Abstract
Introduction
Low-dose-rate (LDR) brachytherapy is a widely used therapeutic option for localised prostate cancer. The aim of this study was to analyse the oncological and functional outcomes after 10 years of experience with brachytherapy for localised prostate cancer.
Material and methods
All patients who underwent brachytherapy between April 2006 and September 2017 were included for analysis. Initial prostate-specific antigen (PSA) levels, tumour stages, Gleason scores, positive biopsies, prostate volumes, dosimetric parameters, and urinary symptoms were noted.
Results
A total of 201 patients underwent brachytherapy between April 2006 and September 2017. Of these patients, 159 had >3 years of oncological and functional follow-up. Only these relevant patients were included in the statistical analysis. This showed a significant, persistent decline in PSA levels (p <0.0001): the mean PSA was 1.2 ng/ml after 6 months, 1.1 ng/ml after 1 year, and 0.49 ng/ml after 3 years. Only 9 patients had tumour recurrence (3 patients with Gleason score 6 and 6 patients with Gleason score 7). After receiving adequate treatment, the patients underwent oncological follow-up.
Important obstructive and irritative complaints were most pronounced during the first 9 months and decreased strongly after 18 months of follow-up.
Conclusions
LDR brachytherapy has excellent oncological outcomes with limited functional inconveniences that are adequately treatable. Our 10 years' experience shows that brachytherapy is a safe and effective method for the treatment of low-risk localised prostate cancer.
Keywords: brachytherapy, prostate cancer, prostate-specific antigen, oncological outcomes, functional outcomes
INTRODUCTION
Prostate cancer remains the second most commonly diagnosed cancer in men and has a substantial impact on global health care [1]. In 2015, the estimated number of new prostate cancer diagnoses worldwide was 1.1 million [2]. Fortunately, there is a wide range of treatment options with excellent disease control.
Patients with clinically localised prostate cancer can be curatively treated with different modalities, such as radical prostatectomy, brachytherapy, and external beam radiotherapy (EBRT). Recently, an increasing number of patients are being observed with active surveillance [3]. Clinical guidelines have been established and published to offer the correct patient-tailored treatment.
Low-dose-rate (LDR) brachytherapy is a widely used therapeutic option for men with localised prostate cancer. In the 1950s and 1960s, there was already an interest in prostate brachytherapy, and when iodine 125 (I-125) became commercially available in 1967, the interest in brachytherapy grew [4]. Later, with the use of ultrasound, the bearing needles could be placed in precise positions in the prostate gland and brachytherapy became a valid alternative therapeutic option for prostate cancer [5].
The use of the guidelines from the European Association of Urology (EAU) and the European Society for Radiotherapy and Oncology (ESTRO) is strongly recommended [6]. The criteria for LDR monotherapy are as follows: stage cT1b-T2a N0 M0, Gleason score 6 with ≤50% of biopsy cores involved in cancer or Gleason score 3 + 4 with ≤33% of biopsy cores involved in cancer, initial prostate-specific antigen (PSA) level ≤10 ng/ml, prostate volume <50 cm3, International Prostatic Symptom Score (IPSS) ≤12, and maximal flow rate >15 ml/min on urinary flow tests [6].
Older studies already stated that there is a significant correlation between the implanted dose and the cancer recurrence rates [7]. The surgeon and the radiation oncologist should attempt to maintain the dose covering 90% of the prostate (D90) at >140 Gy. This leads to a significantly higher biochemical control rate [7].
Before implantation, patients should be counselled about complications related to genitourinary and gastroenterological radiotoxicity, such as urinary incontinence, rectitis, urethritis, and cystitis [6].
Urologists should also be aware that the biochemical follow-up of PSA levels is less straightforward than after radical prostatectomy, as the prostate gland is not resected in radiotherapy. In some patients, for example, we noticed a PSA bounce (i.e., a temporary increase in PSA level after brachytherapy). This phenomenon must be well known to identify a true recurrence versus a simple PSA bounce [8].
The aim of this study was to analyse the oncological and functional outcomes after 10 years of experience with brachytherapy for clinically localised prostate cancer at our centre. The study was approved by the hospital ethical committee (no. OG-057, project 21–17).
