Le Heron et al. demonstrate, using behavioural, physiological and imaging techniques, converging evidence that reduced reward sensitivity underlies apathy in patients with a monogenic form of cerebral small vessel disease. This specific change in effort-based decision making points to potential treatment avenues for apathy.
Keywords: apathy, decision-making, reward, CADASIL, small vessel cerebrovascular disease
Abstract
Apathy is a syndrome of reduced motivation that commonly occurs in patients with cerebral small vessel disease, including those with the early onset form, CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy). The cognitive mechanisms underlying apathy are poorly understood and treatment options are limited. We hypothesized that disrupted effort-based decision-making, the cognitive process by which potential rewards and the effort cost required to obtain them is integrated to drive behaviour, might underlie the apathetic syndrome. Nineteen patients with a genetic diagnosis of CADASIL, as a model of ‘pure’ vascular cognitive impairment, and 19 matched controls were assessed using two different behavioural paradigms and MRI. On a decision-making task, participants decided whether to accept or reject sequential offers of monetary reward in return for exerting physical effort via handheld dynamometers. Six levels of reward and six levels of effort were manipulated independently so offers spanned the full range of possible combinations. Choice, decision time and force metrics were recorded. Each participant’s effort and reward sensitivity was estimated using a computational model of choice. On a separate eye movement paradigm, physiological reward sensitivity was indexed by measuring pupillary dilatation to increasing monetary incentives. This metric was related to apathy status and compared to the behavioural metric of reward sensitivity on the decision-making task. Finally, high quality diffusion imaging and tract-based spatial statistics were used to determine whether tracts linking brain regions implicated in effort-based decision-making were disrupted in apathetic patients. Overall, apathetic patients with CADASIL rejected significantly more offers on the decision-making task, due to reduced reward sensitivity rather than effort hypersensitivity. Apathy was also associated with blunted pupillary responses to incentives. Furthermore, these independent behavioural and physiological markers of reward sensitivity were significantly correlated. Non-apathetic patients with CADASIL did not differ from controls on either task, whilst actual motor performance of apathetic patients in both tasks was also normal. Apathy was specifically associated with reduced fractional anisotropy within tracts connecting regions previously associated with effort-based decision-making. These findings demonstrate behavioural, physiological and anatomical evidence that dysfunctional effort-based decision-making underlies apathy in patients with CADASIL, a model disorder for sporadic small vessel disease. Reduced incentivization by rewards rather than hypersensitivity to effort costs drives this altered pattern of behaviour. The study provides empirical evidence of a cognitive mechanism for apathy in cerebral small vessel disease, and identifies a promising therapeutic target for interventions to improve this debilitating condition.
Introduction
Apathy, a disorder of motivation that manifests as a reduction in goal-directed behaviour, is an increasingly recognized complication of sporadic cerebral small vessel disease (SVD) (van der Mast et al., 2008; Hollocks et al., 2015; Lohner et al., 2017). Apathy is also a cardinal feature of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a monogenic form of SVD caused by mutations within the NOTCH3 gene and characterized by extensive damage to white matter brain regions (Chabriat et al., 2009; Reyes et al., 2009). It occurs in at least 40% of patients with CADASIL (Reyes et al., 2009). Furthermore, the presence of SVD may also modulate the development of apathy in other neurological conditions including Alzheimer’s disease and stroke (Lavretsky et al., 2008; Starkstein et al., 2009; Jonsson et al., 2010; Brodaty et al., 2013). However, the cognitive mechanisms underlying apathy remain poorly understood, despite increased recognition of its prevalence in many neurological and psychiatric conditions and its association with reduced quality of life and poor health outcomes (Lyketsos et al., 2002; Aarsland et al., 2009; Mayo et al., 2009; Treadway and Zald, 2011; Caeiro et al., 2012; Hollocks et al., 2015; Muhammed and Husain, 2016).
Prominent theories of apathy have considered it as a disorder of goal-directed behaviour (Marin, 1991; Levy and Dubois, 2006). However, goal-directed behaviour is itself a complex construct, relying on multiple cognitive processes (Balleine and O’Doherty, 2010). Although this general concept has proved useful, a mechanistic explanation for why goal-directed behaviour is reduced in apathy, based on dysfunction of specific cognitive processes, has remained elusive. Effort-based decision-making is a cognitive process that lies at the heart of normal goal-directed behaviour (Kurniawan et al., 2011; Rushworth et al., 2011). A recent framework has suggested dysfunction of this process as a possible cognitive mechanism underlying apathy (Le Heron et al., 2017). Effort-based decision-making describes the process of integrating information about the costs of actions and potential rewards resulting from them, in deciding whether a behaviour is worth performing (Kurniawan et al., 2011). It is proposed that such evaluations are crucial to understanding individual differences between how people act in daily life. For example, whether it is worth cleaning a house or mowing the lawn varies depending upon how individuals weigh up the costs of performing these actions compared to the potential rewards associated with these behaviours. Many studies have now demonstrated that such costs, rewards, and an integrated value signal (rewards – costs) are encoded neurally to drive goal-directed behaviour (Prevost et al., 2010; Rushworth et al., 2012).
Intriguingly the neural regions that subserve effort-based decision-making, including anterior cingulate cortex (ACC), orbitofrontal cortex (OFC) and ventral striatum (Croxson et al., 2009; Prevost et al., 2010; Schmidt et al., 2012; Klein-Flugge et al., 2016; Chong et al., 2017; Hauser et al., 2017), are the same regions that have been associated with apathy in neuroimaging studies across different modalities and neurological conditions (Kos et al., 2016; Le Heron et al., 2017). Furthermore, animal models of effort-based decision-making have causally linked damage to both ACC and ventral striatum with behavioural changes that resemble the clinical phenotype of apathy. Lesions to these regions induce a seemingly apathetic state in animals, in which they are no longer as willing to invest effort for reward (Salamone et al., 1994; Walton et al., 2002; Hauber and Sommer, 2009). Thus, disrupted effort-based decision-making provides a plausible cognitive mechanism for the reduced goal-directed behaviour that defines apathy. Such a disruption could result from reduced sensitivity to rewarding outcomes, increased sensitivity to effort costs, or a global reduction in willingness to engage, but which (if any) of these factors is related to apathy remains unknown.
Behavioural evidence for disruption of effort-based decision-making associated with apathy (Le Heron et al., 2018) or changes in motivation (Le Bouc et al., 2016) has recently been reported in Parkinson’s disease. Disrupted physiological responses to incentives have also been associated with apathy in this condition (Lawrence et al., 2011; Martinez-Horta et al., 2014; Muhammed et al., 2016). However, no mechanistic studies have been carried out in SVD—whether sporadic or genetic—and it is unclear whether the results from patients with Parkinson’s disease would generalize to a very different clinical group such as patients with SVD. Similarly, there is also a dearth of imaging studies of apathy in SVD compared to other neurodegenerative conditions, such as Parkinson’s disease (Wen et al., 2016) or Alzheimer’s disease (Theleritis et al., 2014). To date, there is only one report of the neural correlates of apathy in CADASIL (Jouvent et al., 2011), and, as far as we are aware, there is no previous report that has examined associations with specific changes in white matter tracts, despite this being the pathognomonic site of pathology in the disorder (Chabriat et al., 2009).
Apathy in sporadic SVD has also been associated with global burden of vascular disease; however, these whole-brain measures do not allow for a mechanistic interpretation of underlying causes, nor account for potential confounding effects of co-existent neurodegenerative pathology (Lavretsky et al., 2008; Jonsson et al., 2010; Kim et al., 2013; Attems and Jellinger, 2014; Hollocks et al., 2015). One recent study has examined the relationship between fractional anisotropy (a measure of white matter tract integrity) and apathy in sporadic SVD, reporting reductions within the anterior cingulum, uncinate fasciculus and fornix associated with apathy (Hollocks et al., 2015). These white matter tracts link brain regions strongly implicated in the development of apathy in other conditions—regions in which the process of effort-based decision-making is instantiated (Le Heron et al., 2017). However, no behavioural measures of decision-making were available in these patients. Overall, empirical evidence for disrupted effort-based decision-making is lacking in SVD, and only sparse in the general apathy literature. Furthermore, to the best of our knowledge, no study in any neurological condition has examined behavioural, physiological and anatomical components of effort-based decision-making in the same group of apathetic patients, limiting the strength of conclusions that can be drawn from the existing body of work.
Sporadic SVD is a significant public health burden and is the most common cause of vascular cognitive impairment and vascular dementia (Pantoni, 2010). However, it occurs in a predominantly elderly population in whom co-existent neurodegenerative pathology is often present, limiting the study of underlying neurobiological mechanisms (Medical Research Council Cognitive Function and Aging Study, 2001; Attems and Jellinger, 2014). CADASIL has very similar pathology and pattern of cognitive impairment to sporadic SVD, but without the co-existent neurodegenerative changes or significant co-morbidities due to the younger age of patients (Charlton et al., 2006; Chabriat et al., 2009). It therefore provides a model of pure SVD and vascular cognitive impairment in which to study mechanistic questions (Charlton et al., 2006; Duering et al., 2013).
