To the Editor: Arteriovenous malformations (AVM), also referred to as cirsoid aneurysm, arteriovenous aneurysm, arteriovenous fistula, and cavernous hemangioma, can occur in any organ in the body. Uterine AVM is a rare entity that is classified as either congenital or acquired. Congenital uterine AVM is thought to develop from a defect during embryologic differentiation leading to abnormal vascular connections, whereas acquired uterine AVM has been reported to occur due to a previous uterine trauma, such as curettage or cesarean delivery, or to be associated with neoplastic disorders, including gestational trophoblastic disease (GTD) and endometrial adenocarcinoma.[1]
Although uterine AVM is uncommon, it can cause irregular uterine bleeding and even can provoke massive life-threatening uterine hemorrhaging; approximately 30% of patients require blood transfusions.[2] Due to the dangerous conditions, promptly diagnosing and treating of uterine AVM are necessary. Therefore, we retrospectively analyzed the diagnosis and management of uterine AVM in 62 women to guide gynecologists a further understanding of the disease, thus help them identify uterine AVM and give the appropriate treatment in time.
The study was approved by the Peking Union Medical College Hospital (PUMCH) ethics committee. All participants provided written informed consent.
All patients who presented to the PUMCH from January 1, 2004 and January 31, 2017 and were diagnosed with uterine AVM using uterine artery angiography or postoperative pathology were eligible for inclusion in this study. Sixty-two patients were included in the study. Clinical data to be extracted from the medical records included patient age, reproductive status, surgical history, clinical presentation, inciting event, or treatment rendered. The color Doppler ultrasound equipment was Philips IU22 or GE Logiq9 with the customary frequencies of 2–12 MHz. Patients conducted uterine artery angiography were referred to the Department of Interventional Radiology. An experienced operator performed the diagnostic angiogram using selective or superselective digital subtraction angiography, followed by the therapeutic intervention that consists of embolization of uterine artery branch or internal iliac artery branch. Embolic agents commonly used during this procedure were absorbable gelatin sponge particles (Gelfoam, 1400–2000 μm, Hangzhou Alicon Pharm SCI and TEC Co. Ltd., Hangzhou, China) or coils (Cook, Bloomington, IN, USA). Then, the prognosis of these patients including remote complications, menstruation, and childbirth was acquired by outpatient or telephone follow-up. The median follow-up was 2.5 years (1 month to 12 years).
All the statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean/median (range) or mean ± standard deviation. Fisher's exact test was adopted to compare group differences for fourfold table data. Statistical significance was defined as P < 0.05.
The mean age of these women was 31.3 years old (18–42 years old). The median numbers of pregnancies and live births among these women were 3 (1–9) and 1 (0–3), respectively. All included patients were diagnosed with acquired uterine AVM secondary to curettage, cesarean section, or GTD. Among the 62 patients, 21 (33.9%) had a history of cesarean section, 53 (85.5%) had a history of curettage, 18 (29.0%) had a history of both cesarean section and curettage, and the other 6 (9.5%) patients had no history of either cesarean section or curettage, including 4 had a history of medical abortion and the other 2 had a history of vaginal delivery. The median numbers of cesarean sections and curettages were 0 (0–3) and 2 (0–8), respectively. Meanwhile, 14 (22.6%) patients had a history of GTD.
Fifty-eight of the 62 patients (93.5%) were referred to the hospital due to various amounts of vaginal bleeding and the other four patients without any symptom were detected to have uterine AVM by medical examination. Among these 58 bleeding patients, the inciting event or procedure was miscarriage, including artificial abortion and medium induction with or without curettage in 45 (77.6%), full-term normal delivery in 3 (5.2%), cesarean section in 3 (5.2%), and GTD in 7 (12.1%). The median interval between the inciting event and the initial vaginal bleeding was 28.5 days (0 day–17 years). With regard for hemorrhage presentation, 46 (79.3%) patients presented with irregular vaginal bleeding, 7 (12.1%) with menorrhagia or menstrual extension and 5 (8.6%) with copious bleeding during curettage. Among these patients, 30 (48.4%) had episodes of excessive bleeding. Thirty-three of the 62 patients (53.2%) suffered hemorrhagic anemia, and the average hemoglobin level was 97.7 ± 28.2 g/L. Nine patients (14.5%) experienced hemorrhagic shock and 25 patients (40.3%) received blood transfusions.