MATERIAL AND METHODS
Study Population
All patients who underwent brachytherapy between April 2006 and September 2017 were included for analysis. Age, initial PSA level, tumour stage, Gleason score, prostate volume, dosimetric parameters, and urinary symptoms were noted. Each patient had strict follow-up consultations every 3 months during the first year, every 6 months during the second and third years, and yearly during further follow-up. At each follow-up, the PSA level was noted, and the patients were actively asked for possible adverse symptoms of genitourinary and gastroenterological radiotoxicity.
We performed disease risk stratification by using the EAU and National Institute for Health Care and Excellence guidelines. Low-risk prostate cancer was defined as clinical stage T1-T2a and Gleason score ≤6 and PSA level <10 ng/ml; intermediate-risk prostate cancer was defined as clinical stage T2b or Gleason score 7 or PSA level 10–20 ng/ml; and high-risk prostate cancer was defined as clinical stage ≥T2b or Gleason score 8–10 or PSA level >20 ng/ml [6, 9].
Treatment technique
After a multidisciplinary oncology board discussion, 2 urologists treated all the patients in collaboration with the same radiation oncologist at 1 hospital. All procedures were performed under general anaesthesia in the dorsal lithotomy position. After intubation, a transurethral catheter was placed to obtain ideal visualisation of the urethra during surgery. Permanent I-125 seeds were implanted under transrectal ultrasound guidance by using a perineal template and an intraoperative planning system (VariSeed 8.0), with minimal radiation dose at the rectum and urethra.
During the procedure, the number of needles and number of seeds implanted were precisely noted in each patient's record sheet. The D90 value of the prostate was calculated, as well as the total activity, percentage of the clinical target volume (CTV) receiving the prescribed dose (V100 prostate), percentage of the CTV receiving 150% of the prescribed dose (V150), dose delivered to 30% of the urethra (D30), dose delivered to 10% of the urethra (D10), and rectal volume receiving 100% of the prescribed dose (V100 rectum).
Salembier et al. defined the following recommended prescription doses: the V100 of the prostate should be at least 95%, the V150 of the prostate should be ≤50%; the D10 of the urethra should be <150% of the prescription dose; the D30 of the urethra should be <130% of the prescription dose; and the maximal rectal dose should be <200 Gy [10].
After a mean of 1 month, a consultation and a computed tomography scan were planned with the radiation oncologist. This is a critical step in post-implant dosimetry and feedback, because there is a small risk of seed loss and seed migration. The migration rates are between 1% and 15% according to the literature [10].
Definitions
As stated before, the prostate gland is not removed in brachytherapy, and urologists should therefore be aware that the biochemical follow-up of PSA levels is less straightforward than after radical prostatectomy. Treatment failure was defined as a persistently increasing PSA level, with positive biopsies leading to salvage therapy, or biochemical failure. Biochemical failure consisted of PSA nadir plus 2 ng/ml (Phoenix definition) [11]. A PSA bounce was defined as a temporary increase of the nadir value by at least 0.2 ng/ml with spontaneous decrease to the nadir value or lower [12]. The time and amplitude of each bounce was noted and analysed.
Statistics
The data are summarised as mean (SD) or as median (interquartile range) depending on the normality of the data. Comparison between continuous variables at different time points was done using a paired t- test. A p-value of <0.05 was considered as statistically significant. The association between categorical variables was assessed using the exact chi-square test (Fisher's exact test for 2 x 2 frequency tables). The results of univariate survival analysis are presented using Kaplan-Meier curves. All statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
Study population
Between April 2006 and September 2017, a total of 201 patients underwent brachytherapy for prostate cancer treatment with a curative intent. Of them, 159 patients had >3 years of post-implantation oncological and functional follow-up, and only these relevant patients were included in the retrospective statistical analysis.
The mean age of the patient population was 69 years with a standard deviation (SD) of 6 years. The mean follow-up duration was 71 months (SD 29 months), and the mean initial PSA level was 7.2 ng/ml (SD 3.1 ng/ml) (Table 1).
