Abstract
Introduction
Prostate cancer is the second most common cancer and a major significant health problem amongst men in the world. Radical prostatectomy with open, laparoscopic and robotic techniques is the gold standard treatment for localized and local advanced disease. There are some risk factors including gleason score, T stage and prostate-specific antigen (PSA) level to predict the biochemical recurrence. We investigated the association with biochemical recurrence and TMPRSS2:ERG fusion in patients who were treated with open radical prostatectomy.
Material and methods
The expression of TMPRSS2:ERG was defined as positive (Group 1) and negative (Group 2). The positive staining of the patients is classified into three groups; weak positive, moderate positive and strong positive. In the statistical analyses; chi squared test and Mann Whitney U were used and p <0.05 was defined as statistical significance.
Results
The present study includes 87 patients, 32 and 55 patients were in group 1 and 2 respectively. The mean age of the patients was 62.81 +5.55 and 64.45 +5.18 in the groups without significant difference. Extraprostatic extension was reported in 27 patients; 11 of these patients were in group 1 and 16 patients were in group 2 (p = 0.60). Biochemical recurrence was detected in 15 patients. Of these patients, 5 were in group 1 and 10 were in group 2 (p = 0.76).
Conclusions
The current study found no association between TMPRSS2:ERG fusion and biochemical recurrence and unfavourable pathological results in Turkish patients. Further research including a large number of patients from different regions of Turkey is needed to investigate the ERG status and biochemical recurrence for patients.
Keywords: biochemical recurrence, prostate cancer, TMPRSS2:ERG fusion
INTRODUCTION
Prostate cancer (PCa) is one of the most common cancers and one of the leading causes of death amongst men in developed countries [1]. Digital rectal examination and prostate-specific antigen (PSA) testing are the screening methods that lead to prostate biopsy for the diagnosis of prostate cancer [2]. Transrectal ultrasound guided prostate biopsy is the gold standard method for histopathological diagnosis. Radical prostatectomy (RP) is a curative treatment for patients with localized PCa [1]. The Gleason score (GS), pathological T staging and serum PSA levels are well established prognostic factors for the prognosis of PCa [3]. However, these parameters do not always predict the clinical outcome of the patients and biochemical recurrence occurs in some patients postoperatively.
The androgen-regulated transmembrane protease serine 2 (TMPRSS2) gene codes for serine protease and is expressed in both normal and malignant prostate tissue [4]. The erythroblastosis virus E26 oncogene homolog (ERG) is a member of the erythroblast transformation specific (ETS) family of oncogenes, that acts as a transcriptional activator and inhibitor, usually controlled by phosphorylation. The TMPRSS2 gene is most commonly fused to ERG (TMPRSS2:ERG) [5]. The TMPRSS2:ERG gene fusion was first described in 2005 by Tomlins et al., as a PCa specific biomarker [6]. The prevalence of TMPRSS2:ERG fusion in prostate cancer is dependent on genetic and ethnic differences [3]. The prevalence is between 50–70% in the United States and Europe and 15.9–29.7% in Asian countries [7].
The aim of the present study is to investigate the relationship between biochemical recurrence, pathological results and TMPRSS2:ERG fusion in Turkish patients who underwent open radical prostatectomy.
MATERIAL AND METHODS
Ninety-four patients who underwent open radical prostatectomy between January 2012 and August 2014 were reviewed retrospectively. The PSA level at diagnosis, age, prostate volume and radical prostatectomy specimen examination including T stage, Gleason score, surgical margin as positive or negative and tumor volume were noted. Patients who had missing data, were older than 75 years old, or had previous radiotheraphy and neoadjuvant androgen deprivation therapy were excluded from the study. The Gleason scoring system and TNM classification were used to grade and stage. High grade prostate cancer (HGPCa) was defined as Gleason score higher than 6.
The paraffin embedded, formalin fixed radical prostatectomy specimens were reviewed by experienced genitourinary pathologists (GG). The best area for Gleason scoring was selected and deparaffinized with xylene for 2 minutes then dehydrated in alcohol with each 99%, 85%, 70% concentrations for 5 minutes and incubated with hydrogen peroxide. Antigen retrieval method was performed in preheated Trilogy buffer for 45 minutes. Then the slides were incubated in the pre-antibody solutions for 10 minutes and incubated with anti-ERG primary antibody antibody (Biocare Medical, LLC, USA) at room temperature for 60 minutes. The DAB detection kit was used for secondary antibodies and the slides were counter-stained with Mayer's hematoxylin.