Here, we examined reward and effort processing in patients with CADASIL, with and without clinical apathy, as well as matched healthy controls. We used behavioural, physiological and anatomical techniques in the same participants to investigate whether disrupted effort-based decision-making underlies apathy in this condition, and specifically whether alterations in this process were associated with reward insensitivity, effort hypersensitivity or global changes in willingness to engage. Participants completed an effort-based decision-making task in which they weighed up offers of monetary reward in return for exerting physical effort. Computational analysis of these choices provided an estimate of each person’s reward and effort sensitivity. This allowed us to determine if any observed changes in decision-making associated with apathy were specific to reward or effort processing. Additionally, a physiological marker of reward sensitivity—the degree of pupillary dilatation to reward—was determined using a previously validated eye movement task (Muhammed et al., 2016). The noradrenergic system is considered to mediate preparation of effort (Varazzani et al., 2015), and thus pupillary dilatation in response to rewards may provide an autonomic marker of motivation (Chiew and Braver, 2014; Manohar and Husain, 2015). This metric was compared between apathy and no-apathy groups, and to each person’s behaviourally derived reward sensitivity. Finally, using a tract-based spatial statistics (TBSS) analysis of diffusion imaging sequences (Smith et al., 2006), we assessed whether apathy is associated with disruption of tracts linking neural regions crucial for effort-based decision-making. Thus, we have used a multimodal approach to investigate whether specific changes in the process of effort-based decision-making underlie the clinical phenotype of apathy in patients with CADASIL, a model of pure SVD.
Materials and methods
Ethics
The study was approved by the local ethics committee and written consent was obtained from all subjects in accordance with the Declaration of Helsinki.
Participants
Nineteen patients with a genetically confirmed diagnosis of CADASIL, all with cysteine changing NOTCH3 mutations, were recruited from two regional specialist centres (in Cambridge and Oxford, UK). Exclusion criteria included physical disability such that a patient was unable to squeeze a hand-held dynamometer or previously documented large vessel stroke. Three patients with a history of lacunar stroke were included because this is an intrinsic feature of CADASIL, and is also a cardinal feature of sporadic SVD (Chabriat et al., 2009; Pantoni, 2010). Nineteen healthy age and gender matched controls were recruited via a local database.
All participants completed the behavioural and eye-tracking experiments. One patient with CADASIL did not undertake an MRI scan due to severe claustrophobia. Additionally, two further patients were excluded following image acquisition, one because of significant movement artefact and the other due to an acute clinical event occurring between the behavioural testing and the time of imaging (which had been delayed by 1 month). Demographics are presented in Table 1.
Table 1.
Demographics
| Measure | Control n = 19 | CADASIL n = 19 | P-value | CADASIL No apathy n = 8 | CADASIL Apathy n = 11 | P-value |
|---|---|---|---|---|---|---|
| Age | 54.5 ± 11.2 | 54.3 ± 10.3 | 0.96 | 55.8 ± 11.1 | 53.3 ± 10.1 | 0.62 |
| Gender, female/male | 12/7 | 13/6 | 0.73 | 6/2 | 7/4 | 0.6* |
| Apathy, LARS, range −36 to 36 | −28 ± 3.6 | −20 ± 11.8 | 0.007 | −29 ± 3.5 | −13.5 ± 11.4 | 0.0018 |
| Apathy, AES, range 0−72 | 30.8 ± 10.3 | 34.6 ± 10.4 | 0.27 | 26.9 ± 5.7 | 40.2 ± 9.6 | 0.0028 |
| GDS-totala, range 0−15 | 1 ± 1.8 | 5.7 ± 5.0 | <0.0001 | 2.1 ± 2.5 | 8.5 ± 4.6 | 0.003 |
| GDS-apathy, range 0−3 | 0.5 ± 0.9 | 1.2 ± 1.1 | 0.04 | 0.25 ± .046 | 1.9 ± 0.87 | <0.0001 |
| GDS-depression, 0−12 | 0.7 ± 1.4 | 4.5 ± 4.2 | <0.0001 | 1.9 ± 2.1 | 6.6 ± 4.3 | 0.012 |
| Quality of life: CANTRIL, range 0−10 | 8.1 ± 1.6 | 6.6 ± 2.2 | 0.017 | 8 ± 0.9 | 5.5 ± 2.3 | 0.010 |
| Quality of life: WHO5, range 0−25 | N/A | 13.8 ± 6.1 | N/A | 18.1 ± 3.5 | 10.6 ± 5.7 | 0.0005 |
Values in bold represent P < 0.05. AES = Apathy Evaluation Scale; GDS = Geriatric Depression Scale; LARS = Lille Apathy Rating Scale.
*Chi-square test.
aData for the GDS was missing for one participant.
Disease, cognitive and questionnaire measures
Apathy was assessed using the Lille Apathy Rating Scale (LARS) (Sockeel et al., 2006), and the Apathy Evaluation Scale (AES) self-report version (Marin et al., 1991; Clarke et al., 2007), which were both systematically administered to improve the sensitivity of the apathy diagnosis. Apathy was diagnosed if either the LARS score was >−22 or the AES score was >37 (equivalent to at least mild-moderate apathy). Physical signs were assessed using the National Institutes of Health Stroke Scale (Lyden, 2017). Baseline cognitive levels were formally assessed using the Addenbrookes’s Cognitive Examination III (ACE-III, Hsieh et al., 2013). Given previous work suggesting cognitive deficits in CADASIL particularly involve executive functions (Charlton et al., 2006), a digit span working memory task (Groth-Marnat, 2000), the Trail-Making Task parts A and B (Tombaugh, 2004), and a design fluency task in which participants were required to generate as many novel designs by connecting up to five dots with straight lines in a 2-min period (Tucha et al., 2012) were also administered. Depressive symptoms were assessed using the Geriatric Depression Scale (GDS), which has been the most extensive index used in SVD apathy research (van der Mast et al., 2008; Ligthart et al., 2012; Hollocks et al., 2015). Quality of life was assessed using a Cantril Ladder (Cantril, 1965), in which participants rated their overall current quality of life on a visual scale ranging from 1 to 10, and the WHO-5 Well-Being Index (Topp et al., 2015).
Effort-based decision-making paradigm
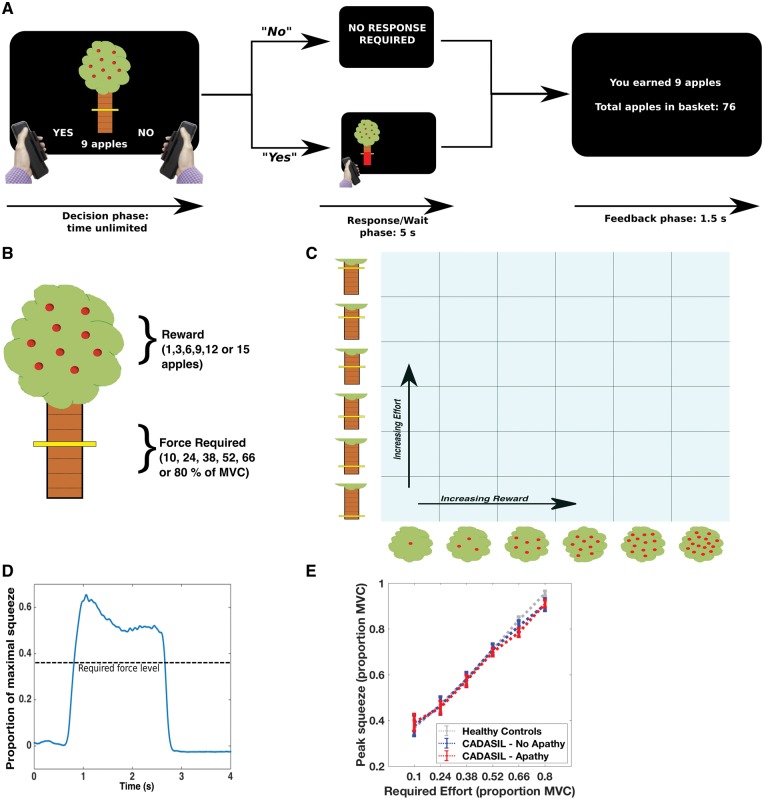
Participants were administered an effort-based decision-making task on a desktop computer running Pyschtoolbox (psychotoolbox.org) implemented within MATLAB (MathWorks, USA). They made sequential decisions of whether to accept an offer of reward in return for exerting effort via individually calibrated handheld dynamometers (SS25LA, BIOPAC Systems; Fig. 1A). Each offer was presented on the screen as a cartoon apple tree. Reward for the current trial was indicated by the number of apples on the tree (1, 3, 6, 9, 12 or 15) and numerically displayed underneath the tree. Each apple was worth 1p. Effort required to obtain the reward was indicated by the height of the yellow bar on the tree trunk, with the six possible levels corresponding to 10, 24, 38, 52, 66 and 80% of a participant’s maximal voluntary contraction (Fig. 1B). The six reward and six effort levels were systematically combined and the resultant 36 conditions were sampled evenly in a pseudo-randomized order across five blocks, for a total of 180 trials (Fig. 1C). This meant all participants received the same offers, presented in the same order.
Figure 1.
Effort-based decision-making task. (A and B) On a trial-by-trial basis participants were presented with offers of reward (apples on an apple tree, with each apple worth 1p) in return for exerting physical effort, ranging between 10% and 80% of a subject’s previously determined maximal voluntary contraction (MVC). If they accepted an offer (by squeezing the left-hand grip) the tree moved to the left or right of the screen, indicating which hand they had to respond with. They had a 5-s window within which to achieve the required force level. If they rejected the offer (by squeezing the right-hand grip) they waited the same 5-s period. (C) Participants worked through 180 trials, which pseudo-randomly, evenly sampled the 6 × 6 decision space over five blocks. (D) Example force trace from a single trial. (E) All groups (healthy controls, CADASIL no apathy and CADASIL apathy) modulated their force output to task requirements: mean ± standard error (SE).
Participants were instructed to weigh up the effort costs against the reward on offer for each trial, and decide ‘If it is worth it—is it worth squeezing that hard for that number of apples?’ If they accepted an offer (by exerting a small squeeze on the left hand-grip) they had to squeeze to the required force and hold above this level for at least 1 s within a 5-s response window, after which they were ‘rewarded’ with the apples on offer. During the squeeze, online force feedback was shown as a red bar that indicated current force relative to the target line. Conversely, if participants rejected an offer (by exerting a small squeeze on the right-hand grip) they waited an equivalent time (to control for temporal discounting effects) before moving onto the next offer. Therefore, on each trial participants decided whether the value of an offer was worth engaging with, compared to doing nothing for the equivalent time (Fig. 1A).