Table 1.
Patient and treatment characteristics
| Variable | Mean (SD) |
|---|---|
| Age (y) | 69 (6) |
| iPSA (ng/ml) | 7.2 (3.1) |
| Prostate volume (g) | 33 (8.9) |
| D90 prostate (Gy) | 166.2 (6) |
| D30 urethra (Gy) | 169 (8.4) |
| V100 rectum (Gy) | 0.16 (0.18) |
| Follow-up period (mo) | 71 (29) |
As stated before, we performed disease risk stratification by using the EAU and National Institute for Health Care and Excellence guidelines. In our study, 79 of the 159 patients (49%) were classified as having low-risk prostate cancer and 80 patients (51%) were classified as having intermediate-risk prostate cancer. There were no patients with high-risk prostate cancer.
Of the patients, 89 (56%) had Gleason 6 prostate cancer, 58 (36.5%) had Gleason 3 + 4 prostate cancer, and 12 (7.5%) had Gleason 4 + 3 prostate cancer. Clinical T1c tumour stage was noted in 129 patients (81%) and clinical T2a tumour stage in 30 patients (19%). A total of 131 patients (82%) had <50% positive biopsies and 28 patients (18%) had >50% positive biopsies. Moreover, 27 patients (17%) had neoadjuvant hormonal therapy, which was mostly done to decrease the prostate size. The mean preoperatively measured prostate volume was 33 g (SD 8.9 g), and the mean D90 value was 166.2 Gy (SD 6 Gy) (Tables 1 and 2).
Table 2.
Tumour characteristics
| Gleason score | Patient group |
|---|---|
| Gleason 6 | 89/159 = 56% |
| Gleason 3 + 4 | 58/159 = 36.5% |
| Gleason 4 + 3 | 12/159 = 7.5% |
| Tumour stage | Patient group |
| cT1c | 129/159 = 81% |
| cT2a | 30/159 = 19% |
| Percentage of positive biopsies | Patient group |
| <50% | 131/159 = 82% |
| ≥50% | 28/159 = 18% |
| Gleason score preoperatively | Percentage of tumour recurrence |
| Gleason 6 | 3/89 = 3.4% |
| Gleason 7 | 6/70 = 8.6% |
Oncological outcomes
We assessed the oncological outcomes by using a correct interpretation (as stated above) of the PSA evolution during follow-up.
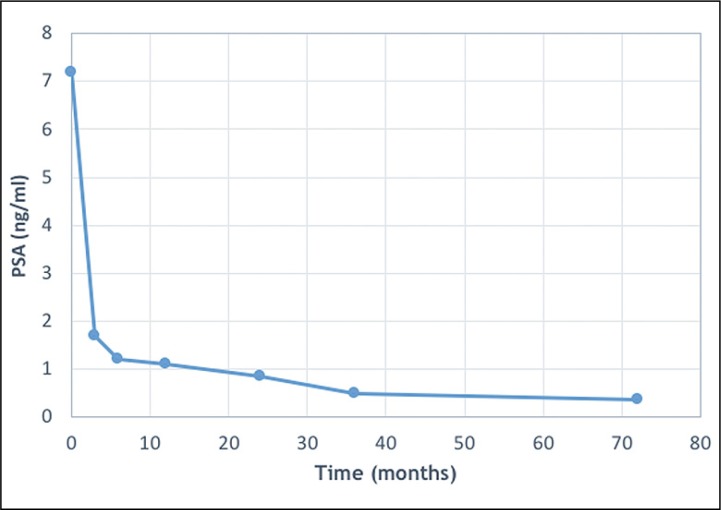
The mean initial PSA level was 7.2 ng/ml, and our analysis showed a significant, persistent decline of the PSA level (paired t-test, p <0.0001): the mean PSA was 1.68 ng/ml after 3 months (n = 159/159), 1.2 ng/ml after 6 months (n = 159/159), 1.1 ng/ml (n = 159/159) after 1 year, 0.83 ng/ml (n = 159/159) after 2 years, 0.49 ng/ml after 3 years (n = 159/159), and 0.34 ng/ml (n = 52/159) after 6 years (Figure 1).