The expression of TMPRSS2:ERG was defined as positive or negative, and positive staining was classified as focal or diffuse. Positive staining of ERG expression was classified based on three grading systems: weak positive, moderate positive and strong positive. The patients were divided into two groups according to the staining of the monoclonal anti-ERG antibody as positive (Group 1) and negative (Group 2). Biochemical recurrence (BCR) was defined as PSA level of ≥0.2 ng/ml after surgery with secondary confirmation.
The data was expressed as mean+standard definition, median value and statistical analyses were performed by MedCalc Statistical Software demo version 17.6 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2017). The comparison of the groups was performed using the kolmogorov smirnov test. In the comparison of the groups, chi squared test and Mann Whitney U were used for statistical difference and p <0.05 was defined as statistical significance.
RESULTS
There were 87 patients with a mean follow-up 44.03 +9.44 months in the study; 32 and 55 patients in Group 1 and 2 respectively. Patients' characteristics are shown in Table 1. The patients' were aged between 50 and 73 years and there was no significant difference between groups. Local advanced disease was reported in 27 patients (31.03%). The proportion of advanced disease was 34.37% (n = 11) and 29.09% (n = 16) in groups 1 and 2 respectively (p = 0.60). Gleason scores of 6, 7 and 8 were reported in in 11, 17 and 4 patients respectively in group 1. High grade prostate cancer was reported in 50.90% of the patients in group 2, which is less than group 1 (65.25%) without significant difference (p = 0.18).
Table 1.
The clinical characteristics of the patients
| Group 1 | Group 2 | p value | |
|---|---|---|---|
| Number of patients (n, %) | 32 (36.78) | 55 (63.21) | |
| Age (years) | 64 | 65 | 0.16 |
| BMI (kg/m2) | 27 | 28 | 0.83 |
| PSA (ng/ml) | 7 | 7 | 0.55 |
| Diabetes Mellitus (n, %) | 8 (25) | 16 (29) | 0.68 |
| Hypertension (n, %) | 7 (21.87) | 11 (20) | 0.83 |
| Gleason score | 7 | 7 | 0.29 |
| Tumor volume (cc) | 2 | 2.2 | 0.73 |
| Follow-up (months) | 36 | 48 | <0.05* |
| Local advanced disease (T3-4) (n, % for group) | 11 (34.75) | 16 (29.09) | 0.60 |
| Positive surgical margin (n, %) | 12 (37.50) | 16 (29.09) | 0.42 |
| HGPCa (n, %) | 21 (65.62) | 28 (50.90) | 0.18 |
| BCR (n, %) | 5 (15.62) | 10 (18.18) | 0.76 |
HGPCa – high grade prostate cancer; BCR – biochemical recurrence; BMI – body mass index
Mann Whitney U test for median values and chi-squared test for percentage were performed
statistically significant
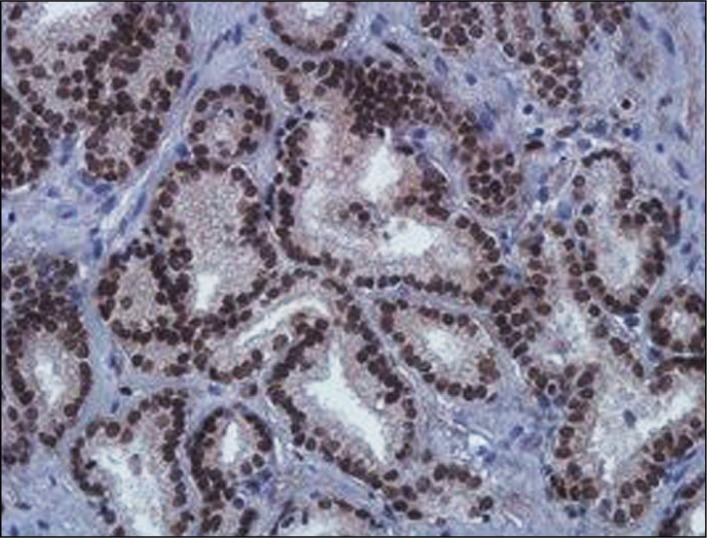
Biochemical recurrence occurred in 15 patients. Of these patients, 5 were in group 1 and 10 were in group 2 (p = 0.76). Of the patients who had biochemical recurrence in group 1, 1 was weak positive, and 4 had moderate positive staining (p:0.09). Table 2 shows the clinical and pathological reports of the patients who had positive staining of ERG according to the grading system. Figure 1 shows the positive staining of ERG.
Table 2.