Before starting the experiment, participants practised each force level with each hand to familiarize themselves with the effort required, and completed a practice block in which they made decisions on the full range of options in the experiment. We included a number of features to reduce potential effects of fatigue on choice, including varying response side (signalled after an offer was accepted), only requiring subjects to squeeze on 75% of accepted trials and allowing subjects to rest between each block of 36 trials. In addition to choice (accept/reject), decision latency and force metrics (sampled at 500 Hz) were recorded for each trial (Fig. 1D and E; see Supplementary material for further details).
Eye movement paradigm
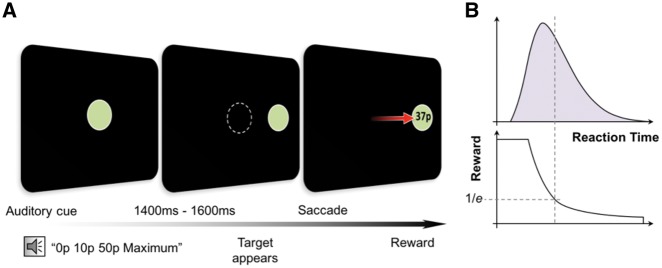
The eye movement task has been described in detail in a previous publication (Muhammed et al., 2016 and Supplementary material). Briefly, participants performed saccadic eye movements from a central fixation point to a peripheral target to earn monetary rewards, while pupil diameter and eye position were monitored using an infrared eye tracker (Eyelink 1000, SR Research; Fig. 2). They were seated 60 cm from a 21-inch CRT (1024 × 768 pixels; 100 Hz refresh rate) in a dimly lit room. Three levels of reward were used: 0p, 10p and 50p; representing the maximal amount available on that trial. Participants were informed of reward level on the current trial at the beginning via an audio cue whilst they maintained central fixation. Following a period of 1400–1600 ms, the central fixation point was extinguished and a peripheral target displayed, signalling participants to saccade to this target. Reward earned (as a proportion of the maximum available for that trial) depended on time to reach this target; however, this amount was dynamically adjusted dependent on performance over the preceding 20 trials. This maintained a consistent difficulty level across the experiment, accounting for potential confounding factors such as fatigue and baseline reaction time and ensuring equal rewards were earned by all participants. Participants performed five blocks of 54 trials each, with the three reward levels interleaved through each block. Disc luminance was matched across all trials so as not to affect pupil dilation. Baseline pupil size was recalculated at the beginning of each trial.
Figure 2.
Eye movement task. (A) Participants fixated on a disc displayed on a desktop monitor linked to an infrared eye tracker. At the beginning of each trial participants heard an auditory cue (‘0p/10p/50p maximum’) informing them the maximal amount they could win on that trial. Following a delay of 1400–1600 ms the central disc disappeared and concurrently a target disc appeared on one side, cueing a saccade to this location. Participants were rewarded with a proportion of the maximum amount available based on the time taken to reach the target. (B) Although the absolute amount of reward varied with reaction time, this was dynamically adjusted according to the mean reaction time of the last 20 trials. Therefore, difficulty level was maintained across the experiment and overall participants received equal reward amounts. Adapted from Muhammed et al. (2016) with permission.
Imaging protocol
Participants were scanned in a 3 T Siemens Verio scanner with a 12-channel head coil between January 2015 and June 2017. They were instructed to lie as still as possible. The neuroimaging protocol included diffusion-weighted (19:52 s), T1-weighted (4:54 s) and T2-FLAIR (4:32 s) sequences. Whole-brain diffusion weighted imaging was performed using an echo-planar sequence (repetition time = 8900 ms, echo time = 91.2 ms, voxel size = 2 mm isotropic, b-value = 1500 s/mm2). Images were obtained using diffusion-sensitizing gradients in 60 isotropically-distributed orientations optimized to evenly sample the whole diffusion sphere, acquired in opposite phase encoding directions (AP and PA) to allow for more robust distortion correction. 4 b = 0 images were obtained in each phase encoding direction.
Statistical analysis
Decision-making task
Choice data were analysed in two separate ways. We used a repeated measures ANOVA to test whether the proportion of offers accepted in each of the 36 (reward × effort) conditions varied as a function of reward, effort, apathy or their interactions. The dependent variable was arcsine transformed because it did not meet assumptions of linearity and a Greenhouse-Geisser correction was applied because assumptions of sphericity were not met (Mauchly’s test: ɛ = 0.317, P < 001). Residuals were examined following the analysis to ensure an approximately normal distribution.
We also used a computational model of choice to estimate, for each participant, the degree that reward and effort changed the value of an offer. We selected the model based on a comparison of candidate models (identified from prior decision-making literature) (Prevost et al., 2010; Chong et al., 2017), using standard methods (minimization of Bayesian information criterion and visual inspection of individual and group model fits to raw data); (Supplementary material and Supplementary Fig. 1).
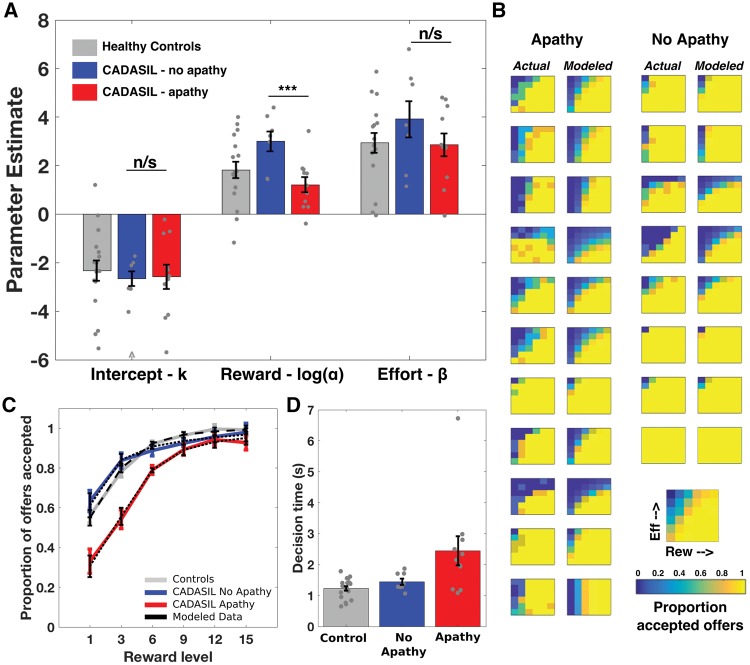
An exponential model closely approximated both individual and group average raw choices (Fig. 4B and C):
| (1) |
Here α estimated the degree that reward (discounted by effort) increased the value of an offer, β estimated the degree that effort reduced the value of an offer, and k the baseline tendency to accept an offer (the value of accepting an offer if zero reward was available). β and k parameter estimates, and log(α), were normally distributed and therefore compared using unpaired t-tests.
Figure 4.
Apathy in CADASIL and reward incentivization. (A) The reward parameter (α) was significantly lower in apathetic compared to non-apathetic patients, whilst neither the effort parameter (β) nor intercept (κ) were significantly different. (B) Actual and modelled choice proportions for each apathetic (left columns) and non-apathetic (right columns) CADASIL patient. (C) Group level choice proportions with increasing reward, with model fits superimposed (black dotted lines). (D) Apathetic patients took longer to make accept/reject decisions than non-apathetic patients or healthy controls. Note that one patient (no apathy) and one control accepted all offers and had outlying (positive) intercepts. For clarity the parameter estimates for these participants are not plotted, however they were included in the analysis (inclusion or exclusion did not change any reported results). ^Outlying value not plotted for clarity (actual value = –18). ***P < 0.01; n/s = not significant.
Decision time was analysed by repeated measures ANOVA with decision-type (accept or reject) a within subject variable and group (CADASIL-apathy, CADASIL-no apathy or control) a between subject variable. Decision time also provides a metric of task engagement. We predicted regions of decision space where subjects were more variable in their choice (and therefore more uncertain) would have longer decision times than ‘easier’ choices (for example regions of decision space that are always accepted or rejected). To test whether decision time was modulated by decision difficulty we split each participant’s decisions into easy (accepted <25% or >75%) and hard (accepted 25–75%), and analysed them by repeated measures ANOVA.
Eye movement task
The eye movement task data were analysed according to a previously published approach (Muhammed et al., 2016). Pupillometry analysis was performed using a one-way ANOVA to test for group differences in the variables of interest (CADASIL-apathy, CADASIL-no apathy and controls), and two-sided t-tests for subsequent comparisons of average pupil responsiveness between groups, with significance level set at P-values of < 0.05. Pearson correlations were used for parametric behavioural outcome comparisons. Time periods for averaging pupil responses were chosen based on past work (Muhammed et al., 2016). Additionally, differences in pupillary reward sensitivity were analysed over time at each millisecond time point using multiple permutation testing (to account for multiple comparison bias).
Diffusion image preprocessing and analysis
All images were analysed in FSL (Smith et al., 2004). Correction for susceptibility induced distortions, eddy currents and subject movement were performed using the FSL tools topup and eddy (Andersson et al., 2003; Smith et al., 2004; Andersson and Sotiropoulos, 2016) (Supplementary material). All images were visually inspected at each stage of processing.
White-matter voxelwise analysis of the fractional anisotropy imaging data was carried out using TBSS (Smith et al., 2006) (Supplementary material). We used randomise (Winkler et al., 2014) to carry out non-parametric voxelwise cross-subject statistics using 5000 permutations, and threshold-free cluster enhancement (TFCE) to correct for multiple comparisons across space. The P-value images for apathy < no apathy contrast in the CADASIL patients are displayed in the ‘Results’ section.
