-1β proteins were co-localized in astrocytes (micrograph in Supplementary Fig. 2B) suggesting that the resolution signalling process is operative within the cells that generate neuroinflammatory molecules. Similar to status epilepticus-exposed mice, ALX/FPR2 and ChemR23/ERV1 were predominantly induced in astrocytes in hippocampal specimens from patients who died 1–49 days after status epilepticus and in chronic epilepsy patients (n = 7; Supplementary Fig. 2E and F) as compared to autoptic control tissue (n = 6).
Epileptogenesis alters mouse hippocampal specialized pro-resolving mediator profiles
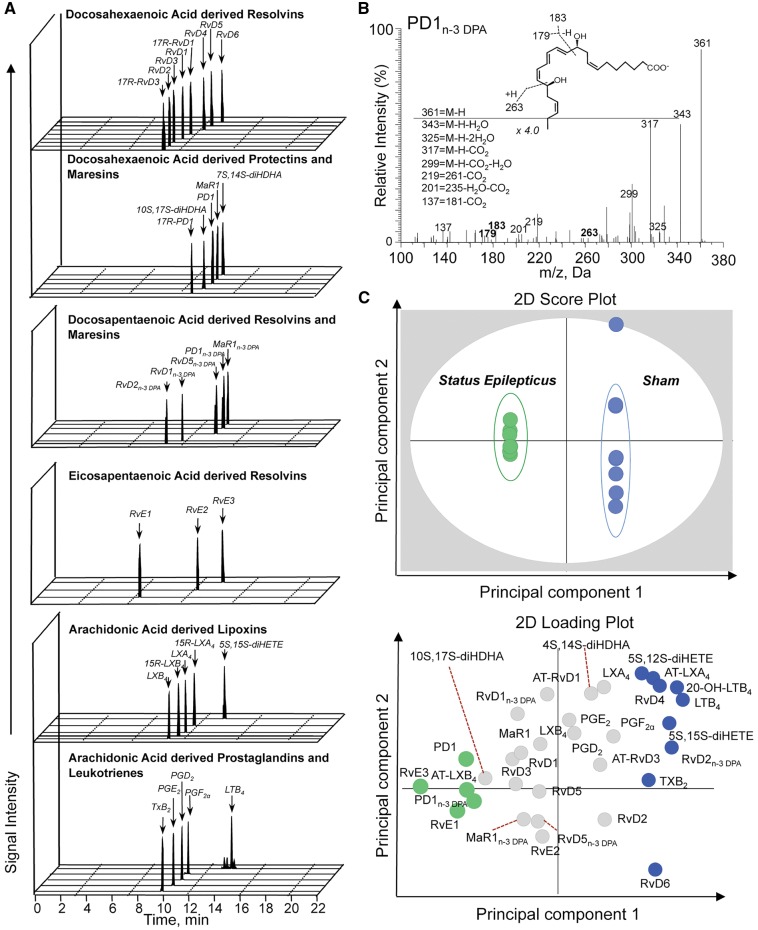
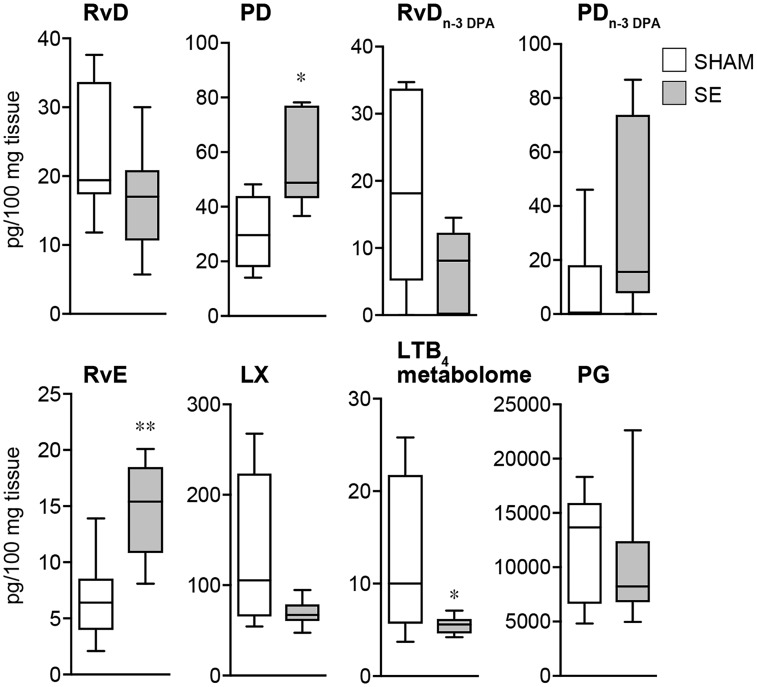
Given that we found an alteration in resolution mechanisms and an upregulation in the expression of specialized pro-resolving mediator biosynthetic enzymes during murine epileptogenesis, we next assessed whether specialized pro-resolving mediator biosynthesis was also altered. Using LC-MS/MS-based lipid mediator profiling we identified mediators from all four major bioactive metabolomes that were identified in accordance to established criteria, including retention times and MS/MS spectra (Dalli et al., 2018) (Fig. 2A and B). These included the DHA-derived resolvins and protectins and the n-3 DPA-derived protectins. Multivariate analysis of lipid mediator profiles obtained from hippocampi of sham mice and epileptogenic mice gave two distinct clusters indicating that lipid mediator production is dysregulated during epileptogenesis (Fig. 2C). Assessment of cumulative levels for the distinct specialized pro-resolving mediator families gave a downregulation of both DHA and n-3 DPA-derived resolvins and the arachidonic acid derived lipoxins. We also observed an upregulation of DHA and n-3 DPA derived protectins and eicosapentaenoic acid derived resolvins (Fig. 3). Assessment of individual mediator concentration gave statistically significant decreases in RvD2n-3 DPA and RvD5n-3 DPA as well as 5S,15S-diHETE, the lipoxin pathway marker, and an upregulation of PD1n-3 DPA in tissues from epileptogenic mice (Supplementary Table 2). These results demonstrate that epileptogenesis leads to dysregulation of specialized pro-resolving mediator production in the brain.