The pathological results according to the staining
| Weak | Moderate | Strong | p value | |
|---|---|---|---|---|
| Number of patients (n) | 3 | 15 | 14 | |
| Local advanced disease (n, % for group) | 1 (33.3) | 6 (40) | 4 (28.57) | 0.81 |
| Positive surgical margin (n, %) | 1 (33.3) | 7 (46.6) | 4 (28.57) | 0.59 |
| HGPCa (n, %) | 1 (33.3) | 11 (73.3) | 9 (64.2) | 0.40 |
| BCR (n, %) | 1 (33.3) | 4 (26.6) | 0 | 0.09 |
The chi-squared test was performed
Figure 1.
Positive nuclear staining of ERG (x400).
DISCUSSION
The TMPRSS2:ERG fusion is the most common genetic alteration in prostate cancer and is detected in approximately 50% of patients with PCa [8]. The other TMPRSS2 fusions with the ETS family of transcription factors are ETV1, ETV4, ETV5 and ELK4; but these are observed in only 5–10% of cases. High sensitivity and specificity techniques including polymerase chain reaction (PCR) and fluorescence in situ hybridization (FISH) have been used for detection of genetic alterations [9]. The disadvantages of these methods are high costs and the need of qualified personnel, therefore they cannot be easily used in clinical practice. Immunohistochemistry is an alternative method and offers the advantages of low cost, and being efficient and simple when comparing the other molecular techniques [10]. The authors reported IHC staining of ERG with a high specificity of 85% and sensitivity of 100% comparing with quantitative PCR in PC specimens obtained from radical prostatectomy [11]. Falzarona et al demonstrated that sensitivity and specificity of ERG protein expression was 96% and 99% according to FISH positive cases [9].
The prevalence of gene fusion is different in studies. The authors reported the prevalence of TMPRSS2:ERG fusion as 47% and the highest prevalence was in Europe (54%), followed by North America (48%) and Asia (23%) in a meta-analysis [12]. Hogland et al. [13] reported the prevalence of positive staining of ERG by immunohistochemistry as 65% in patients from the Netherlands. Authors from the USA found the prevalence to be 33% [9]. The authors demonstrated that ERG fusions were more common in young than in elderly patients with prostate cancer [14]. Yılmaz et al. [7] reported the prevalence of TMPRSS2:ERG fusion as 46.5% in Turkish patients. We found the prevalence of ERG expression to be 36.78%. The small number of patients may be the reason for the low prevalence.
The relationship between TMPRSS2:ERG fusion and clinical outcome is not clear in the literature [13]. The authors investigated the ERG fusion using the FISH method and reported that there was no association between ERG fusion and biochemical recurrence, metastasis and overall survival after radical prostatectomy [15]. Additionally, the investigators reported that there was no relation between ERG positivity and age, Gleason score, stage and positive surgical margin [13]. The authors found that ERG expression had no prognostic value about recurrence and there was no association for ERG expression with any of the clinicopathological variables including Gleason score and pT stage [16]. Pettersson et al. [12] found that the ERG fusion is associated with stage but not with biochemical recurrence or lethal disease. Using the FISH method, the authors reported that ERG status is unrelated to PSA relapse but associated with tumor stage and Gleason score in their study [17].
On the contrary, the authors demonstrated that TMPRSS2:ERG fusion is associated with a high Gleason score, distant metastases and death [18]. In another study from Spain showed an association between ERG immunostaining and high stage, young age and disease progression [19]. In the present study, we found no significant difference between groups for stage, Gleason score, positive surgical margin, HGPCa, PSA level and age. However, the percentage of local advanced disease, HGPCa, and positive surgical margins were detected more often in patients with positive immunostaining of ERG; the biochemical recurrence rate was more common in patients without ERG staining than the others (18.18% and 15.62%) without significant difference.
The limitation of this study was the small number of patients from a single center data pool. Thus, the results may not be representative of overall Turkish patients. The other limitation is a lack of preoperative rectal examination findings of the patients. To best of our knowledge, this is the first study investigating the relationship between ERG immunostaining and pathological outcome and biochemical recurrence for Turkish patients.