As a global metric of white matter integrity the mean fractional anisotropy for each participant was extracted from their skeletonized image, and group differences were tested for using a one-way ANOVA.
Data availability
Anonymized data are available on request.
Results
Demographic and mean values on questionnaire and baseline cognitive measures in the three groups are shown in Table 1 and Table 2, respectively. The percentage of CADASIL patients with apathy was 58%. CADASIL was associated with a reduced quality of life compared to controls, driven by the apathy group: F(2,37) = 8.7, P = 0.001; mean difference: CADISIL-apathy versus CADISIL-no apathy = 2.5, P = 0.01; mean difference: CADISIL-apathy versus healthy controls = 2.6, P = 0.001; mean difference: CADISIL-no apathy versus healthy controls = 0.1, P = 0.98. CADASIL was also associated with a higher level of depressive symptoms than controls, again driven by the apathy group: F(2,36) = 17.2, P < 0.001; mean difference: CADISIL-apathy versus CADISIL-no apathy = 4.7, P = 0.002; mean difference: CADISIL-apathy versus healthy controls = 5.9, P < 0.001; mean difference: CADISIL-no apathy versus healthy controls = 1.2, P = 0.53. Within the CADASIL group, apathy was not associated with significantly worse performance on baseline cognitive testing of executive functions, although they did trend towards a lower score on a global metric of cognition (ACE-III). There were no differences in motor signs between apathetic and non-apathetic patients.
Table 2.
Baseline cognitive testing
| Measure | Control | CADASIL | P-value | CADASIL No apathy | CADASIL Apathy | P-value |
|---|---|---|---|---|---|---|
| ACE | 96.6 ± 2.4 | 92.3 ± 5.3 | 0.003 | 94.9 ± 2.4 | 90.3 ± 6.1 | 0.064 |
| Digit Span | 6.1 ± 0.8 | 6.1 ± 0.8 | 6 ± 0.8 | 0.74 | ||
| Design Fluency | 30.7 ± 6.6 | 25.4 ± 9.3 | 0.053 | 28.6 ± 5.4 | 23 ± 11.0 | 0.20 |
| TMT − Aa | N/A | 30 ± 15.7 | N/A | 22.8 ± 8.0 | 35.8 ± 18.2 | 0.08 |
| TMT − Ba | N/A | 86.7 ± 54.3 | N/A | 65 ± 27.1 | 104.1 ± 65.1 | 0.13 |
| TMT − B-Aa | N/A | 56.7 ± 41.4 | N/A | 42.3 ± 25.2 | 68.3 ± 49 | 0.19 |
Values are mean ± SE. Values in bold represent P < 0.05. ACE = Addenbrooke’s Cognitive Examination III; TMT = Trail Making Task.
aData for the Trail Making Task was missing for one participant.
Apathetic CADASIL patients rejected more offers
First, we analysed the overall acceptance rates in the three groups using a one-way ANOVA. CADASIL patients with apathy accepted significantly fewer offers than patients without apathy or healthy controls, whilst acceptance rates did not differ between controls and non-apathetic patients [group effect: F(2,37) = 4.6, P = 0.017; mean difference: CADISIL-apathy versus CADISIL-no apathy = 0.14, t(17) = 2.5, P = 0.018; mean difference: CADISIL-apathy versus healthy controls = 0.13, t(17) = 2.77, P = 0.009; mean difference: CADISIL-no apathy versus healthy controls = 0.01, t(17) = 0.24, P = 0.81; Fig. 3A and B]. This global metric of offers accepted correlated significantly with the action initiation subscale of the LARS (ρ = 0.60, n = 19, P = 0.007; Fig. 3C).
Figure 3.
Effort-based decision-making in CADASIL apathy. (A and B) Apathetic patients accepted significantly fewer offers than non-apathetic patients or healthy controls. (C) The proportion of offers accepted was significantly correlated with the action initiation subscale of the LARS. (D) There was a significant apathy × reward interaction on offer acceptance. This plot shows the proportion of offers accepted as reward increases (collapsed across effort). Apathetic patients accepted fewer offers when rewarding outcomes were low, but performed the same as non-apathetic patients when rewards were high. (E) In contrast, there was no apathy × effort interaction. This plot shows offer acceptance as effort level increases (collapsed across reward). (F) The difference in offers accepted between no apathy and apathy groups occurred in a distinct region of decision space—where offers were for low rewards, irrespective of effort required. A, D and E show mean ± SE. **P < 0.05; ***P < 0.01; n/s = not significant.
Reduced incentivization by rewards in apathetic patients
To investigate the factors driving reduced acceptance of offers in the apathetic group, we used a repeated measures ANOVA to analyse how the proportional acceptance rate in each cell of the sampled 6 × 6 decision space (Fig. 1C) varied with reward, effort and apathy. The difference in responding was not global across the sampled decision space, but rather varied with reward, but not effort. There was a significant two-way interaction between apathy and reward (Fig. 3D), but not between apathy and effort (Fig. 3E): Reward × Apathy, F(1.7,29.4) = 4.3, P = 0.028; Effort × Apathy, F(1.6,26.9) = 0.40, P = 0.63.
That is, apathetic patients rejected significantly more offers when reward was low, but showed no difference compared to non-apathetic patients at high reward levels, irrespective of what the effort costs were (Fig. 3F). There were also significant main effects of apathy [F(1,17) = 5.0, P = 0.038], reward [F(1.7,29.4) = 38.4, P < 0.001] and effort [F(1.6,26.9) = 21.7, P < 0.001], and a two-way interaction between reward and effort [F(5.9,100.2) = 3.6, P = 0.003]. There was no three-way interaction between apathy, reward and effort [F(5.9,100.2) = 1.3, P = 0.25].
Next we compared the parameter estimates for reward (α), effort (β) and intercept (κ) obtained from the computational model of choice fitted to each participant’s decisions (Fig. 4A). Modelled data closely approximated raw choice data, both for each CADASIL patient (Fig. 4B) and for group average choice proportions (Fig. 4C). The reward sensitivity parameter estimate—which quantified the degree that the rewarding outcome of the action increased the value of an offer—was significantly reduced in apathetic CADASIL patients compared to the non-apathetic patients {mean difference [log(α)] = 1.67, t(17) = 3.32, P = 0.004}. In contrast, there was no significant difference between apathy and no apathy patients for the effort sensitivity parameter estimate [the degree that effort reduced the value of an offer: mean difference (β) = 0.47, t(17) = 0.50, P = 0.62] or for the intercept [the baseline tendency to accept an offer: mean difference (κ) = 0.84, t(17) = 0.50, P = 0.63].
Apathetic patients took longer to make effort-based decisions
The time each participant took to accept or reject an offer varied significantly by group, with both controls and non-apathetic CADASIL patients significantly faster than apathetic patients [main effect of group: F(2,33) = 5.8, P = 0.007; mean difference: CADISIL-apathy versus CADISIL-no apathy = 0.85 s, t(17) = 2.04, P = 0.05; mean difference: CADISIL-apathy versus healthy controls = 1.1 s, t(28) = 3.39, P = 0.002; mean difference: CADISIL-no apathy versus healthy controls = 0.27s, t(25) = 0.70, P = 0.49; Fig. 4D]. In contrast to this effect, apathetic patients were not significantly slower than non-apathetic patients on a neuropsychological measure of processing speed (Trail-Making Task B – A, P = 0.2, Table 1), nor did they show differences in reaction time when performing the eye-tracking task (P = 0.69, Supplementary Fig. 3C). Both apathetic and non-apathetic CADASIL patients showed the expected modulation of decision time by choice difficulty, taking longer to make hard compared to easy choices: F(1,29) = 44.76, P < 0.001; mean difference (hard - easy): CADISIL-apathy = 0.41 s, t(9) = 4.17, P = 0.002; mean difference: CADISIL-no apathy = 0.74s, t(5) = 3.93, P = 0.01. This difficulty effect was not significant in the control group [mean difference: healthy controls = 0.1s, t(15) = 1.43, P = 0.17], leading to an interaction between group and difficulty [F(2,29) = 9.1, P = 0.001; Supplementary Fig. 2A].
Participants in both CADASIL groups and the control group parametrically modulated their force output to task requirements, with no force × group interaction [main effect of force level: F(1.8,60.2) = 517.5, P < 0.001; force × group interaction F(3.5,60.2) = 1.4, P = 0.26, Fig. 1E]. Participants on average achieved required force levels >95% of the time (mean success rate for healthy controls, CADISIL-no apathy and CADISIL-apathy = 98.6%, 96.2% and 96.7%, respectively [CADISIL-apathy versus CADISIL-no apathy: mean difference = 0.5%, t(17) = 0.3, P = 0.75, Supplementary Fig. 2B]. Participants’ choices did not systematically change across the course of the experiment, with no effect of block number on acceptance rate in control [F(4,179) = 0.41, P = 0.8], non-apathetic [F(4,179) = 0.81, P = 0.52] or apathetic participants [F(4,179) = 0.15, P = 0.96, Supplementary Fig. 2C–E]. Furthermore, although apathetic patients reported higher levels of depressive symptoms (Table 1), there was no main effect of depression on proportion of offers accepted [F(1,16) = 2.2, P = 0.16], nor any interactions between depression and either reward or effort [Reward × Depression: F(1.6,25.4) = 0.47, P = 0.59; Effort × Depression: F(1.5,24.8) = 0.65, P = 0.49; Supplementary material].
