Abstract
Introduction
To compare earlier and later patient groups with Fournier's gangrene, specifically with the incidence of rising antibiotic resistance rates in mind. Primary endpoints were to compare therapy, outcomes, and resistance rates.
Material and methods
A multicentric, retrospective, multi-national study was performed. Two groups with different time frames of treatment were defined: Group 1 (n = 50) and Group 2 (n = 104). Demographics and outcomes were analysed using Student-t test, chi-square test, or Fisher exact test. Survival data were estimated using the Kaplan Meier method and compared by Log rank testing.
Results
There were no significant demographic differences. Nor was there any significant difference in therapy or outcomes in the groups except for the duration of intensive care unit treatment, which lasted a mean 6.3 days in Group 1 and 11.5 days in Group 2 (p = 0.018). Survival time did not improve over the years (p = 0.268). We fortunately did not observe an increased rate of multi-resistant organisms (p = 1.000). This study's limitations are mainly due to its retrospective study design.
Conclusions
Despite increasing antibiotic resistance rates worldwide, it was not apparent in our population. But the situation for these patients is alarming, since final outcome failed to improve over the last ten years despite more intensive critical-care therapy.
Keywords: Fournier's gangrene, urological infections, multi-resistant pathogens, outcome measurements
INTRODUCTION
Fournier's gangrene (FG) is a very rare, life-threatening, necrotizing infection affecting the perineum, perineal region and genitals [1, 2]. As it occurs so seldom, most of the limited knowledge about FG arises from retrospective single-institutional studies with very small patient cohorts [3–15]. Unfortunately, FG also has a poor prognosis. Early studies of FG reported a 20 to 88% mortality rate [1; 16–18], but two studies from 2017 calculated a mortality rate of 25 to 26% [2, 11].
Multi-drug-resistant organisms, including MRSA, are emerging pathogens in FG. Chia et al. described in their retrospective analysis of FG patients that 21% had a multi-drug-resistant organism, with MRSA being the most common pathogen. Those with a multi-drug-resistant organism were also more likely to have a poor outcome (42% versus 28%) [5]. Since rising levels of antibiotic resistance rates are a serious problem in urogenital infections worldwide [19], FG is likely affected by that development as well, and, therefore, the morbidity and mortality of FG patients.
In light of rising antibiotic resistance rates and the paucity of data on FG in general, we performed a multicentric multi-national retrospective study comparing an earlier and later patient group. Primary endpoints were to compare therapy, outcome, and resistance rates. Secondary endpoints were the identification of risk factors for death and multi-resistant pathogens.
MATERIAL AND METHODS
Development of the study and study population
This study was designed according to the guidelines in the synthesis of qualitative research (ENTREQ) found on the equatornetwork.org, an international initiative providing robust reporting guidelines [20].
We performed a multicentric retrospective study at ten centres. Four of these were German University Medical Centres, four German hospitals providing tertiary care, and two tertiary-care hospitals in Austria. Patient data were collected retrospectively and consecutively until December 2016. Two groups with different treatment time frames were defined: Group 1 was treated between January 2006 and December 2010, and Group 2 between January 2011 and December 2016. All patients treated in these time frames were included and analysed. Data from patients' treatment records included age, sex, body mass index (BMI), human immunodeficiency virus (HIV) status, patient history of diabetes, alcohol abuse, as well as colorectal cancer, usage of scoring systems for FG, blood count, CRP, serum urea, HbA1c, sepsis or septic shock (according to SIRS criteria, see section 2.2. below for definition), intensive care unit (ICU) treatment and duration of ICU treatment, surgery data as well as number of wound toilets and other wound procedures like vacuum-assisted wound closure (VAC), urinary diversions used, all microbiological information including antibiotics and antimycotics used, death and finally, duration of inpatient treatment. The data was documented with the database program Microsoft Excel and then transferred to an SPSS 24.0 data bank for statistical analysis.
All study procedures followed the ethical standards of the institutional and/or national research committee and complied with the 1964 Helsinki Declaration and its later amendments for comparable ethical standards. For this type of study, formal consent was not required.
Definitions and statistical analysis
Sepsis was defined as proven infection in the presence of systemic inflammatory response syndrome (SIRS). Its criteria are fever (≥38.0 degrees Celsius) or hypothermia (≤36.0 degrees Celsius), tachycardia (≥90/min), tachypnea (≥20/m in) and leukocytosis (≥12.000/μl) or leukocytopenia (≤4.000/μl). If the blood culture was negative, all four of the SIRS criteria had to be fulfilled to diagnose sepsis. If the blood culture was positive, two SIRS criteria sufficed to diagnose sepsis. Septic shock meant severe sepsis with hypotension despite adequate fluid resuscitation.