Figure 1.
Evolution of mean prostate-specific antigen (PSA) levels.
The mean PSA nadir was reached at a mean of 55 months (SD 20 months). In 73 cases (46%), we saw a PSA bounce at a mean follow-up of 17.6 months. The PSA increased by an average of 0.46 ng/ml at these bounces. There were no on-going bounces at the time of analysis.
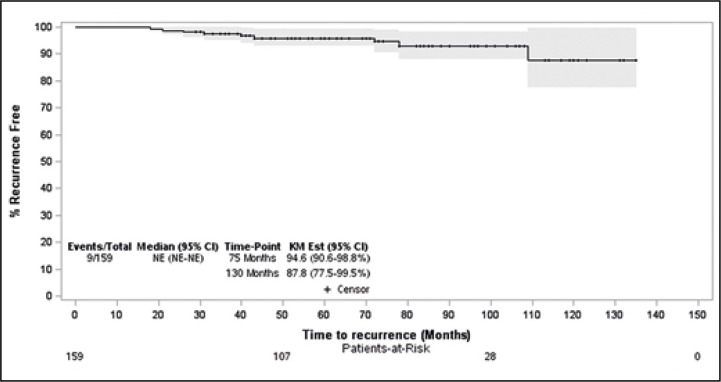
A total of 9 patients (5.7%) had a treatment failure after a mean of 49 months (Figure 2). All patients had PSA progression and proven tumour recurrence in their prostate biopsies. Of these 9 patients, 3 had low- risk prostate cancer and a Gleason score of 6 preoperatively (3 of 89, 3.4%) (Table 2). The remaining 6 patients had intermediate-risk prostate cancer and a Gleason score of 7 preoperatively (6 of 70, 8.6%) (Table 2). Tumour recurrence was treated with salvage radical prostatectomy in 3 patients and with hormonal therapy in 6 patients. Thereafter, their oncological follow-up remained under control. One patient died, but not because of prostate cancer progression.
Figure 2.
Kaplan-Meier curve of recurrence-free survival.
CI – confidence interval; NE – not estimable; KM – Kaplan-Meier; patient-at risk: all patients (159) who were icluded for statistical analysis
There was no significant association between the preoperative Gleason scores and tumour recurrence; however, patients with >50% positive biopsies had a higher, but not statistically significant (p = 0.052), chance of developing treatment failure and tumour recurrence. Furthermore, there were no significant differences between initial PSA level and tumour recurrence and there were no significant differences between D90 and tumour recurrence. Patients who were younger had a statistically significant higher chance of developing a PSA bounce [67.4 years (mean age of patients developing a bounce) vs. 70.1 years (mean age of patients developing no bounce), p = 0.0040].
Functional outcomes
A total of 41 patients (26%) had initial obstructive and irritative complaints, which were most pronounced during the first 9 months after brachytherapy. These patients were mainly treated with anticholinergics and alpha-blockers. A transurethral resection of the prostate was performed in 4 patients. The number of complaints decreased after 18 months of follow-up (n = 10/159, 6%). Of the 159 patients, 2 (1.3%) had bothersome rectitis complaints.
Only 7 patients reported de novo bothersome erectile dysfunction; however, there was no significant correlation with the D90 of the prostate and the D30 of the urethra.
DISCUSSION
The goal of this study was to report the oncological and functional outcomes after LDR brachytherapy in 159 patients with >3 years of follow-up. Of them, 79 patients (49%) were classified as having low-risk prostate cancer and 80 patients (51%) were classified as having intermediate-risk prostate cancer before surgery. The main objective of LDR brachytherapy is to provide adequate cure and prevent local failure. At our centre, only 9 patients (5.7%) developed biopsy-proven and biochemical recurrence according to the Phoenix definition. Our results support those of previous reports with large patient numbers, showing that brachytherapy is a valuable alternative first-line treatment for prostate cancer [11, 13, 14]. After 10 years, only 1 death was noted, and this was not due to prostate cancer progression.