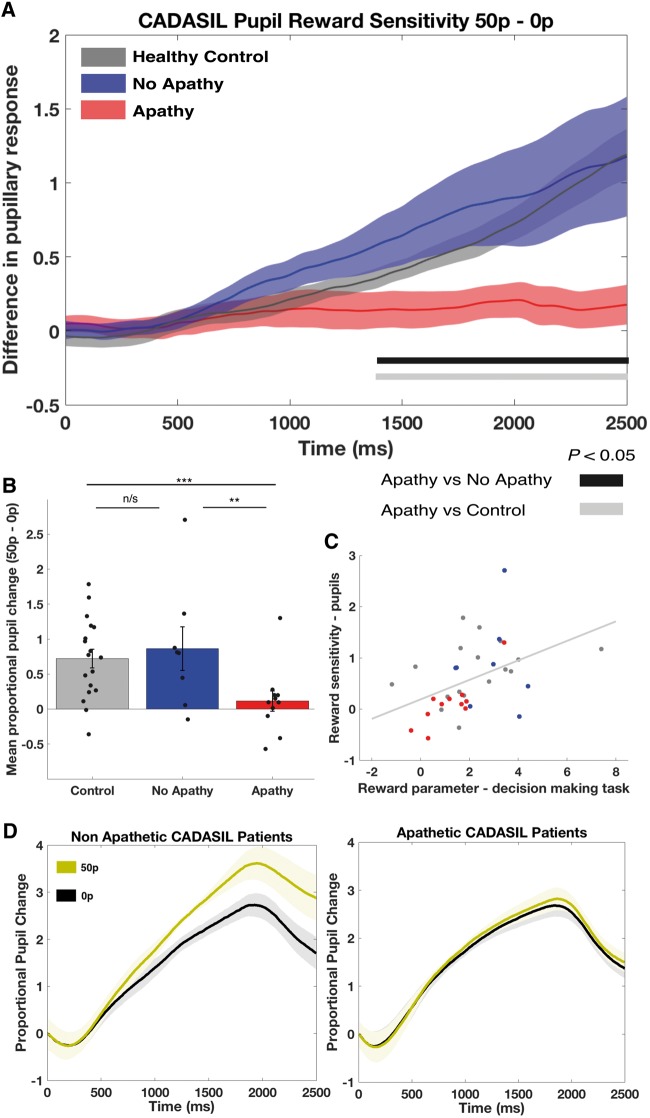
Apathetic CADASIL patients showed reduced autonomic responses to reward
Apathy in CADASIL was associated with blunted pupillary responses to reward, as measured by the proportional change in pupil size between high reward (50p) and low reward (0p) conditions of the eye movement task (Fig. 5A and B). There was a significant main effect of group on this metric: F(2,34) = 4.3, P = 0.021. This was driven by a reduced change in pupil size as reward increased in the apathy group, compared to both the no apathy group [mean difference in proportional change = 0.75 ± 0.29, t(17) = 2.39, P = 0.029] and the healthy matched controls [mean difference = 0.61 ± 0.24, t(27) = 2.94, P = 0.007; Fig. 5B]. There was no difference between non-apathetic CADASIL patients and controls [mean difference = 0.14 ± 0.27, t(24) = 0.49, P = 0.63]. Importantly, there was no significant difference in baseline pupil size between groups [F(2,34) = 0.68, P = 0.51], meaning the reduced response associated with apathy could not simply be explained by these patients having greater dilatation at the start of the experiment. Furthermore, there was no general deficit in pupil motility in the apathetic group (Fig. 5D). There was also no significant effect of depression on the proportional change in pupil size [mean difference (low depression - high depression) = 0.44 ± 0.37, t(16) = 1.2, P = 0.25].
Figure 5.
Autonomic responses to reward in CADASIL apathy. (A) Apathetic CADASIL patients had a significantly diminished pupillary response to increasing incentives compared to both non-apathetic patients and healthy controls. Reward sensitivity (indexed as the difference in pupil dilatation between the high and no reward conditions) was assessed at each millisecond time point using multiple permutation testing (to correct for multiple comparisons). Apathetic patients had significantly blunted pupil responses compared to non-apathetic patients and healthy controls from ~1380 ms to the end of each trial. (B) Mean reward sensitivity (time period 1400–2400 ms) was significantly lower in the apathetic group compared to non-apathetic patients and controls. (C) Physiological reward sensitivity, as indexed by pupillary response to reward, was significantly correlated with the reward parameter estimate (α) from the effort-based decision-making task; red dots = CADASIL apathy; blue dots = CADASIL no apathy; grey dots = controls. (D) Actual (proportional) changes in pupil dilatation for high (gold) and no (black) reward conditions, for non-apathetic and apathetic groups. Both groups demonstrate significant pupillary dilatation to auditory cue, however in the apathy group this response is not modulated by reward level. Mean ± SE shown in A and B. **P < 0.05; ***P < 0.01; n/s = not significant.
The degree of pupillary dilation to rewards was significantly correlated with the behavioural metric of reward sensitivity derived from the effort-based decision-making task (R = 0.45, n = 36, P = 0.006; Fig. 5C). Therefore, a reduction in reward incentivization on the decision-making task was associated with reduced physiological responses to reward on an independent eye movement task, and both these metrics were significantly diminished in the apathy group.
Apathy not associated with changes in saccadic velocity
There was no significant difference in peak saccadic velocity between apathetic and non-apathetic CADASIL patients or healthy controls [group difference (repeated measures ANOVA): F(2,34) = 0.24, P = 0.79; mean (no apathy) = 545 ± 25°/s; mean (apathy) = 523 ± 21°/s, P = 0.58]. There was a significant main effect of reward on velocity, with participants reaching a higher peak saccadic velocity as reward level increased [mean 0p = 525°/s; mean 10p = 531°/s; mean 50p = 534°/s, F(2,68) = 6.8, P = 0.002; Supplementary Fig. 3A]. There was no Reward × Group interaction, indicating this effect was present across all groups: F(4,68) = 1.43, P = 0.24. There was also no correlation between reward sensitivity indexed by change in saccadic velocity (50p – 0p) and the reward parameter estimate from the decision-making task (R = 0.074, n = 36, P = 0.678; Supplementary Fig. 3B).
Reduced white matter integrity in CADASIL apathy: tract-based spatial statistics
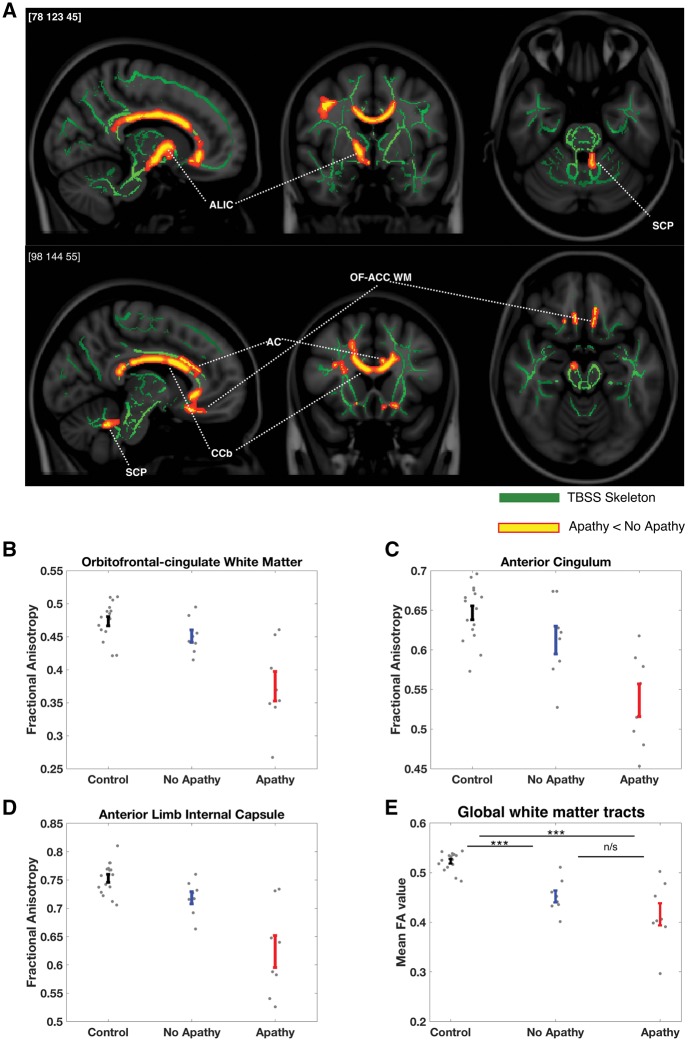
Three apathetic CADASIL patients were excluded from the imaging analysis [because of claustrophobia (one), excessive movement (one) and an acute abnormality on the scan (one)]. This left 16 CADASIL patients (eight apathetic, eight non-apathetic), and their 16 matched controls. Apathy was associated with significantly reduced fractional anisotropy in the left anterior cingulum, bilateral orbitofrontal-anterior cingulate white matter tracts, right anterior internal capsule, the body of the corpus callosum and the left superior cerebellar peduncle (Fig. 6A). There was also a significant reduction within a region of the right corona radiata. There were no regions where fractional anisotropy was significantly higher in apathetic compared to non-apathetic CADASIL patients.
Figure 6.
Changes in white matter tract integrity associated with apathy in CADASIL. (A) Apathetic CADASIL patients (n = 8) had significantly reduced white matter fractional anisotropy compared to non-apathetic CADASIL patients (n = 8) within the right anterior cingulum (AC), bilateral orbitofrontal-anterior cingulate white matter (OFC-ACC WM), right anterior limb of the internal capsule (ALIC), body of the corpus callosum (CCb) and left superior cerebellar peduncle (SCP): P < 0.05, TFCE-corrected in red-yellow, against the study-specific white matter tract skeleton in green. (B–D) The associated error bar plots (mean ± SE) show the average fractional anisotropy values from significant regions (from the apathy versus no apathy contrast) for control (n = 16) and CADASIL patients. Fractional anisotropy in the apathy group appears to be reduced compared with both non-apathetic patients and controls at each location, whereas control and non-apathetic patient values overlapped considerably. (E) In contrast, global mean fractional anisotropy values were reduced in both apathetic and non-apathetic CADASIL patients compared to controls, but did not significantly differ from each other.***P < 0.01; n/s = not significant.