Mixed flora was defined as presence of two or more pathogens in the microbiological specimen.
For each numeric variable, the numeric distribution was preliminarily assessed by the Kolmogorov-Smirnov test. Descriptive statistics were analysed with mean and standard deviation for normal distribution or with median and interquartile range (IQR) for non-parametric data. For parametric continuous variables the Student-t test was used and for parametric categorical variables the chi-square test or the Fisher exact test was used. To assess risk factors, the univariate Cox regression method was used, and significance was tested with the Wald statistic. Kaplan-Meier plots were used to estimate median overall survival, and univariate comparisons were performed using the log rank test. All reported p-values were based on a two-sided hypothesis, p <0.05 was considered to be significant. All statistical calculations were performed using statistical package for the Social Sciences 24.0 software (SPSS Inc., Chicago, Ill., USA).
RESULTS
Demographic characterization of the study population
We were able to include a total of 154 patients from ten centres retrospectively. All of these patients were male and none presented evidence of HIV infection. Group 1 consists of 50 patients and Group 2 of 104. Table 1 compares the two groups in terms of demographics; we observed no significant difference between the groups.
Table 1.
Demographic characteristics of the study population (n = 154)
| Group 1 01/2006-12/2010 n = 50 |
Group 2 01/2011-12/2016 n = 104 |
p value | Statistic Test | |
|---|---|---|---|---|
| Age (Mean; SD) | 59.7; 13.5 | 64.2; 13.9 | 0.672 | T test |
| Sex | 50 males | 104 males | constant | – |
| BMI (Mean; SD) | 30.4; 8.01 | 30.9; 8.72 | 0.351 | Chi square |
| Diabetes mellitus (n; %) | 17; 34.0 | 43; 41.3 | 0.719 | Fisher exact |
| HIV (n; %) | 0 | 0 | constant | – |
| Alcohol abuse (n; %) | 9; 18.0 | 19; 18.3 | 1.000 | Fisher exact |
| Colorectal cancer (n; %) | 1; 2.0 | 7; 6.7 | 0.435 | Fisher exact |
1 – 30 missing concerning body mass index (=BMI); 2 – 48 missing concerning Body mass index; HIV – human immunodeficiency virus
Comparison of therapy and outcome between the groups
Table 2 provides an overview of the comparison of therapy and outcome between groups. Interestingly, the only significant difference was in the duration of intensive care unit treatment, that is, Group 2 spent significantly longer (p = 0.018; T test) in critical care. Furthermore, there was no significant difference in the application of urinary diversion (p = 0.151; Chi-square test). Suprapubic catheterisation was the most frequent urinary diversion in both groups (Group 1: 22; 44%; Group 2: 60; 57.7%).
Table 2.
Comparison of therapy and outcome in both groups (n = 154)
| Group 1 01/2006-12/2010 n = 50 |
Group 2 01/2011-12/2016 n = 104 |
p value | Statistic Test | |
|---|---|---|---|---|
| Number of wound toilets (mean; SD) | 3.7; 3.5 | 4.5; 4.7 | 0.315 | T test |
| VAC therapy | 19; 38.0 | 52; 50.0 | 0.172 | Fisher exact |
| Hyperbaric oxygenation | 3; 6.0 | 13; 12.5 | 0.269 | Fisher exact |
| Colostomy | 8; 16.0 | 18; 17.3 | 1.000 | Fisher exact |
| Sepsis | 26; 52.0 | 66; 63.5 | 0.810 | Fisher exact |
| Septic shock | 13; 26.0 | 36; 34.6 | 1.000 | Fisher exact |
| ICU treatment | 29; 58.0 | 75; 72.1 | 0.096 | Fisher exact |
| Duration of ICU treatment (mean; SD) | 6.3; 8.4 | 11.5; 18.5 | 0.018 | T test |
| Duration of inpatient treatment (mean; SD) | 24.7; 19.4 | 27.5; 22.3 | 0.456 | T test |
VAC – vacuum-assisted wound closure; ICU – intensive care unit
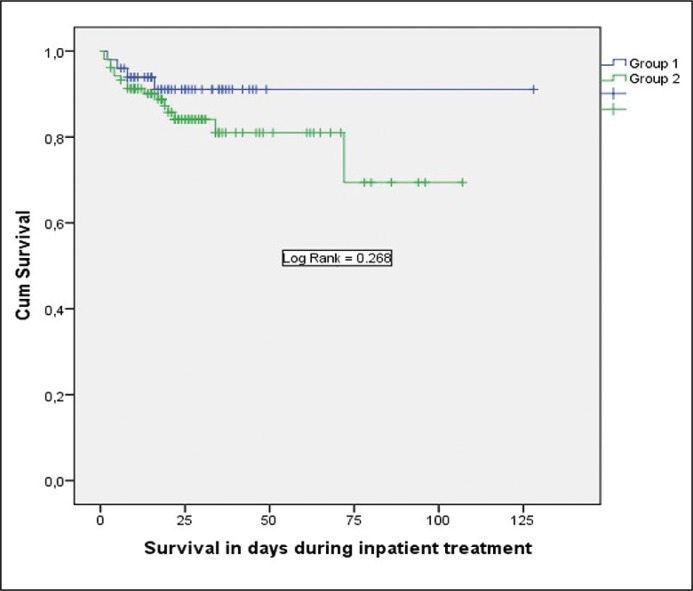
Although Group 2 was treated longer in an intensive care unit, there was no significant difference in survival, as Figure 1 illustrates. Four patients in Group 1 died (8%) and 16 in Group 2 (15.4%) during inpatient treatment.