Recent studies with a large number of patients also demonstrated a comparative oncological analysis between low-risk and intermediate-risk patients [11, 15]. No statistically significant difference was seen, and our study also confirms this finding. A recent study also stated that although fewer reports are available about patients with intermediate-risk prostate cancer, LDR brachytherapy should be considered a primary treatment strategy in this patient group [8]. There was not much evidence about prognostic factors for tumour recurrence. Patients with >50% positive biopsies were at a higher risk of developing treatment failure; however, the difference was not statistically significant in our series. Nevertheless, recent studies and the EAU-ESTRO-International Society of Geriatric Oncology (SIOG) guidelines on prostate cancer showed that the percentage of positive biopsies is a risk factor for treatment failure [6, 8].
Our analysis showed that younger patients have a statistically higher chance of developing a PSA bounce (67.4 years vs. 70.1 years, p = 0.0040). This was already confirmed by multiple other studies; however, the aetiology of this phenomenon is not yet known [16-19]. The recent study by Kindts et al. [8] stated that a bounce is likely linked with inflammation, radiation, prostatitis, or vascular fibrosis.
The second goal of this study was to assess the functional outcomes after surgery. Besides the fact that I-125 seed LDR brachytherapy is a valid therapeutic option for localised prostate cancer, patients are more and more interested in their postoperative quality of life. Therefore, the possible adverse effects of this treatment should be taken into account. We did not use any validated questionnaires to assess these possible genitourinary and gastroenterological radiotoxicity symptoms; however, all patients were actively asked about these adverse events during each follow-up visit. Bothersome voiding symptoms were most pronounced during the first months after surgery and were adequately treated. The complaints decreased strongly after 18 months of follow-up. A recent large study showed a peak IPSS during the first months and a consistent return towards baseline levels during further follow-up [11]. The same was concluded about the patients' quality of life (both urinary related and bowel related), with a decrease during the first months but a recovery during further follow-up. Potency preservation was comparable to previous studies, in which no significant long-term treatment-induced declines were noted [11, 20, 21].
Furthermore, the Expanded Prostate Cancer Index Composite, which was used in the ProtecT (Prostate Testing for Cancer and Treatment) trial showed that LDR brachytherapy had better sexual and urinary outcomes than radical prostatectomy and better bowel outcomes than EBRT [22].
The limitations of this study are its retrospective nature with inherent biases, the small study sample, a single institution nature and the fact that no validated questionnaires were used to assess postoperative functional outcomes. On the other hand, the strengths of this study are the long persistent follow-up and that the seed implantation was done by the same team of urologists in collaboration with the same single radiation oncologist.
CONCLUSIONS
LDR brachytherapy has excellent oncological outcomes with limited functional inconveniences that are adequately treatable. Our 10 years' experience shows that brachytherapy is a safe and effective method for the treatment of localised prostate cancer. The use of the EAU-ESTRO-SIOG Guidelines on Prostate Cancer is strongly recommended.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Wong MC, Goggins WB, Wang HH, et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70:862–874. doi: 10.1016/j.eururo.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network . Prostate Cancer, Practice Guidelines in Oncology. 2016. v. 3.2016. [Google Scholar]
- 4.Whitmore WF, Jr, Hilaris B, Grabstald H. Retropubic implantation of iodine 125 in the treatment of prostate cancer. J Urol. 1972;108:918–920. doi: 10.1016/s0022-5347(17)60906-6. [DOI] [PubMed] [Google Scholar]