Figure 2.
Dysregulated lipid mediator profiles in hippocampus of mice during epileptogenesis. Multiple reaction monitoring (A) for identified mediators in murine brains. (B) MS/MS spectrum employed for the identification of PD1n-3 DPA. (C) Octagonal-partial least square discriminant analysis of hippocampal lipid mediator profiles (top) 2D score plot; (bottom) 2D loading plot. Cumulative concentrations of bioactive mediator families identified in hippocampi from mice following status epilepticus and sham mice. Lipid mediators were isolated, identified and quantified using lipid mediator profiling in the hippocampus of mice 72 h post-status epilepticus (n = 16) and in sham mice (n = 8). See ‘Materials and methods’ section for details. (C) P < 0.05 sham versus status epilepticus using Mann Whitney U-test.
Figure 3.
Epileptogenesis alters specialized pro-resolving mediator profiles in the hippocampus. The levels of DHA- and DPA-derived protectins and resolvins, EPA-derived resolvins and AA-derived lipoxins were identified and quantified using LC-MS/MS-based liquid mediator profiling (see ‘Materials and methods’ section for details) in the hippocampus of mice 72 h post-status epilepticus and in sham mice. Data are presented as box-and-whisker plots depicting median, interquartile interval, minimum and maximum (n = 8 mice/group). RvDn-3 DPA (P = 0.053); LX (P = 0.075); *P < 0.05; **P < 0.01 by Mann Whitney U-test.
PD1n-3 DPA regulates neuroinflammation
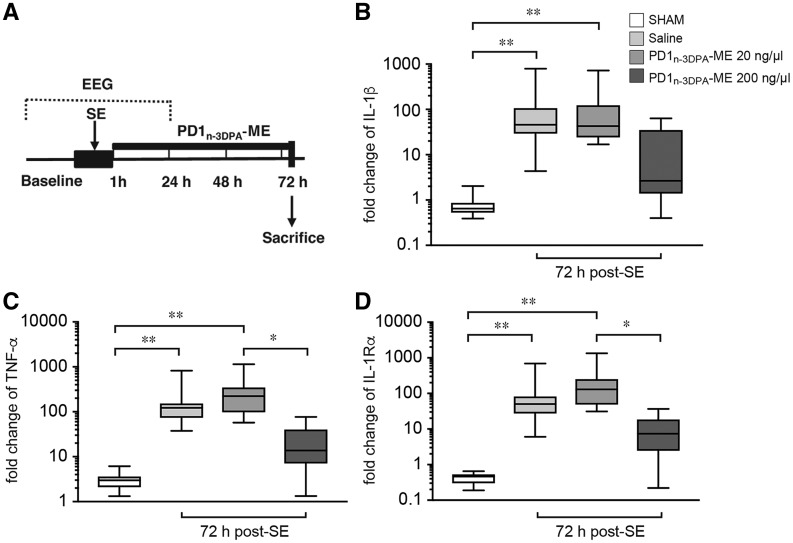
Given that PD1n-3 DPA was markedly upregulated during epileptogenesis, we next investigated whether this mediator was involved in controlling neuroinflammation. Thus, we administered this mediator as its methylester form (PD1n-3 DPA-ME), which is rapidly converted to the endogenous mediator in biological tissues. Administration of PD1n-3 DPA-ME (20 or 200 ng/µl) significantly reduced in a dose-dependent manner hippocampal Il1b and Tnfa mRNAs expression after status epilepticus when compared to mice administered vehicle only (Fig. 4). We also found that administration of this mediator reduced the expression of inflammation-dampening mediator IL-1Ra in brains from epileptogenic mice, likely because neuroinflammation was efficiently blunted by PD1n-3 DPA-ME. Of note, PD1n-3 DPA-ME administration did not modify status epilepticus onset, duration and severity (Supplementary Fig. 3). Together these results indicate that PD1n-3 DPA administration controls the onset and propagation of neuroinflammation during epileptogenesis.