Figure 1.
Comparison of survival between groups in days during inpatient treatment.
Surgical techniques were very heterogenic. Interestingly, only two patients (4%) received an orchiectomy in Group 1 while 20 patients (19.2%) had an orchiectomy in Group 2.
Causative pathogens and multi-resistant organisms We tended to detect mixed flora in the wound specimens from both groups. Table 3 shows an overview of the causative organisms identified microbiologically in the wound specimens. We fortunately detected no significant difference in multi-resistant organisms (Group 1: 4; 8%; Group 2: 10; 9.6%; p = 1.000; Fisher exact test). The initial antibiotic treatment was resistogram fair in over half of the patients (Group 1: 26; 52%; Group 2: 59; 56.7%; p = 1.000; Fisher exact test). On the whole, first-line antimycotic treatment was seldom applied (Group 1: 2; 4%; Group 2: 9; 8.7%).
Table 3.
Comparison of causative organisms in both groups in n and % (n = 154)
| Causative organism | Group 1 01/2006-12/2010 n = 50 |
Group 2 01/2011-12/2016 n = 104 |
|---|---|---|
| No pathogen detection | 10; 20.0 | 35; 33.7 |
| Mixed flora | 23; 46.0 | 50; 48.1 |
| Streptococcus spp. | 6; 12.0 | 6; 5.7 |
| Staphylococcus spp. | 5; 10.0 | 5; 4.9 |
| Enterococcus spp. | 4; 8.0 | 6; 5.7 |
| Citrobacter spp. | 1; 2.0 | 0; 0 |
| Pseudomonas spp. | 1; 2.0 | 0; 0 |
| Candida spp. | 0; 0 | 2; 1.9 |
Concerning primary antibiotic treatment in Group 1, 22 patients (44%) received a combination therapy, mostly (n = 15; 30%) with a ß-lactamase inhibitor plus aminoglycoside (n = 7; 14%) or ß-lactamase inhibitor plus fluorchinolone. Nineteen patients (38%) received therapy with a ß-lactamase inhibitor, four (8%) with a cephalosporine, three (6%) with a flourchinolone and two (4%) with a carbapenem, respectively. On the whole, in 15 patients (30%) metronidazole was also added to initial therapy in Group 1. Furthermore, in Group 2, 75 patients had initial combination therapy (72.1%), mostly with ß-lactamase inhibitor plus aminoglycoside (n = 60; 57.7%) or ß-lactamase inhibitor plus a fluoroquinolone (n = 15; 14.4%). Seventeen patients (16.3%) had antibiotic therapy with a ß-lactamase inhibitor, six (5.8%) with a carbapenem, five (4.8%) with a cephalosporine and one (1.0%) with a fluoroquinolone, respectively. Metronidazole was added to the initial therapy in 60 patients (57.7%) in Group 2.
Risk factors for worse outcome
Univariate Cox regression failed to identify a risk factor for death in either group or in the entire study population. Nor did the search for risk factors for multi-resistant pathogens yield any indication. Our analysis parameters were age, body mass index (BMI), diabetes mellitus, alcohol abuse, colorectal-cancer, leucocyte count, CRP, thrombocyte count, urea, HbA1c, sepsis, septic shock, intensive care unit treatment, duration of intensive care unit treatment, number of wound toilets, vacuum wound therapy, hyperbaric oxygenation, colostomy and the type of urinary diversion.
DISCUSSION
We conducted a retrospective multicentric study of FG including 154 patients. To our knowledge, this is the largest study population for this rare disease reported so far. For analysis we defined two groups who underwent different therapy timeframes, since our primary study endpoint was to compare them in terms of therapy and outcome.