We used these white matter regions showing significant differences between apathetic and non-apathetic patients to define masks at each location. Mean fractional anisotropy values extracted from these masks for the healthy controls and both patient groups suggest that disruptions in these white matter tracts were specific to the apathetic status of the CADASIL patients (Fig. 6B–D and Supplementary Fig. 4). Furthermore, apathetic and non-apathetic CADASIL patients did not differ in mean fractional anisotropy extracted across the entire TBSS skeleton [mean difference (fractional anisotropy) = 0.036, P = 0.15]. Rather, there was a general effect of disease with fractional anisotropy in both groups significantly lower than the controls [mean difference (no apathy versus controls) = 0.071, P < 0.001; mean difference (apathy versus controls) = 0.11, P < 0.001; Fig. 6E]. Finally, we tested whether fractional anisotropy values within the apathy-associated regions were associated with behavioural reward sensitivity. Fractional anisotropy values within orbitofrontal-anterior cingulate (OFC-ACC) white matter and the anterior limb of the internal capsule significantly predicted behavioural reward sensitivity, after first controlling for the effect of apathy. That is, patients with reduced white matter integrity within these regions also had lower sensitivity to rewarding outcomes on the decision-making task (OFC-ACC: ΔR2 = 0.2, F = 5.3, P = 0.04; ALIC: ΔR2 = 0.3, F = 5.8, P = 0.03; Supplementary material).
Discussion
This study provides evidence that disrupted effort-based decision-making underlies apathy in patients with CADASIL, with reduced reward sensitivity being a key marker that distinguished apathetic from motivated patients. Overall, apathetic individuals rejected more offers of reward for effort, but this change was not global across the sampled decision space. Rather it manifested only in a certain region of the reward-effort decision space: where rewarding outcomes were low (Fig. 3). Computational modelling of these decisions confirmed this change in responding was driven by reduced reward incentivization in the apathy group (Fig. 4), a finding that was mirrored by the blunted autonomic responses to rewards in these same patients as indexed by pupillary response to potential rewards (Fig. 5). Furthermore, white matter integrity was reduced within the apathy group specifically within tracts connecting neural regions crucial for reward processing and effort-based decision-making (Fig. 6).
Together these results demonstrate, through converging behavioural, physiological and anatomical methods, evidence for a specific cognitive mechanism underlying the reduced goal-directed behaviour that defines apathy in CADASIL. The concordance of the imaging findings with a previous study of apathy in sporadic SVD (Hollocks et al., 2015) suggest that these results may also be translatable to SVD more generally. Furthermore, the similar behavioural and physiological findings previously demonstrated in Parkinson’s disease (Muhammed et al., 2016; Le Heron et al., 2018) point to disrupted effort-based decision-making as a cognitive mechanism that may underlie apathy, across neurodegenerative diseases.
The change in effort-based decision-making associated with apathy manifested in the behavioural task as reduced engagement with a subset of offers—those in which the rewarding outcomes were low. Put another way, apathetic patients were prepared to exert as much effort as non-apathetic patients and healthy controls, even at high effort requirements, if the reward was high. This suggests that when apathetic patients fail to engage with a potential activity, it is not because the costs associated with acting are too high, but rather the outcome value of the action is not sufficient to drive behaviour towards it. Importantly, this reward insensitivity was also evident in the physiological responses of apathetic patients to reward. Their autonomic (pupil) responses to increasing reward levels were blunted in comparison to both non-apathetic patients and healthy controls. Furthermore these two markers of reward sensitivity (behavioural and physiological) were themselves correlated across all participants, consistent with them both measuring the same reward metric.
Importantly, non-apathetic patients and healthy controls did not differ in their behavioural or autonomic responses. Therefore, the observed changes were not simply a general feature of having CADASIL, and nor could they be explained by other confounders such as age or gender, which were matched between groups, or depression, which was not associated with significantly altered responses. Rather, reduced incentivization by reward was a specific feature of the apathetic state. Evidence for such reward insensitivity in apathy has previously been demonstrated following stroke (Rochat et al., 2013) and in Parkinson’s disease (Lawrence et al., 2011; Martinez-Horta et al., 2014; Muhammed et al., 2016; Le Heron et al., 2018). It suggests that the failure to activate goal-directed behaviour in apathy may relate to altered processing of reward information about potential actions (Levy and Glimcher, 2012; Rushworth et al., 2012), or in the subsequent integration of reward with effort costs to drive behaviour (Holroyd and Yeung, 2012).
Not all aspects of the reward-related response were significantly altered in patients with apathy. Specifically, peak saccadic velocities to the rewarding target were no different compared to either non-apathetic patients or healthy controls, an observation that has also been reported in Parkinson’s disease (Muhammed et al., 2016). Similarly, in the behavioural decision-making task their motor responses to the rewarding target were also normal. Therefore, although apathetic patients were less sensitive to reward value (whether indexed by choice or pupillary dilatation), once they had accepted an offer (behavioural task) or were cued to saccade (eye tracking task), their motor responses were unimpaired. One frequently emphasized feature of apathy is that it is a failure of self-generated goal-directed behaviour (Levy and Dubois, 2006; Sockeel et al., 2006; Robert et al., 2009). In both the paradigms used in the current study, motor responses were externally cued (by the appearance of a force level on a cartoon tree or a peripheral visual target, respectively), which may have effectively bypassed the disrupted mechanisms underlying apathy. This demonstrates that not all components of effortful behaviour for reward are disrupted in apathy and points to a potentially important, simple therapeutic strategy—providing external cues or prompts for action within an apathetic patient’s environment.
Prominent theories of behaviour have identified separate systems that can lead to actions. Whilst goal-directed systems explicitly represent information about action outcomes (rewards) and costs in determining whether to engage, habitual systems rely on simpler, previously learnt stimulus-response associations (Balleine and O’Doherty, 2010). Indeed, the technique of behavioural activation, which is thought to work via the same processes underlying habitual systems (instrumental conditioning), has been shown to be effective in treating depression (Ekers et al., 2014). Because of the relative ease of instituting such interventions, in our view, they would certainly be worth exploring further as potential non-pharmacological treatments for apathy. Such a strategy obviates the need for an effort-based decision to be made, activating behaviour independent of goal-directed systems, and could theoretically be tailored to an individual’s psychosocial backdrop and previous interests, for example directing behaviour towards hobbies that they have previously engaged with.
Another potential therapeutic strategy that might be important to explore is the possibility of increasing reward sensitivity. The findings in this study demonstrate that apathetic patients are not incentivized by low reward outcomes—the types of outcome associated with many of the everyday activities these patients fail to engage with. Modulating the reward system to amplify the incentivizing effects of reward could be achieved pharmacologically, with a number of neuromodulatory systems providing potential targets. This has been shown to occur with dopaminergic therapy in Parkinson’s disease (Le Bouc et al., 2016; Muhammed et al., 2016; Le Heron et al., 2018) and following basal ganglia stroke (Adam et al., 2013), whilst manipulation of serotonergic (Callegari et al., 2016) and cholinergic (Devos et al., 2014) systems, which are also closely linked to effort-based decision-making (Nunes et al., 2013), has also been shown to improve apathy. Whether such manipulations would have any impact on apathy in CADASIL or sporadic SVD remains to be established.
Apathetic patients with CADASIL were slower to make decisions about whether to accept or reject offers. Although this could be caused by a general slowing in processing speed associated with damage to frontal white matter connections (O’Sullivan et al., 2005), there were no significant differences in performance either on the paper-based trail making test, or reaction time for saccade initiation in the eye tracking task. This suggests that, in fact, the increased decision latency is a feature of changes in the decision process itself, consistent with the broader hypothesis of disrupted effort-based decision-making underlying apathy.
Anatomically, apathy was associated with reduced white matter integrity within specific regions: anterior cingulum, orbitofrontal-anterior cingulate white matter, anterior limb of the internal capsule and the body of the corpus callosum. Furthermore, white matter integrity in two of these regions (orbitofrontal-anterior cingulate white matter and anterior limb of internal capsule) was directly associated with behavioural reward sensitivity, after controlling for the presence of apathy. In contrast, apathy was not associated with overall burden of white matter disease (assessed by each participant’s mean global skeletonized fractional anisotropy value). Instead this metric was altered as a function of disease itself. These findings link the behavioural and physiological evidence of disrupted effort-based decision-making in CADASIL apathy to disruption of tracts that connect brain areas strongly implicated in this cognitive process (Prevost et al., 2010; Neubert et al., 2015; Klein-Flugge et al., 2016; Hauser et al., 2017).
These anatomical findings are consistent with studies of apathy in different conditions. The anterior cingulum is closely associated with the overlying anterior cingulate cortex, and reduced fractional anisotropy within this tract has been linked to apathy in sporadic SVD (Hollocks et al., 2015) and Alzheimer’s disease (Kim et al., 2011), as well as reduced motivation in healthy people (Bonnelle et al., 2016). The identified ventral white matter region associated with OFC-ACC lies medial to the uncinate fasciculus and may represent the amygdalofugal pathway, a tract that links amygdala and regions including ventral striatum and medial frontal cortex (Miller et al., 2010; Mori et al., 2017). The anterior limb of the internal capsule contains fibres projecting between anterior and mediodorsal thalamus and prefrontal and anterior cingulate cortex and has recently been associated with apathy in frontotemporal lobar degeneration (Lansdall et al., 2018). Strokes within these regions of the thalamus are also an important subcortical cause of apathy (Carrera and Bogousslavsky, 2006). Reduced fractional anisotropy in the body of the corpus callosum has been associated with apathy in progressive supranuclear palsy (Agosta et al., 2014) and other frontotemporal dementias (Lansdall et al., 2018). Furthermore, the medial cortical and subcortical regions connected by these tracts have been consistently associated with apathy across multiple disorders and imaging modalities (Lanctôt et al., 2007; Schroeter et al., 2011; Huang et al., 2013; Le Heron et al., 2017). It is within these regions that the core processes of effort-based decision-making to drive motivated behaviour are thought to be instantiated (Migneco et al., 2001; Croxson et al., 2009; Kennerley et al., 2009; Schroeter et al., 2011; Levy and Glimcher, 2012; Rushworth et al., 2012; Le Heron et al., 2017).