Figure 4.
PD1n-3 DPA regulates components of the inflammatory response in the hippocampus during epileptogenesis. (A) Experimental design applied to status epilepticus-exposed mice to study the effect of PD1n-3 DPA-ME on proinflammatory and anti-inflammatory mediators. One hour after status epilepticus onset, animals were randomized into a treatment arm injected intracerebroventricularly with either 20 ng/µl or 200 ng/µl PD1n-3 DPA-ME (n = 6–9) and a saline-injected group (n = 14). PD1n-3 DPA-ME or saline was injected for three consecutive days starting 1 h after status epilepticus onset. Mice were sacrificed 72 h post-status epilepticus (2 h after the last injection of PD1n-3DPA-ME or saline) for RT-qPCR analysis. Panels show mRNA levels of Il1b (IL-1β, B), Tnfa (TNF-α, C) Il1rn (IL-1Rα, D) in the hippocampus of mice treated with 20 ng/μl or 200 ng/μl dose of PD1n-3 DPA-ME (n = 6–9) or saline (n = 14) and in sham mice (n = 12). Reference genes were Mfsd5, Brap and Bcl2l13. Data are presented on a log10 scale as box-and-whisker plots depicting median, interquartile interval, minimum and maximum (n = number of mice).*P < 0.05; ** P < 0.01 by Kruskal-Wallis test with Dunn’s post hoc correction.
Effect of PD1n-3 DPA on early pathological consequences of status epilepticus
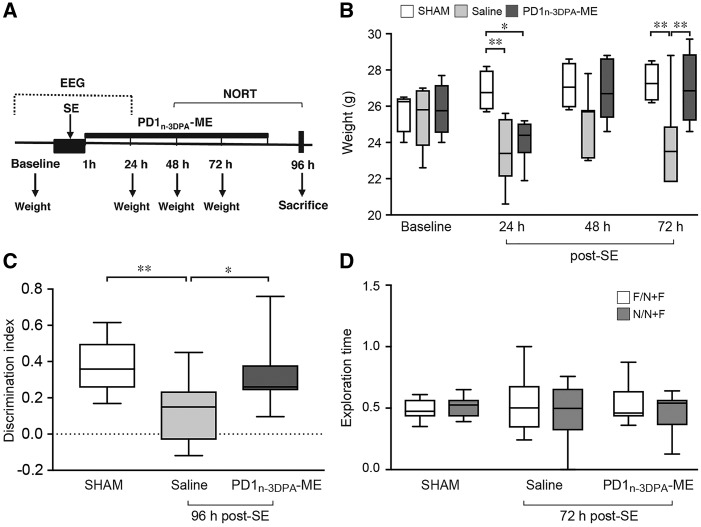
We next tested whether the protective mechanisms engaged by PD1n-3 DPA also translated to improvements in weight loss provoked by status epilepticus. Mice were administered PD1n-3 DPA-ME or vehicle intracerebroventricularly for 4 days starting 1 h after status epilepticus induction (Fig. 5A). Mice exposed to status epilepticus and injected with vehicle (n = 7) displayed a persistent loss of weight after status epilepticus compared to sham mice (n = 9; Fig. 5B). In contrast, in mice given PD1n-3 DPA-ME (n = 6), weight returned to those in sham mice after 48 h and this effect was sustained through 72 h after status epilepticus (Fig. 5B). The baseline weight measured the day before surgery was similar in the three experimental groups (sham, 26.8 ± 0.3g; saline, 24.4 ± 0.4g; treatment, 25.9 ± 0.6g).
Figure 5.
PD1n-3 DPA rescued weight loss and cognitive deficit during epileptogenesis. (A) Experimental design applied to status epilepticus-exposed mice to study the effect of PD1n-3 DPA-ME on animal’s weight and recognition memory. One hour after status epilepticus onset, mice were randomized into PD1n-3 DPA-ME (200 ng/μl) or saline group. Mice were injected intracerebroventricular for four consecutive days starting 1 h after status epilepticus onset. (B) The weight of mice at baseline (the day before surgery) and at consecutive times after status epilepticus. Mice were weighed daily (between 8:00 and 9:00 am) after administering saline (n = 7) or PD1n-3 DPA-ME (200 ng/μl; n = 6). Sham mice (n = 9). Data are presented as box-and-whisker plots depicting median, interquartile interval, minimum and maximum (n = number of mice). *P < 0.05; **P < 0.01 by Kruskal-Wallis test with Dunn’s post hoc correction. (C and D) The mice performance in the novel object recognition test (NORT) starting 48 h post-status epilepticus. (C) The discrimination index [the ratio between the difference in time spent with the novel and the familiar object (N−F) and the sum of total exploration time (N+F)]. The test was performed 96 h after status epilepticus in mice injected with saline (n = 12) or PD1n-3 DPA-ME (n = 11) and compared to sham mice (n = 18). (D) The exploration time (the ratio between the time spent exploring the object and the total exploration time) during the familiarization phase when the two objects were identical. Data are presented as box-and-whisker plots depicting median, interquartile interval, minimum and maximum (n = number of mice). *P < 0.05; **P < 0.01 versus sham or saline by one-way ANOVA with Tukey’s post hoc test.