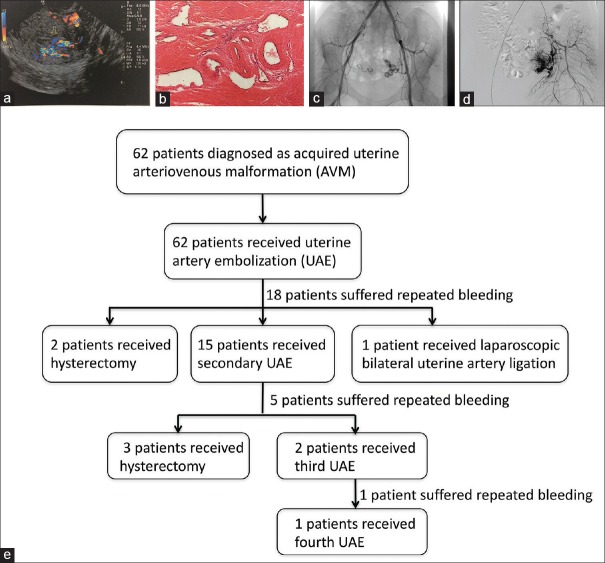
In 52 patients, color Doppler imaging revealed a hypoechogenic structure within the myometrium that exhibited high-velocity and low-impedance flow [Figure 1a], suggesting that uterine AVM had developed in these patients. In the eight patients who were not examined by color Doppler imaging and two (3.6%) patients in whom color Doppler imaging revealed no AVM, the results of selective uterine artery angiography confirmed these cases to be uterine AVM. It is worth mentioning that four patients with no symptoms were found to have uterine AVM on color Doppler imaging. Among these four patients, one of them came to our hospital for follow-up after chemotherapy due to GTD, one for subsequent visit after artificial abortion, and the other two for pregnancy termination.
Figure 1.
Images of uterine AVM acquired from different examinations. (a) Color Doppler ultrasonography shows hypoechogenic structures within the myometrium with high-velocity, low-impedance flow. (b) Histopathology image showing the dilated and irregularly shaped blood vessel in the uterine muscular layer (hematoxylin-eosin staining, original magnification × 60). (c and d) Images taken from the same patient. (c) Bilateral uterine artery angiography shows the left uterine AVM. (d) Selective left uterine artery angiography shows a clearer image of AVM. (e) Management procedures of patients diagnosed with uterine AVM. AVM: Arteriovenous malformations.
All patients who underwent selective uterine artery angiography, imaging showed a well-visualized AVM with tortuous expansion of the blood vessels and early filling veins [Figure 1c and 1d].
Hysterectomy was performed in five patients (8.1%) in whom postoperative histopathological analysis confirmed the presence of dilated and irregularly shaped blood vessels in the uterine muscular layer [Figure 1b].
The management flowchart is displayed in Figure 1e. All the women received support for symptoms and other care, including anti-infection, hemostasis, or fluid-supplement therapy.
All patients initially underwent pelvic angiography with therapeutic uterine artery embolization (UAE) using absorbable gelatin sponges or coils. However, 18 of 62 patients (29%) had recurrent uterine bleeding resulting in a subsequent procedure at a median time of 4 months (3 days to 8 years). Second procedures included a second embolization (15), hysterectomy (2), or laparoscopic bilateral uterine artery ligation (1). Bleeding was controlled after the first embolization procedure in 44 of 62 patients (71.0%). In another ten patients (16.1%), bleeding was controlled by the second embolization procedure, while one patient (1.6%) required a third embolization and one patient (1.6%) needed a fourth embolization to control the bleeding. Another three patients underwent hysterectomy after a second embolization. After every UAE procedure, an angiography was performed to confirm that the uterine artery was blocked.
Among these 62 patients, 59 (95.2%) underwent bilateral UAE for uterine bleeding, while 3 (4.8%) underwent unilateral UAE due to the difficulty of performing embolization in the opposite artery. One of these three had recurrent bleeding because of reperfusion of the contralateral artery and underwent a second embolization of the opposite artery 5 months later, while the other two stopped bleeding after the first unilateral UAE. There was no significant difference in the success rate of hemostasis between patients who underwent unilateral and bilateral embolization (66.7% [17/59] vs. 71.2% [1/3], respectively; P = 1.0).
Moreover, curettage or lesion resection was performed in 11 patients after UAE to treat other obstetric conditions, such as intrauterine remainder (6), cesarean scar pregnancy (3), and pregnancy termination (2). In these cases, UAE was not only performed to block the fistula and stanch bleeding but also as a management practice to prevent recurrent bleeding during the subsequent intrauterine operation.
In all, 19 patients (30.6%) reported pelvic pain and 13 patients (21.0%) reported fever for a few days shortly after the operation, and these were regarded as postembolization syndrome and were easily controlled. One patient (1.6%) was suspected to have a pulmonary embolism due to low blood oxygen saturation after UAE. This patient underwent an urgent tracheal intubation and mechanical ventilation for 2 days until the blood oxygen saturation returned to normal.
Among the 57 patients who underwent UAE in which the uterus was preserved, 16 were lost to follow-up, 3 took contraceptive measures, and 10 subsequently became pregnant. The mean time between the embolization procedure and pregnancy was 26.5 months (range: 13–60 months). Among these patients, seven underwent cesarean section and gave birth to a single healthy baby and two patients delivered vaginally. One patient is currently 8-week pregnant.
The first case of uterine AVM was reported by Dubreuil and Loubat in 1926.[3] Acquired uterine AVMs tend to occur in women of reproductive age. The most common presenting of uterine AVM is hemorrhage of unknown causes, including menorrhagia or menstrual extension and copious bleeding during curettage and irregular vaginal bleeding. Some patients even present with shock due to sudden or massive vaginal bleeding and curettage does not stop the bleeding or may even exacerbate this condition.