CONCLUSIONS
In conclusion, ERG immunostaining has not predicted the unfavourable outcome of radical prostatectomy specimens and biochemical recurrence during the follow-up period in the current study. We need further research including a larger number of patients from different regions of Turkey with multicenter data to investigate the clinical importance of ERG for Turkish patients with prostate cancer.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Çaliskan S, Keles MO, Kaba S, Koca O, Akyuz M, Ozturk MI, Karaman MI. Active surveillance or radical prostatectomy? Which treatment is best? Bratisl Lek Listy. 2016;117:468–471. doi: 10.4149/bll_2016_091. [DOI] [PubMed] [Google Scholar]
- 2.Çaliskan S, Sungur M. Fibrinogen and D-dimer levels in prostate cancer: Preliminary results. Prostate Int. 2017;5:110–112. doi: 10.1016/j.prnil.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noh BJ, Sung JY, Kim YW, Chang SG, Park YK. Prognostic value of ERG, PTEN, CRISP3 and SPINK1 in predicting biochemical recurrence in prostate cancer. Oncol Lett. 2016;11:3621–3630. doi: 10.3892/ol.2016.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavukçu HH, Mangır N, Ozyürek M, Turkeri L. Preliminary Results of Noninvasive Detection of TMPRSS2:ERG Gene Fusion in a Cohort of Patients With Localized Prostate Cancer. Korean J Urol. 2013;54:359–363. doi: 10.4111/kju.2013.54.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pflueger D, Rickman DS, Sboner A, et al. N-myc Downstream Regulated Gene-1(NDRG1) is Fused to ERG in Prostate Cancer. Neoplasia. 2009;11:804–811. doi: 10.1593/neo.09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 7.Yılmaz O, Berber U, Okçelik S, Soydan H, Ateş F, Karademir K. TMPRSS2-ERG gene fusion in Turkish patients with localized prostate cancer: results of radical prostatectomy specimens. Turk J Urol. 2016;42:60–63. doi: 10.5152/tud.2016.94763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleischman A, Saramaki OR, et al. Prevalance and prognostic significance of TMPRSS2-ERG gene fusion in lymph node positive prostate cancers. Prostate. 2014;74:1647–1654. doi: 10.1002/pros.22882. [DOI] [PubMed] [Google Scholar]
- 9.Falzarano SM, Zhou M, Carver P, et al. ERG gene reaarrangement status in prostate cancer detected by immunohistochemistry. Virschow Arch. 2011;459:441–447. doi: 10.1007/s00428-011-1128-4. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Kim SH, Joung JY, Lee GK, Hong EK, Kang KM. Overexpression of ERG and Wild-Type PTEN Are Associated with Favorable Clinical Prognosis and Low Biochemical Recurrence in Prostate Cancer. PLOS One. 2015;10:1–13. doi: 10.1371/journal.pone.0122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Leenders GJ, Boormans JL, et al. Antibody EPR3864 is specific for ERG genomic fusions in prostate cancer: implications for pathological practice. Mod Pathol. 2011;24:1128–1138. doi: 10.1038/modpathol.2011.65. [DOI] [PubMed] [Google Scholar]
- 12.Pettersson A, Graff RE, Bauer SR, et al. THE TMPRSS2:ERG Rearrangement, ERG Expression and Prostate Cancer Outcomes: A Cohort Study and Meta-Analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1497–1509. doi: 10.1158/1055-9965.EPI-12-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W. DNA alterations in the tumor genome and their associations with clinical outcome in prostate cancer. Asian J Androl. 2016;18:533–542. doi: 10.4103/1008-682X.177120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weischenfeldt J, Simon R, Feuerbach L, et al. Integrative genomic analyses reveal androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell. 2013;23:159–170. doi: 10.1016/j.ccr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Gopalan A, Leversha MA, Satagopan JM, Zhou Q, Al-Ahmadie HA, Fine SW. TMPRSS2-ERG gene fusion is not associated with outcome inpatients treated by prostatectomy. Cancer Res. 2009;15:1400–1406. doi: 10.1158/0008-5472.CAN-08-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva MP, Barros-Silva JD, Ersvær E, Kildal W, Hveem TS, Pradhan M. Cancer Prognosis Defined by The 8q, PTEN and ERG. Translational Oncology. 2016;9:575–582. doi: 10.1016/j.tranon.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minner S, Enodien M, Sirma H, et al. ERG Status Is Unrelated to PSA Recurrence in Radically Operated Prostate Cancer in the Absence of Antihormonal Therapy. Clin Cancer Res. 2011;17:5878–5888. doi: 10.1158/1078-0432.CCR-11-1251. [DOI] [PubMed] [Google Scholar]
- 18.Demichelis F, Fall K, Perner S, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–4599. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 19.Tello AF, Juanpere N, deMuga S, et al. Association of ERG and TMPRSS2-ERG With Grade, Stage and Prognosis of Prostate Cancer is Dependent on Their Expression Levels. Prostate. 2015;75:1216–1226. doi: 10.1002/pros.23004. [DOI] [PubMed] [Google Scholar]