To determine whether there PD1n-3 DPA also improved cognitive function, we performed the novel object recognition test (NORT) as a measure of non-spatial recognition memory. During the recognition phase of the NORT, control mice (n = 18) spent significantly more time exploring the novel object as compared to the familiar one (∼70% and 30% of total exploration time, respectively), thus yielding a discrimination index of 0.37 ± 0.03 (Fig. 5C). By contrast, mice injected with saline and exposed to status epilepticus (n = 12) spent equal time exploring previously presented and novel objects; hence the discrimination index was 0.13 ± 0.05 (Fig. 5C). This deficit was significantly rescued in mice treated with PD1n-3 DPA-ME (n = 11) since they spent more time exploring the novel object thus yielding a discrimination index similar to control mice (Fig. 5C). The deficit in the NORT was not a result of changes in spontaneous motor activity since the distance covered and the velocity of mice in the open field were not affected by status epilepticus or the treatment (sham, 3061 ± 192 cm; 5.1 ± 0.3 cm/s; saline, 3364 ± 574 cm; 5.3 ± 0.6 cm/s; PD1n-3 DPA-ME, 3890 ± 613 cm; 6.9 ± 1.2 cm/s). As depicted in Fig. 5D, the three groups of mice spent equal time (∼50%) exploring the two identical objects during the familiarization phase, therefore excluding that the changes in discrimination index were due to different objects’ exploration times.
At the end of the behavioural test, the mice were killed for histopathological brain analysis. We did not detect significant differences in any of the cellular hallmarks measured in status epilepticus-exposed mice treated with PD1n-3 DPA-ME versus the corresponding vehicle-injected mice (Supplementary Fig. 4). In particular, there was an attenuation of the increased number of ectopic doublecortin-positive cell in the hilus (Supplementary Fig. 4A) as well as of astrogliosis and microgliosis (Supplementary Fig. 4B) in status epilepticus-exposed mice treated with by PD1n-3 DPA-ME versus vehicle, although this effect did not reach statistical significance. We measured a significant neuronal cell loss in the hippocampus (CA1, CA3) and the kainic acid-injected amygdala as assessed by counting Nissl-stained and Fluoro-Jade-positive neurons in status epilepticus-exposed mice treated with vehicle (Supplementary Fig. 4C and D). No significant neuroprotection was measured in PD1n-3 DPA-ME versus vehicle injected status epilepticus-exposed mice (Supplementary Fig. 4C and D).
PD1n-3 DPA reduced ensuing epileptic seizures
To test whether PD1n-3 DPA also regulates spontaneous seizures in mice, we administered the PD1n-3 DPA-ME (n = 9) or saline (n = 12) for 4 days starting 1 h after status epilepticus induction and cortical and hippocampal EEG activities were measured continuously for 21 days to detect the onset and frequency of spontaneous seizures (Fig. 6A and B). Although the onset of spontaneous seizures was not significantly modified by PD1n-3 DPA-ME (Fig. 6C), the mediator significantly reduced the cumulative number of daily seizures after disease onset which is reflected by an average 50% reduction of daily seizure frequency (Fig. 6C and D). The mean seizure duration was also significantly reduced in treated mice versus vehicle mice (Fig. 6B and C). As a result, the total time spent in seizures was reduced by 3-fold in mice treated with PD1n-3 DPA-ME (Fig. 6C). The reduction in the average seizure duration persisted at Day 16 from epilepsy onset in PD1n-3 DPA-ME-treated mice versus vehicle mice (vehicle, 41.4 ± 3.8 s; PD1n-3 DPA-ME, 30.1 ± 2.2, P < 0.05) despite the cumulative number of seizures at this day was similar in the two experimental groups (Fig. 6D).
The extent of neuronal cell loss in the hippocampus and amygdala in the epileptic mice treated with PD1n-3 DPA-ME during epileptogenesis was similar to vehicle-injected mice (Supplementary Fig. 5), in accordance with the evidence at 96 h post-status epilepticus (Supplementary Fig. 4C). We did not detect Fluoro-Jade-positive neurons in the epileptic mice at the end of EEG recordings indicating that neurodegeneration was complete.