Patients with FG are considered to be an absolute emergency, which must be treated immediately in an interdisciplinary manner. An immediate radical operation with accompanying antibiotic therapy is inevitable for those affected.
Interestingly, we identified no significant differences in outcome or therapy except for the duration of ICU treatment, which was significantly longer in the more recent treatment group. There are two possible conclusions: On the one hand, patients in group 2 were more seriously ill, and thus required more intensive care. On the other hand, we can assume that critical-care medicine and ICU therapy were administered to improve patient outcome. However, although the two groups' demographics did not significantly differ, and despite group 2's more intensive care, patient outcome and mortality rates exhibited no significant change. We therefore must conclude that despite more intensive treatment and progress made in critical-care medicine, the outcome of FG did not change over the last several years. FG is a severe disease; we identified a mortality rate of 15.4% during inpatient treatment in group 2 and even higher mortality rates in more recent investigations [2;, 11]. Therapy of FG needs to be improved. One approach might be to treat these patients in high volume centres, e.g., Sorensen et al. reported that hospitals treating more than one FG per year had an adjusted 42–84% lower mortality rate (p <0.0001) [7] and Osburn et al. recommend that more acute patients be transferred to high-volume centres [1]. The high-volume centre approach makes sense as such institutions are capable of conducting adequately-powered prospective clinical studies to develop a more effective management strategy for FG. Yet, there are still two problems with this solution: Firstly, is the patient stable enough to be transferred to a centre? Secondly, would not time be lost for rapid surgical treatment during the transfer, as we know that early surgical debridement is one of the most effective methods to improve survival rates [11]? It might seem redundant, but to answer these two questions and to optimise FG management, further prospective investigations are mandatory.
Luckily, we observed no significant difference in multi-resistant organisms (Group 1:4; 8%; Group 2:10; 9.6%; p = 1.000) despite evidence that they are a growing problem in FG [5]. But due to rising antibiotic resistance rates especially in urogenital infections and gram-negative bacteria, the problem of multi-drug-resistant organisms will continue to become more evident, e.g. Actinobacter baumanii, which is very often multi-resistant, was the only microorganism associated with an increased mortality rate in a recent study by Yilmazlar et al. [2].
Furthermore, our study population revealed no significant risk factors for death or multi-resistant pathogens, however, we assume that no one used a prognostic scoring system for FG like the Fournier's gangrene severity index (FGSI), Laboratory Risk Indicator for necrotizing fasciitis (LRINEC) or neutrophil lymphocyte ratio (NLR) in our study centres [6]. All of the prognostic scoring systems for FG are controversial [4, 6, 8, 9, 13]. The main problem is that these prognostic factors and scoring systems are all based on retrospective data – thus the scoring systems in particular require prospective analysis to determine their efficacy in daily clinical practice.
Our study is limited by fact that we only addressed inpatient treatment, but there might have been further complications, secondary infections, or mortality during follow up. In their retrospective multicentre study in northern Germany, Czymek et al. reported that patients with FG suffer from persistent physical and mental-health problems for a long time after their primary hospital stay and must receive long-term care from various specialists, as otherwise the disease leads to an increase in the duration of morbidity and a decrease in quality of life [10]. Consequentially, further studies should also seek the most efficient follow-up regimen for managing FG; an interdisciplinary approach would seem appropriate for addressing all of the different physical and mental health problems and quality of life issues.
Due to the retrospective study design, the long study period and multicentric approach, there are some other limitations to our study, e.g. microbiological techniques were heterogenic and changed over the time as well as heterogeneity of surgical techniques.
In closing we must assume that our study has other limitations too, mainly due to selection bias and its retrospective study design.
CONCLUSIONS
Despite increasing antibiotic resistance rates worldwide, we did not detect this phenomenon in one of the largest study populations of Fournier's gangrene in Europe. Nevertheless, the situation is alarming, since even with more intensive critical-care treatment, outcome has not improved over the last ten years. Additionally, this disease's outcome is difficult to predict. Further prospective multicentre studies are absolutely essential to optimise the management of this severe and rare disease. These studies should also address problems such as the optimal follow up of FG patients and quality-of-life issues.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank all participating centres for providing their data.