Although speculative, these apathy-associated white matter changes may provide a clue as to why reward modulation of pupil response is reduced in apathetic CADASIL patients. Pupil dilatation is influenced by noradrenergic projections arising with the locus coeruleus (Hou et al., 2005). However, the fact apathetic patients do not show a general deficit in pupil motility suggests the changes observed in this group may be more upstream. The locus coeruleus receives dopaminergic projections from the ventral tegmental area (VTA) (Samuels et al., 2006). In turn, the VTA has widespread afferent projections from prefrontal and cingulate cortex, subcortical and brainstem structures, which influence the firing rate of VTA neurons and subsequent dopamine release (Haber and Knutson, 2010). Additionally, it has been demonstrated previously that ACC and OFC lesions impair effort and reward-related autonomic arousal in both primates and humans (Critchley et al., 2003; Reekie et al., 2008; Manohar and Husain, 2016). Any compromise of projections from these regions to VTA, such as secondary to the reduced white matter integrity present in the apathy group, could theoretically alter regulation of locus coeruleus neurons by the VTA, leading to the observed loss of modulation by reward in the pupil responses of apathetic CADASIL patients.
In interpreting the findings from this study it is important to consider strengths and potential limitations. The lack of significant differences on measures of executive function as well as the specific rather than general changes in decision-making associated with apathy suggest the observations in the decision-making paradigm are unlikely to be explained by a confounding cognitive problem. Further, the apathetic group modulated their force production to force requirements, and showed the expected relationship between decision time and choice difficulty. The study protocol controlled for other potential confounders such as fatigue and temporal discounting. Baseline pupil size did not differ by group in the eye-tracking task, and the normal saccadic responses in the apathetic group is consistent with them attending to the task in the same manner as other participants. The sample size of 19 patients with CADASIL (and 16 for the imaging component) is a potential limitation and the findings in the study should be weighted against this. Although we note that it is difficult to perform extensive behavioural and imaging studies in this rare patient group, the results would merit replication in a different sample. It will be important in particular to replicate the tractography findings. However, CADASIL remains a rare condition (Chabriat et al., 2009) and there is no reason to consider the sampled population to be unrepresentative. Additionally, by sampling a smaller group we were able to perform a more in-depth, mechanistically focussed experiment, in a population that provides a model of pure vascular cognitive impairment (Charlton et al., 2006). The concordance of results across modalities strengthens the conclusions we can draw, as do the strong parallels we found between the behavioural and physiological responses of apathetic CADASIL patients and Parkinson’s disease patients performing the same tasks (Muhammed et al., 2016; Le Heron et al., 2018).
This study provides behavioural, physiological and anatomical evidence that disrupted effort-based decision-making underlies apathy in patients with CADASIL. Specifically, apathetic responses were characterized by reduced incentivization by the rewarding outcomes of actions. These changes manifested in apathetic patients rejecting lower reward offers in the decision-making task—offers that likely correspond to the rewarding outcomes of many everyday activities. This suggests that the reduced goal-directed behaviour that characterizes apathy occurs not because of the costs of making actions, but because patients do not derive sufficient drive from the outcomes of these actions. The concordance of the results with studies in other populations fits with a transdiagnostic model of apathy, in which the syndrome develops due to disruption of specific cognitive mechanisms (here reward incentivization and effort-based decision-making), irrespective of the underlying disease state. The reduced quality of life associated with apathy in this and other studies underlines the importance of developing treatments for it—treatments that this work suggests should be targeted at increasing the incentivising value of rewards.
Supplementary Material
Acknowledgements
We would like to thank all patients who volunteered their time to take part in this study.
Glossary
Abbreviations
- ACC
anterior cingulate cortex
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- OFC
orbitofrontal cortex
- SVD
cerebral small vessel disease
Funding
C.L.H. was funded by a University of Oxford Christopher Welch Scholarship in Biological Sciences, a University of Oxford Clarendon Scholarship and a Green Templeton College Partnership award. K.M. was funded by a Wellcome Trust Clinical Research Training Fellowship. G.D. acknowledges funding from the Medical Research Council UK (MR/K006673/1). H.S.M. is supported by an NIHR Senior Investigator award and his work is supported by infrastructure provided by the Cambridge University Hospital Trusts NIHR Biomedical Research Centre. His work on apathy in SVD is supported by a Priority Programme Grant from the Stroke Association (TSA PPA 2015-02). MH was funded by a grant from the Wellcome Trust (098282)
Competing interests
The authors report no competing interests.
References
- Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord 2009; 24: 2175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R, Leff A, Sinha N, Turner C, Bays P, Draganski B, et al. Dopamine reverses reward insensitivity in apathy following globus pallidus lesions. Cortex 2013; 49: 1292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Svetel M, Lukić MJ, Copetti M, Davidovic K, et al. Clinical, cognitive, and behavioural correlates of white matter damage in progressive supranuclear palsy. J Neurol 2014; 261: 913–24. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003; 20: 870–88. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016; 125: 1063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease—lessons from pathology. BMC Med 2014; 12: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 2010; 35: 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Manohar S, Behrens T, Husain M. Individual differences in premotor brain systems underlie behavioral apathy. Cereb Cortex 2016; 26: 807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouc R, Rigoux L, Schmidt L, Degos B, Welter M-L, Vidailhet M, et al. Computational dissection of dopamine motor and motivational functions in humans. J Neurosci 2016; 36: 6623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodaty H, Liu Z, Withall A, Sachdev PS. The longitudinal course of post-stroke apathy over five years. J Neuropsychiatry Clin Neurosci 2013; 25: 283–91. [DOI] [PubMed] [Google Scholar]
- Caeiro L, Ferro JM, Figueira ML. Apathy in acute stroke patients. Eur J Neurol 2012; 19: 291–7. [DOI] [PubMed] [Google Scholar]
- Callegari I, Mattei C, Benassi F, Krueger F, Grafman J, Yaldizli Ö, et al. Agomelatine improves apathy in frontotemporal dementia. Neurodegener Dis 2016; 16: 352–6. [DOI] [PubMed] [Google Scholar]
- Cantril H. The patterns of human concerns. New Brunswick: Rutgers University Press; 1965. [Google Scholar]
- Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology 2006; 66: 1817–23. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol 2009; 8: 643–53. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Morris RG, Nitkunan A, Markus HS. The cognitive profiles of CADASIL and sporadic small vessel disease. Neurology 2006; 66: 1523–6. [DOI] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Dissociable influences of reward motivation and positive emotion on cognitive control. Cogn Affect Behav Neurosci 2014; 14: 509–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T, Apps M, Giehl K, Sillence A, Grima LL, Husain M. Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol 2017; 15: e1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DE, van Reekum R, Simard M, Streiner DL, Freedman M, Conn D. Apathy in dementia: an examination of the psychometric properties of the apathy evaluation scale. J Neuropsychiatry Clin Neurosci 2007; 19: 57–64. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar B, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 2003; 126: 2139–52. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TEJ, Rushworth MFS. Effort-based cost-benefit valuation and the human brain. J Neurosci 2009; 29: 4531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D, Moreau C, Maltete D, Lefaucheur R, Kreisler A, Eusebio A, et al. Rivastigmine in apathetic but dementia and depression-free patients with Parkinson’s disease: a double-blind, placebo-controlled, randomised clinical trial. J Neurol Neurosurg Psychiatry 2014; 85: 668–74. [DOI] [PubMed] [Google Scholar]
- Duering M, Gonik M, Malik R, Zieren N, Reyes S, Jouvent E, et al. Identification of a strategic brain network underlying processing speed deficits in vascular cognitive impairment. Neuroimage 2013; 66: 177–83. [DOI] [PubMed] [Google Scholar]
- Ekers D, Webster L, Van Straten A, Cuijpers P, Richards D, Gilbody S. Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLoS One 2014; 9: e100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth-Marnat G. Neuropsychological assessment in clinical practice: a guide to test interpretation and integration. New York: Wiley; 2000. [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision-making. Cereb Cortex 2009; 19: 2240–47. [DOI] [PubMed] [Google Scholar]
- Hauser TU, Eldar E, Dolan RJ. Separate mesocortical and mesolimbic pathways encode effort and reward learning signals. Proc Natl Acad Sci USA 2017; 114: E7395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron C, Apps MAJ, Husain M. The anatomy of apathy: a neurocognitive framework for amotivated behaviour. Neuropsychologia 2017. doi: 10.1016/j.neuropsychologia.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron C, Plant O, Manohar SG, Ang Y, Jackson M, Lennox G, et al. Distinct effects of apathy and dopamine on effort-based decision-making in Parkinson’s disease. Brain 2018; 141: 1455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks MJ, Lawrence AJ, Brookes RL, Barrick TR, Morris RG, Husain M, et al. Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain 2015; 138: 3803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci 2012; 16: 122–8. [DOI] [PubMed] [Google Scholar]
- Hou RH, Freeman C, Langley RW, Szabadi E, Bradshaw CM. Does modafinil activate the locus coeruleus in man? Comparison of modafinil and clonidine on arousal and autonomic functions in human volunteers. Psychopharmacology 2005; 181: 537–49. [DOI] [PubMed] [Google Scholar]
- Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the addenbrooke’s cognitive examination iii in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord 2013; 36: 242–50. [DOI] [PubMed] [Google Scholar]
- Huang C, Ravdin LD, Nirenberg MJ, Piboolnurak P, Severt L, Maniscalco JS, et al. Neuroimaging markers of motor and nonmotor features of Parkinson’s disease: an 18f fluorodeoxyglucose positron emission computed tomography study. Dement Geriatr Cogn Disord 2013; 35: 183–96. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Edman Å, Lind K, Rolstad S, Sjögren M, Wallin A. Apathy is a prominent neuropsychiatric feature of radiological white-matter changes in patients with dementia. Int J Geriatr Psychiatry 2010; 25: 588–95. [DOI] [PubMed] [Google Scholar]
- Jouvent E, Reyes S, Mangin JF, Roca P, Perrot M, Thyreau B, et al. Apathy is related to cortex morphology in CADASIL: a sulcal-based morphometry study. Neurology 2011; 76: 1472–7. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci 2009; 21: 1162–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kang SJ, Kim C, Kim GH, Jeon S, Lee JM, et al. The effects of small vessel disease and amyloid burden on neuropsychiatric symptoms: a study among patients with subcortical vascular cognitive impairments. Neurobiol Aging 2013; 34: 1913–20. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lee DY, Choo IH, Seo EH, Kim SG, Park SY, et al. Microstructural alteration of the anterior cingulum is associated with apathy in Alzheimer disease. Am J Geriatr Psychiatry 2011; 19: 644–53. [DOI] [PubMed] [Google Scholar]
- Klein-Flugge MC, Kennerley SW, Friston K, Bestmann S. Neural signatures of value comparison in human cingulate cortex during decisions requiring an effort-reward trade-off. J Neurosci 2016; 36: 10002–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos C, van Tol MJ, Marsman JB, Knegtering H, Aleman A. Neural correlates of apathy in patients with neurodegenerative disorders, acquired brain injury, and psychiatric disorders. Neurosci Biobehav Rev 2016; 69: 381–401. [DOI] [PubMed] [Google Scholar]
- Kurniawan IT, Guitart-Masip M, Dolan RJ. Dopamine and effort-based decision-making. Front Neurosci 2011; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctôt KL, Moosa S, Herrmann N, Leibovitch FS, Rothenburg L, Cotter A, et al. A spect study of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord 2007; 24: 65–72. [DOI] [PubMed] [Google Scholar]
- Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, Vázquez Rodríguez P, Wilcox A, Wehmann E, et al. White matter change with apathy and impulsivity in frontotemporal lobar degeneration syndromes. Neurology 2018; 90: e1066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Zheng L, Weiner MW, Mungas D, Reed B, Kramer JH, et al. The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int J Geriatr Psychiatry 2008; 23: 1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Goerendt IK, Brooks DJ. Apathy blunts neural response to money in Parkinson’s disease. Soc Neurosci 2011; 6: 653–62. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol 2012; 22: 1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006; 16: 916–28. [DOI] [PubMed] [Google Scholar]
- Ligthart SA, Richard E, Fransen NL, Eurelings LSM, Beem L, Eikelenboom P, et al. Association of vascular factors with apathy in community-dwelling elderly individuals. Arch Gen Psychiatry 2012; 69: 636–42. [DOI] [PubMed] [Google Scholar]
- Lohner V, Brookes RL, Hollocks MJ, Morris RG, Markus HS. Apathy, but not depression, is associated with executive dysfunction in cerebral small vessel disease. PLoS One 2017; 12: e0176943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P. Using the national institutes of health stroke scale: a cautionary tale. Stroke 2017; 48: 513–19. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002; 288: 1475–83. [DOI] [PubMed] [Google Scholar]
- Manohar SG, Husain M. Reduced pupillary reward sensitivity in Parkinson’s disease. NPJ Park Dis 2015; 1: 15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar SG, Husain M. Human ventromedial prefrontal lesions alter incentivisation by reward. Cortex 2016; 76: 104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991; 3: 243–54. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy evaluation scale. Psychiatry Res 1991; 38: 143–62. [DOI] [PubMed] [Google Scholar]
- Martinez-Horta S, Riba J, de Bobadilla RF, Pagonabarraga J, Pascual-Sedano B, Antonijoan RM, et al. Apathy in Parkinson’s disease: neurophysiological evidence of impaired incentive processing. J Neurosci 2014; 34: 5918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo NE, Fellows LK, Scott SC, Cameron J, Wood-Dauphinee S. A longitudinal view of apathy and its impact after stroke. Stroke 2009; 40: 3299–307. [DOI] [PubMed] [Google Scholar]
- Migneco O, Benoit M, Koulibaly PM, Dygai I, Bertogliati C, Desvignes P, et al. Perfusion brain spect and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: a study in Alzheimer’s disease and nondemented patients. Neuroimage 2001; 13: 896–902. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Saint Marie LR, Breier MR, Swerdlow NR. Pathways from the ventral hippocampus and caudal amygdala to forebrain regions that regulate sensorimotor gating in the rat. Neuroscience 2010; 165: 601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Kageyama Y, Hou Z, Aggarwal M, Patel J, Brown T, et al. Elucidation of white matter tracts of the human amygdala by detailed comparison between high-resolution postmortem magnetic resonance imaging and histology. Front Neuroanat 2017; 11: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammed K, Husain M. Clinical significance of apathy in Parkinson’s disease. EMJ Neurol 2016; 4: 56–63. [Google Scholar]
- Muhammed K, Manohar S, Ben Yehuda M, Chong TT-J, Tofaris G, Lennox G, et al. Reward sensitivity deficits modulated by dopamine are associated with apathy in Parkinson’s disease. Brain 2016; 139: 2706–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert F-X, Mars RB, Sallet J, Rushworth MFS. Connectivity reveals relationship of brain areas for reward-guided learning and decision-making in human and monkey frontal cortex. Proc Natl Acad Sci 2015; 112: E2695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuropathology Group. Medical Research Council Cognitive Function and Aging Study Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 2001; 357: 169–75. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD. Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neurosci Biobehav Rev 2013; 37: 2015–25. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Barrick TR, Morris RG, Clark CA, Markus HS. Damage within a network of white matter regions underlies executive dysfunction in CADASIL. Neurology 2005; 65: 1584–90. [DOI] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- Prevost C, Pessiglione M, Metereau E, Clery-Melin ML, Dreher JC. Separate valuation subsystems for delay and effort decision costs. J Neurosci 2010; 30: 14080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reekie YL, Braesicke K, Man MS, Roberts AC. Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proc Natl Acad Sci U S A 2008; 105: 9787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S, Viswanathan A, Godin O, Dufouil C, Benisty S, Hernandez Z, et al. Apathy: a major symptom in Cadasil. Neurology 2009; 72: 905–10. [DOI] [PubMed] [Google Scholar]
- Robert P, Onyike CU, Leentjens AFG, Dujardin K, Aalten P, Starkstein S, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry 2009; 24: 98–104. [DOI] [PubMed] [Google Scholar]
- Rochat L, Van Der Linden M, Renaud O, Epiney JB, Michel P, Sztajzel R, et al. Poor reward sensitivity and apathy after stroke Implication of basal ganglia. Neurology 2013; 81: 1674–80. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Kolling N, Sallet J, Mars RB. Valuation and decision-making in frontal cortex: one or many serial or parallel systems? Curr Opin Neurobiol 2012; 22: 946–55. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron 2011; 70: 1054–69. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res 1994; 65: 221–9. [DOI] [PubMed] [Google Scholar]
- Samuels ER, Hou RH, Langley RW, Szabadi E, Bradshaw CM. Comparison of pramipexole and amisulpride on alertness, autonomic and endocrine functions in healthy volunteers. Psychopharmacology 2006; 187: 498–510. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Lebreton M, Clery-Melin M-L, Daunizeau J, Pessiglione M. Neural Mechanisms underlying motivation of mental versus physical effort. PLoS Biol 2012; 10 Available from: file::/WOS:000300951500011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Vogt B, Frisch S, Becker G, Seese A, Barthel H, et al. Dissociating behavioral disorders in early dementia—an FDG-PET study. Psychiatry Res Neuroimaging 2011; 194: 235–44. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: S208–19. [DOI] [PubMed] [Google Scholar]
- Sockeel P, Dujardin K, Devos D, Denève C, Destée A, Defebvre L. The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: validation in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2006; 77: 579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mizrahi R, Capizzano AA, Acion L, Brockman S, Power BD. Neuroimaging correlates of apathy and depression in Alzheimer’s disease. J Neuropsychiatr 2009; 21: 259–65. [DOI] [PubMed] [Google Scholar]
- Theleritis C, Politis A, Siarkos K, Lyketsos CG. A review of neuroimaging findings of apathy in Alzheimer’s disease. Int Psychogeriatr 2014; 26: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004; 19: 203–14. [DOI] [PubMed] [Google Scholar]
- Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom 2015; 84: 167–76. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 2011; 35: 537–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucha L, Aschenbrenner S, Koerts J, Lange KW. The five-point test: reliability, validity and normative data for children and adults. PLoS One 2012; 7: e46080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mast RC, Vinkers DJ, Stek ML, Bek MC, Westendorp RGJ, Gussekloo J, et al. Vascular disease and apathy in old age. the leiden 85-plus study. Int J Geriatr Psychiatry 2008; 23: 266–71. [DOI] [PubMed] [Google Scholar]
- Varazzani C, San-Galli A, Gilardeau S, Bouret S. Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. J Neurosci 2015; 35: 7866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MFS. The role of rat medial frontal cortex in effort-based decision-making. J Neurosci 2002; 22: 10996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M-C, Chan LL, Tan LCS, Tan EK. Depression, anxiety, and apathy in Parkinson’s disease: insights from neuroimaging studies. Eur J Neurol 2016; 23: 1001–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are available on request.