Supplementary results are included in the Supplementary material.
Discussion
Neuroinflammation is ignited with a rapid onset by various epileptogenic insults in both humans and animal models and persists in brain areas where seizures originate and spread (Gershen et al., 2015; Butler et al., 2016; Koepp et al., 2017; Terrone et al., 2017; van Vliet et al., 2018). We reasoned that the dynamics of this response might point to inefficient mechanisms of resolution in the diseased brain tissue. Hence, lack of resolution may be a key factor for the pathological consequences of neuroinflammation in epilepsy (Vezzani et al., 2015).
We tested the novel hypothesis that the active pro-resolving brain response mediated by fatty acid derivatives is inefficient in epilepsy, thereby contributing to persistent neuroinflammation and the consequent seizure generation, neuropathology and neurological deficits. We focused our investigations in the hippocampus, a key epileptogenic area, in a well-established murine model of epileptogenesis where combinations of anti-inflammatory drugs were proven to block disease progression (Iori et al., 2017).
Similar to other epileptogenesis models (De Simoni et al., 2000; Dhote et al., 2007; Maroso et al., 2011; Jiang et al., 2013; Walker et al., 2017), we found an early and lasting post-injury induction of the ictogenic cytokines IL-1β and TNF-α (Vezzani et al., 2011b; Balosso et al., 2013). Notably, we also determined the lack of activation of LOX enzymes and specialized pro-resolving mediator for hours to days post-insult, indicating a delayed and transient activation of the pro-resolving response as compared to neuroinflammation. Lipid mediator profiling demonstrated differential regulation in pro-resolving mediator concentrations during epileptogenesis, that is characteristic of a failed resolution response (Serhan, 2007, 2017), thus indicating that neuroinflammation contributing to epilepsy may arise from an inability of the neuronal tissue to engage these host protective pathways leading to tissue damage and neuronal impairment.
Notably, PD1n-3 DPA was the most abundant lipid mediator during epileptogenesis suggesting it might be a key player in the tissue attempt to engage resolution pathways. Accordingly, early post-injury administration of PD1n-3 DPA-ME significantly reduced neuroinflammation in our murine model, in line with previous findings that PD1n-3 DPA and its precursor n-3 DPA (Ferdinandusse et al., 2001) exert powerful anti-inflammatory effects (Dalli et al., 2013). This effect was associated with a drastic reduction in the number and duration of spontaneous seizures after the onset of the disease. Since PD1n-3 DPA-ME therapeutic effect occurred after treatment termination, we conclude that the drug mediates a genuine anti-epileptogenic action rather than providing a mere symptomatic control of seizures. Importantly, PD1n-3 DPA-ME improved animal’s weight recovery after status epilepticus and rescued cognitive deficit, a major neurological comorbidity in epilepsy (Mazarati et al., 2017). Since neuroinflammation contributes to both seizures and cognitive dysfunctions (Aronica et al., 2017; Mazarati et al., 2017), the anti-inflammatory properties of PD1n-3 DPA-ME are likely to chiefly mediate its therapeutic effects. An unavoidable limitation of the murine model is the short latency for the development of spontaneous seizures; therefore, the treatment necessarily overlaps with the status epilepticus. We have carefully quantified status epilepticus onset, severity and duration and these parameters were not changed by the early treatment with PD1n-3 DPA-ME. Moreover, PD1n-3 DPA-ME did not reduce neuronal cell loss in the hippocampus which is a direct consequence of status epilepticus. Nevertheless, one cannot exclude there might be a treatment effect on some acute modifications induced by status epilepticus, which play a role in the therapeutic outcomes.
n-3 DPA is known to reduce the activation of microglia, sphingomyelinase, caspase-3 and oxidative stress in aged rats, and as a consequence to attenuate age-related deficit in spatial learning and long-term potentiation (Kelly et al., 2011). Similarly, the DHA-derived NPD1 inhibited these molecular processes in a model of Alzheimer’s disease (Zhao et al., 2011) and also reduced hippocampal excitability and seizures in rodents (Musto et al., 2011, 2015). Since these molecular events are strictly associated with neuroinflammation and play a role in seizure generation (Balosso et al., 2008; Aronica et al., 2017; Pauletti et al., 2017), they may be implicated in the antiepileptogenic effects of PD1n-3 DPA-ME.
Histopathological brain evaluation in PD1n-3 DPA-ME-treated mice indicate a mild reduction in dentate hilus ectopic neurogenesis and in glial cells activation which are both implicated in epileptogenesis (Scharfman and McCloskey, 2009), but no neuroprotection was observed in forebrain. Differently, n-3 DPA exerted neuroprotective effects in the hippocampus of aged rats after 56 days of diet supplementation (Kelly et al., 2011), suggesting that a more prolonged post-injury treatment with PD1n-3 DPA-ME is required to attain neuroprotective effects.