References
- 1.Osburn N, Hapson LA, Holt SK, Gore JL, Wessells H, Voelzke BB. Low-volume versus High-volume centers and management of Founier's Gangrene in Washington State. J Am Coll Surg. 2017;224:270–275. doi: 10.1016/j.jamcollsurg.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Yilmazlar T, Gulcu B, Isik O, Oztruk E. Microbiological aspects of Fournier's gangrene. Int J Surg. 2017;40:135–138. doi: 10.1016/j.ijsu.2017.02.067. [DOI] [PubMed] [Google Scholar]
- 3.Furr J, Watts T, Street R, Cross B, Slobodov G, Patel S. Contemporary trends in the inpatient management of Fournier's gangrene: Predictors of length of stay and mortality based on population-based sample. Urology. 2017;102:79–84. doi: 10.1016/j.urology.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Bjurlin MA, O'Grady T, Kim DY, Divakaruni N, Drago A, Blumetti J, Hollowell CM. Causative pathogens, antibiotic sensitivity, resistance patterns, and severity in a contemporary series of Fournier's gangrene. Urology. 2013;4:752–758. doi: 10.1016/j.urology.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Chia L, Crum-Cianflone NF. Emergence of multi-drug resistant organisms (MDROs) causing fournier's gangrene. J Infect. 2018;76:243–248. doi: 10.1016/j.jinf.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Bozkurt O, Sen V, Demir O, Esen A. Evaluation of the utility of different scoring systems (FGSI, LRINEC and NLR) in the management of Fournier's gangrene. Int Urol Nephrol. 2015;47:243–248. doi: 10.1007/s11255-014-0897-5. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen MD, Krieger JD. Fournier's gangrene: Epidemiology and outcomes in the general US population. Urol Int. 2016;97:249–259. doi: 10.1159/000445695. [DOI] [PubMed] [Google Scholar]
- 8.Roghmann F, von Bodman C, Löppenberg B, Hinkel A, Palisaar J, Noldus J. Is there a need for the Fournier's gangrene severity index? Comparison of scoring systems for outcome prediction in patients with Fournier's gangrene. BJU Int. 2012;110:1359–1365. doi: 10.1111/j.1464-410X.2012.11082.x. [DOI] [PubMed] [Google Scholar]
- 9.Tarchouli M, Bounaim A, Essarghini M, et al. Analysis of prognostic factors affecting mortality in Fournier's gangrene: A study of 72 cases. Can Urol Assoc J. 2015;9:800–804. doi: 10.5489/cuaj.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czymek R, Kujath P, Bruch HP, et al. Treatment, outcome and quality of life after Fournier's gangrene: a multicentre study. Colorectal Dis. 2013;15:1529–1536. doi: 10.1111/codi.12396. [DOI] [PubMed] [Google Scholar]
- 11.Hong KS, Yi HJ, Lee RA, Kim KH, Chung SS. Prognostic factors and treatment outcomes for patients with Fournier's gangrene: a retrospective study. Int Wound J. 2017;14:1352–1358. doi: 10.1111/iwj.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang LM, Su YJ, Lai YC. The evaluation of microbiology and prognosis of Fournier's gangrene in past five years. Springer Plus. 2015;4:14. doi: 10.1186/s40064-014-0783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doluoglu ÖG, Karagöz MA, Kilinc MF, et al. Overview of the different scoring systems in Fournier's gangrene and assessment of prognostic factors. Turk J Urol. 2016;42:190–196. doi: 10.5152/tud.2016.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkut P, Isik Ö, Öztürk E, Gülcü B, Ercan İ, Yılmazlar T. Gender does not affect the prognosis of Fournier's gangrene: a case-matched study. Ulus Travma Acil Cerrahi Derg. 2016;22:541–544. doi: 10.5505/tjtes.2016.27095. [DOI] [PubMed] [Google Scholar]
- 15.Ferretti M, Saji AA, Phillips J. Fournier's gangrene: A review and outcome comparison from 2009 to 2016. Adv Wound Care. 2017;6:289–295. doi: 10.1089/wound.2017.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eke N. Fournier's gangrene: a review of 1726 cases. Br J Surg. 2000;87:718–728. doi: 10.1046/j.1365-2168.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 17.Yeniyol CO, Suelozgen T, Arslan M, et al. Fournier's gangrene: experience with 25 patients and use of Fournier's gangrene severity index score. Urology. 2004;64:218e–222. doi: 10.1016/j.urology.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 18.Stone HH, Martin JD., Jr Synergistic necrotizing cellulitis. Ann Surg. 1972;175:702–711. doi: 10.1097/00000658-197205000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai T, Verze P, Brugnolli A, et al. Adherence to European Association of Urology Guidelines on Prophylactic Antibiotics: An Important Step in Antimicrobial Stewardship. Eur Urol. 2016;69:276–283. doi: 10.1016/j.eururo.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181–188. doi: 10.1186/1471-2288-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]