- 5.Holm HH, Juul N, Pederson JF, Hansen H, Stroyer I. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. J Urol. 1983;130:283–2861. doi: 10.1016/s0022-5347(17)51108-8. [DOI] [PubMed] [Google Scholar]
- 6.Mottet N, van den Bergh RCN, Briers E. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Available at: https://uroweb.org/guideline/prostate-cancer/Accessed February 2018. [Google Scholar]
- 7.Stock RG, Stone NN. Importance of post-implant dosimetry in permanent prostate brachytherapy. Eur Urol. 2002;41:434–439. doi: 10.1016/s0302-2838(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 8.Kindts I, Stellamans K, Billiet I, Pottel H, Lambrecht A. 125I brachytherapy in younger prostate cancer patients. Outcomes in low-and intermediate-risk disease. Strahlenther Onkol. 2017;193:707–713. doi: 10.1007/s00066-017-1142-9. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health Care and Care Excellence . Prostate Cancer: Diagnosis and Management. Clinical guideline [CG175] 2014. Available at: https://www.nice.org.uk/guidance/cg175 Accessed February 2018. [Google Scholar]
- 10.Salembier C, Lavagnini P, Nickers P, et al. Tumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother Oncol. 2007;83:3–10. doi: 10.1016/j.radonc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Langley SEM, Soares R, Uribe J, et al. Long-term oncological outcomes and toxicity in 597 men aged ≤60 years at time of low-dose-rate brachytherapy for localised prostate cancer. BJU Int. 2018;121:38–45. doi: 10.1111/bju.13946. [DOI] [PubMed] [Google Scholar]
- 12.Mazeron R, Bajard A, Montbarbon X, et al. Permanent 125I-seed prostate brachytherapy: early prostate specific antigen value as a predictor of PSA bounce occurrence. Radiat Oncol. 2012;7:46. doi: 10.1186/1748-717X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potters L, Morgenstern C, Calugaru E, et al. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol. 2005;173:1562–1566. doi: 10.1097/01.ju.0000154633.73092.8e. [DOI] [PubMed] [Google Scholar]
- 14.Morris WJ, Keyes M, Spadinger I, et al. Population-based 10-year oncological outcomes after low- dose-rate brachytherapy for low-risk and intermediate-risk prostate cancer. Cancer. 2013;119:1537–1546. doi: 10.1002/cncr.27911. [DOI] [PubMed] [Google Scholar]
- 15.Kollmeier MA, Fidaleo A, Pei X, et al. Favourable long-term outcomes with brachytherapy-based regimens in men ≤60 years with clinically localized prostate cancer. BJU Int. 2013;111:1231–1236. doi: 10.1111/j.1464-410X.2012.11663.x. [DOI] [PubMed] [Google Scholar]
- 16.Critz FA, Williams WH, Levinson AK, et al. Prostate specific antigen bounce after simultaneous irradiation for prostate cancer: the relationship to patient age. J Urol. 2003;170:1864–1867. doi: 10.1097/01.ju.0000091644.41330.2a. [DOI] [PubMed] [Google Scholar]
- 17.Mazeron R, Bajard A, Montbarbon X, et al. Permanent 125I-seed prostate brachytherapy: early prostate specific antigen value as a predictor of PSA bounce occurrence. Radiat Oncol. 2012;7:46. doi: 10.1186/1748-717X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caloglu M, Ciezki J. Prostate-specific antigen bounce after prostate brachytherapy: review of a confusing phenomenon. Urology. 2009;74:1183–1190. doi: 10.1016/j.urology.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Stock RG, Stone NN, Cesaretti JA. Prostate-specific antigen bounce after prostate seed implantation for localized prostate cancer: descriptions and implications. Int J Radiat Oncol Biol Phys. 2003;56:448–453. doi: 10.1016/s0360-3016(02)04470-x. [DOI] [PubMed] [Google Scholar]
- 20.Cesaretti JA, Kao J, Stone NN, Stock RG. Effect of low dose-rate prostate brachytherapy on the sexual health of men with optimal sexual function before treatment: analysis of > or = 7 years of follow-up. BJU Int. 2007;100:362–367. doi: 10.1111/j.1464-410X.2007.07016.x. [DOI] [PubMed] [Google Scholar]
- 21.Keyes M, Pickles T, Crook J, et al. Effect of aging and long-term erectile function after iodine-125 prostate brachytherapy. Brachytherapy. 2015;14:334–341. doi: 10.1016/j.brachy.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer M, Guedea F, Suarez JF, et al. Quality of life impact of treatments for localized prostate cancer: cohort study with a 5-year follow-up. Radiother Oncol. 2013;108:306–313. doi: 10.1016/j.radonc.2013.05.038. [DOI] [PubMed] [Google Scholar]