Overall our data support the concept that resolution of neuroinflammation and therapeutic effects on seizures and cognition can be attained by enhancing brain endogenous pro-resolving mediators. A potential clinical approach is to increment the dietary intake of omega-3-polyunsaturated fatty acids, which are known to be beneficial for health (Cash et al., 2014). Animal studies suggest that n-3 polyunsaturated fatty acids raise the seizure threshold but the clinical studies have provided so far limited effects. This might be because of insufficient doses or too short periods of administration (Taha et al., 2010). Long-term supplementation may be required to produce high enough levels of the active lipids in the brain. An advantage of this approach is the safety profile of diet supplementation. Since we observed induction of pro-resolving receptor expression in the hippocampus of patients who had status epilepticus or with drug-resistant seizures, treatments that enhance the availability of endogenous receptor ligands might be warranted. An alternative approach is to develop stable analogues of specialized pro-resolving mediator, including PD1n-3DPA, to overcome the limitation that they are metabolically unstable. A notable example is the proven efficacy in a phase II clinical trial of a stable analogue of resolvin E1 to treat dry eye symptoms. In this frame, our new evidence of the anti-epileptogenic effect of PD1n-3 DPA-ME highlights novel opportunity for drug discovery. However, the brain penetration of these potential new drugs should be improved for their therapeutic application in epilepsy, unless intrathecal application is conceived. These drugs might in principle be used as adjunctive therapy in patients exposed to epileptogenic injuries or with first presentation of seizures to decrease the burden of the disease and ameliorate its clinical course.
Supplementary Material
Acknowledgements
We thank Dr Elena Colombo for her contribution to immunohistochemistry at the beginning of this study. We thank Alessandro Soave for figure editing.
Glossary
Abbreviations
- DHA
docosahexaenoic acid
- DPA
n-3 docosapentaenoic acid
- LOX
lipoxygenase
- PD1n-3 DPA
n-3 DPA-derived protectin D1
Funding
F.F. was a recipient of a fellowship from UCB Biopharma. This work was supported by European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n. 602102 (EPITARGET to A.V., M.C.W., E.A.V., E.A.), Citizen United for Research in Epilepsy (CURE) (A.V.), the Epilepsy Research UK (M.C.W.), and in part by NIH grants RO1 NS086423 and RO1NS039587 (M.P.). This work was also supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant number: 107613/Z/15/Z), funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant number: 677542), and the Barts Charity (Grant number: MGU0343). The Norwegian Research Council is acknowledged for funding to T.V.H. (FRIPRO-FRINATEK 230407).
Competing interests
This research was partially funded by UCB Pharma. P.F, C.V, K.L and R.M.K are full-time employees of UCB Pharma. The remaining authors report no competing interests.
References
- Aronica E, Bauer S, Bozzi Y, Caleo M, Dingledine R, Gorter JA et al. Neuroinflammatory targets and treatments for epilepsy validated in experimental models. Epilepsia 2017; 58 (Suppl 3): 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Boer K, van Vliet EA, Redeker S, Baayen JC, Spliet WG et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol Dis 2007; 26: 497–511. [DOI] [PubMed] [Google Scholar]
- Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia 2011; 52 (Suppl 3): 26–32. [DOI] [PubMed] [Google Scholar]
- Aursnes M, Tungen JE, Vik A, Colas R, Cheng C-YC, Dalli J et al. Total synthesis of the lipid mediator PD1n-3 DPA: configurational assignments and anti-inflammatory and pro-resolving actions. J Nat Prod 2014; 77: 910–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T et al. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain 2008; 131: 3256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balosso S, Ravizza T, Aronica E, Vezzani A. The dual role of TNF-alpha and its receptors in seizures. Exp Neurol 2013; 247C: 267–71. [DOI] [PubMed] [Google Scholar]
- Bertani I, Iori V, Trusel M, Maroso M, Foray C, Mantovani S et al. Inhibition of IL-1β signaling normalizes NMDA-dependent neurotransmission and reduces seizure susceptibility in a mouse model of creutzfeldt-jakob disease. J Neurosci 2017; 37: 10278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer K, Jansen F, Nellist M, Redeker S, van den Ouweland AM, Spliet WG et al. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res 2008; 78: 7–21. [DOI] [PubMed] [Google Scholar]
- Butler T, Li Y, Tsui W, Friedman D, Maoz A, Wang X et al. Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia 2016; 57: e191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JL, Norling LV, Perretti M. Resolution of inflammation: targeting GPCRs that interact with lipids and peptides. Drug Discov Today 2014; 19: 1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristante E, McArthur S, Mauro C, Maggioli E, Romero IA, Wylezinska-Arridge M et al. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc Natl Acad Sci USA 2013; 110: 832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep 2013; 3: 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Walker ME, Serhan CN. Lipid mediator metabolomics via lc-ms/ms profiling and analysis. Methods Mol Biol 2018; 1730: 59–72. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci 2000; 12: 2623–33. [DOI] [PubMed] [Google Scholar]