As a noninvasive imaging procedure, color Doppler ultrasonography is universally used to diagnose uterine AVM. The characteristic of uterine AVM observed by color Doppler ultrasonography is classically hypoechoic tortuous spaces in the myometrium indicating vascular flow and spectral analysis of the vessels shows low-impedance and high-velocity flow.[4] It is difficult to distinguish uterine AVM from GTD using color Doppler ultrasonography. Thus, the serial beta-human chorionic gonadotropin levels are measured to exclude possible GTD. Meanwhile, in some patients, acquired uterine AVM is coexistence with other pregnancy-related conditions, such as intrauterine residual, cesarean scar pregnancy, or termination of pregnancy for various reasons. When color Doppler ultrasonography identifies potential uterine AVM in these pregnancy-related conditions, UAE should be conducted before intrauterine operation to prevent the possible postoperative hemorrhage. Pelvic angiography remains “gold standard” for diagnosis of uterine AVM.[5]
Management of uterine AVMs depends on many factors including the patient's hemodynamic status, age, and desire for future fertility. In the earliest reports, hysterectomy and ligation of the internal iliac or the uterine arteries were the preferred treatment. Since the first UAE procedure reported by Forssman et al.,[6] UAE gradually became widely accepted due to its preservation of fertility.
Although UAE had the ideal effect of closing the fistula and arresting bleeding, it was not always successful. According to our study, the success rate of the first UAE treatment for uterine AVM is 71% (44/62).
Fertility after UAE remains speculative. Despite advances in therapeutic techniques and embolic agents, pregnancy following successful embolization of uterine AVMs remains rare.[2] Decreased vascularization of the placenta has been proposed as being the main cause of adverse pregnancy outcomes following embolization. In our study, ten patients had successful pregnancies after first or second embolization, which appears to have no impact on their fertility, with nine single live births by cesarean section or vaginal delivery. Some patients had already had babies and chose contraceptive measures, and it is, therefore, possible that childbearing capacity was retained by more patients. Moreover, some patients with uterine AVM also had other obstetric conditions that may have influenced their fertility. Therefore, we agree with the view that UAE does not impair fertility. Meanwhile, arterial flow to the ovary is likely to be transiently occluded during UAE but may be reestablished in the longer term. Despite this occlusion, the incidence of clinically apparent injury to ovarian function is low.[7]
Uterine AVM is a rare and dangerous clinical condition, and it should be included in the differential diagnosis of patients with excessive postpartum or postinstrumentation bleeding. Color Doppler ultrasonography can aid in the diagnosis and clinical management of these patients. To prevent possible hemorrhage after an intrauterine operation, all these patients with a history of uterine trauma or neoplastic disorder are recommended to undergo color Doppler ultrasonography to exclude uterine AVM. Finally, UAE is a safe and effective therapy for treating uterine AVM and should be used as an initial treatment for women of childbearing age who wish to preserve their fertility.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Picel AC, Koo SJ, Roberts AC. Transcatheter arterial embolization with n-butyl cyanoacrylate for the treatment of acquired uterine vascular malformations. Cardiovasc Intervent Radiol. 2016;39:1170–6. doi: 10.1007/s00270-016-1328-z. doi: 10.1007/s00270-016-1328-z. [DOI] [PubMed] [Google Scholar]
- 2.Vijayakumar A, Srinivas A, Chandrashekar BM, Vijayakumar A. Uterine vascular lesions. Rev Obstet Gynecol. 2013;6:69–79. doi: 10.3909/riog0207. [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming H, Ostor AG, Pickel H, Fortune, DW Arteriovenous malformations of the uterus. Obstet Gynecol. 1989;73:209–14. [PubMed] [Google Scholar]
- 4.Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovács S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity/arteriovenous malformations. Am J Obstet Gynecol. 2016;214:731.e1–731.e10. doi: 10.1016/j.ajog.2015.12.024. doi: 10.1016/j.ajog.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Szpera-Goździewicz A, Gruca-Stryjak K, Bręborowicz GH, Ropacka-Lesiak M. Uterine arteriovenous malformation – Diagnosis and management. Ginekol Pol. 2018;89:276–9. doi: 10.5603/GP.a2018.0047. doi: 10.5603/GP.a2018.0047. [DOI] [PubMed] [Google Scholar]
- 6.Forssman L, Lundberg J, Scherstén T. Conservative treatment of uterine arteriovenous fistula. Acta Obstet Gynecol Scand. 1982;61:85–7. doi: 10.3109/00016348209156958. [DOI] [PubMed] [Google Scholar]
- 7.Kaump GR, Spies JB. The impact of uterine artery embolization on ovarian function. J Vasc Interv Radiol. 2013;24:459–67. doi: 10.1016/j.jvir.2012.12.002. doi: 10.1016/j.jvir.2012.12.002. [DOI] [PubMed] [Google Scholar]