- DeSena AD, Do T, Schulert GS. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J. Neuroinflammation 2018; 15: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, O’Brien TJ, Jette N, Scheffer IE, de Curtis M et al. Epilepsy. Nat Rev Dis Primers 2018; 4: 18024. [DOI] [PubMed] [Google Scholar]
- Dhote F, Peinnequin A, Carpentier P, Baille V, Delacour C, Foquin A et al. Prolonged inflammatory gene response following soman-induced seizures in mice. Toxicology 2007; 238: 166–76. [DOI] [PubMed] [Google Scholar]
- Ferdinandusse S, Denis S, Mooijer PA, Zhang Z, Reddy JK, Spector AA et al. Identification of the peroxisomal beta-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. J Lipid Res 2001; 42: 1987–95. [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press; 2008 [Google Scholar]
- Gershen LD, Zanotti-Fregonara P, Dustin IH, Liow JS, Hirvonen J, Kreisl WC et al. Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein. JAMA Neurol 2015; 72: 882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins KE, DeMars KM, Singh J, Yang C, Cho HS, Frankowski JC et al. Neurovascular protection by post-ischemic intravenous injections of the lipoxin A4 receptor agonist, BML-111, in a rat model of ischemic stroke. J Neurochem 2014; 129: 130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Avery A, Vandewoude S. Environmental enrichment for laboratory rodents. ILAR J 2005; 46: 148–61. [DOI] [PubMed] [Google Scholar]
- Iori V, Iyer AM, Ravizza T, Beltrame L, Paracchini L, Marchini S et al. Blockade of the IL-1R1/TLR4 pathway mediates disease-modification therapeutic effects in a model of acquired epilepsy. Neurobiol Dis 2017; 99: 12–23. [DOI] [PubMed] [Google Scholar]
- Jiang J, Quan Y, Ganesh T, Pouliot WA, Dudek FE, Dingledine R. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci USA 2013; 110: 3591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med 2012; 18: 1087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H. Intractable epilepsy (IE) and responses to anakinra, a human recombinant IL-1 receptor antagonist (IL-1Ra): case reports. J Clin Cell Immunol 2016; 7: 456–60. [Google Scholar]
- Kelly L, Grehan B, Chiesa AD, O’Mara SM, Downer E, Sahyoun G et al. The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol Aging 2011; 32: 2318.e1–15. [DOI] [PubMed] [Google Scholar]
- Kenney-Jung DL, Vezzani A, Kahoud RJ, LaFrance-Corey RG, Ho ML, Muscardin TW et al. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol 2016; 80: 939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel Hesselink JM. Fundamentals of and critical issues in lipid autacoid medicine: a review. Pain Ther 2017; 6: 153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P, Dingledine R, Aronica E, Bernard C, Blümcke I, Boison D et al. Commonalities in epileptogenic processes from different acute brain insults: do they translate? Epilepsia 2018; 59: 37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp MJ, Årstad E, Bankstahl JP, Dedeurwaerdere S, Friedman A, Potschka H et al. Neuroinflammation imaging markers for epileptogenesis. Epilepsia 2017; 58 (Suppl 3): 11–19. [DOI] [PubMed] [Google Scholar]
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med 2011; 365: 919–26. [DOI] [PubMed] [Google Scholar]
- Librizzi L, Noé F, Vezzani A, de Curtis M, Ravizza T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann Neurol 2012; 72: 82–90. [DOI] [PubMed] [Google Scholar]
- Loscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov 2013; 12: 757–76. [DOI] [PubMed] [Google Scholar]
- Luo C-L, Li Q-Q, Chen X-P, Zhang X-M, Li L-L, Li B-X et al. Lipoxin A4 attenuates brain damage and downregulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res 2013; 1502: 1–10. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem 2003; 278: 43807–17. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J et al. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 2011; 8: 304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini AC, Berta T, Forner S, Chen G, Bento AF, Ji R-R et al. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. J Neuroinflammation 2016; 13: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Lewis ML, Pittman QJ. Neurobehavioral comorbidities of epilepsy: role of inflammation. Epilepsia 2017; 58 (Suppl 3): 48–56. [DOI] [PubMed] [Google Scholar]
- Musto AE, Gjorstrup P, Bazan NG. The omega-3 fatty acid-derived neuroprotectin D1 limits hippocampal hyperexcitability and seizure susceptibility in kindling epileptogenesis. Epilepsia 2011; 52: 1601–08. [DOI] [PubMed] [Google Scholar]
- Musto AE, Walker CP, Petasis NA, Bazan NG. Hippocampal neuro-networks and dendritic spine perturbations in epileptogenesis are attenuated by neuroprotectin d1. PLoS One 2015; 10: e0116543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noé FM, Polascheck N, Frigerio F, Bankstahl M, Ravizza T, Marchini S et al. Pharmacological blockade of IL-1β/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis 2013; 59: 183–93. [DOI] [PubMed] [Google Scholar]
- Pauletti A, Terrone G, Shekh-Ahmad T, Salamone A, Ravizza T, Rizzi M et al. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain 2017; 140: 1885–99 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pernhorst K, Herms S, Hoffmann P, Cichon S, Schulz H, Sander T et al. TLR4, ATF-3 and IL8 inflammation mediator expression correlates with seizure frequency in human epileptic brain tissue. Seizure 2013; 22: 675–8. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J. Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res 2005; 63: 27–42. [DOI] [PubMed] [Google Scholar]
- Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J Clin Invest 2017; 127: 169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T, Balosso S, Vezzani A. Inflammation and prevention of epileptogenesis. Neurosci Lett 2011; 497: 223–30. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Boer K, Redeker S, Spliet WG, van Rijen PC, Troost D et al. The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiol Dis 2006; 24: 128–43. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis 2008; 29: 142–60. [DOI] [PubMed] [Google Scholar]
- Romano M, Cianci E, Simiele F, Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur J Pharmacol 2015; 760: 49–63. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, McCloskey DP. Postnatal neurogenesis as a therapeutic target in temporal lobe epilepsy. Epilepsy Res 2009; 85: 150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 2007; 25: 101–37. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 2017; 31: 1273–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Dalli J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med 2017, in press. doi: 10.1016/j.mam.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008; 8: 349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park C-K, Xu Z-Z et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 2012; 26: 1755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F-J, Zhang C-Q, Chen X, Wei Y-J, Li S, Liu S-Y et al. Downregulation of CD47 and CD200 in patients with focal cortical dysplasia type IIb and tuberous sclerosis complex. J Neuroinflamm 2016; 13: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha AY, Burnham WM, Auvin S. Polyunsaturated fatty acids and epilepsy. Epilepsia 2010; 51: 1348–58. [DOI] [PubMed] [Google Scholar]
- Taha AY, Trepanier M-O, Ciobanu FA, Taha NM, Ahmed M, Zeng Q et al. A minimum of 3 months of dietary fish oil supplementation is required to raise amygdaloid afterdischarge seizure thresholds in rats–implications for treating complex partial seizures. Epilepsy Behav EB 2013a; 27: 49–58. [DOI] [PubMed] [Google Scholar]
- Taha AY, Zahid T, Epps T, Trepanier M-O, Burnham WM, Bazinet RP et al. Selective reduction of excitatory hippocampal sharp waves by docosahexaenoic acid and its methyl ester analog ex-vivo. Brain Res 2013b; 1537: 9–17. [DOI] [PubMed] [Google Scholar]
- Terrone G, Salamone A, Vezzani A. Inflammation and epilepsy: preclinical findings and potential clinical translation. Curr Pharm Des 2017; 23: 5569–76. [DOI] [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol 2011a; 7: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology 2013; 69: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Lang B, Aronica E. Immunity and inflammation in epilepsy. Cold Spring Harb Perspect Med 2015; 6: a022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 2011b; 25: 1281–9. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci USA 2000; 97: 11534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T et al. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia 2002; 43 (Suppl 5): 30–5. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 2015; 96: 70–82. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Aronica E, Vezzani A, Ravizza T. Review: Neuroinflammatory pathways as treatment targets and biomarker candidates in epilepsy: emerging evidence from preclinical and clinical studies. Neuropathol Appl Neurobiol 2018; 44: 91–111. [DOI] [PubMed] [Google Scholar]
- Walker LE, Frigerio F, Ravizza T, Ricci E, Tse K, Jenkins RE et al. Molecular isoforms of high-mobility group box 1 are mechanistic biomarkers for epilepsy. J Clin Invest 2017; 127: 2118–32. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang X, Zhu M, Hjorth E, Cortés-Toro V, Eyjolfsdottir H, Graff C et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement 2015; 11: 40–50.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang A, Min Z, Xiong Y, Yan Q, Zhang J et al. Lipoxin A4 inhibits the production of proinflammatory cytokines induced by beta-amyloid in vitro and in vivo. Biochem Biophys Res Commun 2011; 408: 382–7. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Li X. Polyunsaturated fatty acids modify mouse hippocampal neuronal excitability during excitotoxic or convulsant stimulation. Brain Res 1999; 846: 112–21. [DOI] [PubMed] [Google Scholar]
- Xu Z-Z, Liu X-J, Berta T, Park C-K, Lü N, Serhan CN et al. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann Neurol 2013; 74: 490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med 2010; 16: 592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ et al. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS One 2011; 6: e15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.