Abstract
Adjuvant endocrine hormonal therapy (EHT) is highly effective and appropriate for nearly all breast cancer patients with hormone receptor-positive tumors, which represent 75% of all breast cancer diagnoses. Long-term use of EHT reduces recurrence rates and nearly halves the risk of death during the second decade after diagnosis. Despite the proven benefits, about 33% of women receiving EHT do not take their medication as prescribed. This causes an increase in the risk for recurrence and death.
To promote adherence to EHT among breast cancer patients, this study will develop and pilot-test an intervention consisting of 1) a bilingual, culturally tailored, personalized, interactive smartphone application (app); and 2) support from a patient navigator. The control group will receive usual care. This 2-group randomized control trial will recruit 120 breast cancer patients receiving EHT at the Mays Cancer Center at UT Health San Antonio. The two-year study will have 3-time assessments (baseline, 3 and 6 months).
This theory-based intervention will empower patients' self-monitoring and management. It will facilitate patient education, identification/reporting of side effects, delivery of self-care advice, and simplify communication between the patient and the oncology team. The ultimate goal of this innovative multi-communication intervention is to improve overall survival and life expectancy, enhance quality of life, reduce recurrence, and decrease healthcare cost. The anticipated outcome is a scalable, evidence-based, and easily disseminated intervention with potentially broad use to patients using EHT and other oral anticancer agents.
Keywords: Breast cancer, Endocrine hormone therapy, Adherence, Smartphone app, Patient navigation
1. Introduction
Breast cancer is the most common cancer diagnosis among U.S. women, comprising nearly one-third of new female cancer diagnoses [1,2]. Of the 330,080 women diagnosed with breast cancer each year, approximately 75% will have hormone receptor-positive breast cancers (ER+ and/or PgR+) [1,3,4]. Shifts in oncological practice have seen a rise in self-administered adjuvant hormone therapy pills after primary treatment (i.e., surgery, chemotherapy, radiation) [5,6]. Although considered a prolonged self-management strategy, adjuvant hormone therapy with Tamoxifen and aromatase inhibitors (AIs) is highly effective and appropriate for nearly all women with ER+ and PgR + tumors [3,4]. In this context medication adherence has become increasingly important, given the association between sustained daily adherence and improved recurrence-free survival outcomes [5,[7], [8], [9]]. Nevertheless, adherence is suboptimal and many women discontinue hormone therapy prematurely [3,4,7,9].
Women with optimal adherence to Tamoxifen and AIs (more than 80% of doses taken) and more cumulative years of adherence experience the greatest reductions in recurrent risk [3,7,[9], [10], [11]]. However, adherence to adjuvant therapy in post-menopausal breast cancer patients ranges from 15% to 55% for Tamoxifen and 31%–73% for AIs [12]. Moreover, an estimated one-third of women who initiate endocrine hormone therapy (EHT) discontinue the drug before five years, which is the recommended duration of EHT [3,4,13,14]. One of the main reasons reported by patients for their non-adherence is the adverse side effects of EHT. Even though common side effects, such as nausea, hot flashes, sleep problems and sexual dysfunction are not life threatening, they can be very distressing for some patients, negatively affecting their quality of life [5,16]. Therefore, prevention and management of side effects is considered a priority to promote treatment adherence [16].
Both suboptimal adherence (not taken as prescribed) and discontinuation of EHT before completion of the fifth year are associated with a decline in survival as well as shorter time to recurrence, increased medical costs and low quality of life due to disease progression and treatment [3,4,13].
Minority and low-income breast cancer patients are disproportionally affected by EHT non-initiation, discontinuation, and nonadherence [4]. Research suggests that anxiety and patient beliefs about EHT, including perceived risks, benefits, and necessity, are tied to adherence and persistence with therapy [[3], [4], [5],7,15]. Poor patient provider interactions at time of diagnosis and/or treatment decision-making—including not assessing the patient's understanding of the need for the prescribed treatment, its purpose and the importance of adherence, failure to discuss potential treatment side effects and how to manage them, low degree of provider support, inadequate patient opportunity to ask questions and make decisions, and low patient self-efficacy in interactions—have been shown to impact persistence with hormone therapy [3,5,16].
Given that minority patients are less likely to adhere to medication regimens than white patients [4,17,18], improving medication adherence may be a means to address healthcare outcome disparities [4].
Patient navigation, a recognized care coordination strategy, has proven effective in supporting timely access to cancer care for minority patients including Latinas, addressing specific barriers, improving adherence to treatment across the care continuum and facilitating quality of care [19,20]. A recent study showed strong evidence that patient navigation reduced not only time to treatment but improved compliance with adjuvant therapy, including EHT, among breast cancer patients in a public hospital serving underserved minority women [21]. In addition, a systematic review of health information technology self-management interventions among diabetic patients found that incorporating the human interaction factor (i.e., patient navigator, personal educational videos, motivational text messages) was the common feature among the most successful interventions, providing evidence that combining new technology with the human interaction component can improve health outcomes among vulnerable population groups [22].
Studies have shown that self-efficacy and social support have a positive effect on drug adherence [[23], [24], [25], [26], [27], [28], [29], [30], [31]]. Self-efficacy, the belief in one's ability or confidence to successfully perform a task or behavior (i.e., taking their medication as prescribed, when traveling, not feeling well or experiencing side effects) [32], has been shown to influence adherence to EHT, with lower levels of perceived self-efficacy associated with decreased rates of adherence, while higher levels of perceived self-efficacy are associated with higher adherence rates [[23], [24], [25], [26]]. Social support from friends and family is also an important determinant of ongoing EHT adherence. Women who have higher levels of social support had greater adherence compared with women with lower levels of social support from family and friends [24,[27], [28], [29], [30], [31]].
The rapid proliferation of mobile smartphones offers unprecedented opportunities to improve U.S. health outcomes and reach special groups such as breast cancer patients by delivering tailored interventions to improve patients' adherence to EHT and empower them to take an active role in their healthcare.
We describe the protocol of a randomized controlled study aiming at developing and pilot testing a bilingual, culturally tailored, personalized, interactive smartphone application (app) in combination with patient navigation to improve adherence to EHT among breast cancer patients in a patient population with a large minority component.
2. Methods
2.1. Study design
This two-year study will use a 2-group parallel, randomized, control trial to enroll 120 breast cancer patients who are prescribed EHT and are attending the breast clinic at the Mays Cancer Center (MCC), a National Cancer Institute-designated cancer center at UT Health San Antonio to test the feasibility and effectiveness of a bilingual, culturally tailored, personalized, interactive mobile smartphone app in combination with patient navigation to improve adherence to EHT. The intervention group will receive two components: 1) a culturally sensitive, personalized and easy to use smartphone app; and 2) a patient navigator who will provide educational, psychosocial support and reinforcement, address common barriers, and facilitate the interaction with the medical team as needed. The control group will receive the usual care and information provided by the MCC's breast clinic and pharmacy to patients undergoing EHT (which does not include support from a patient navigator). The app and navigation support will be based in Social Cognitive Theory [32] and principles of motivational interviewing [33] and feature basic components of individual empowerment, motivation and engagement, including knowledge, attitudes, skills, peer modeling, social support/reinforcement and self-efficacy beliefs and expectations [[32], [33], [34]].
2.2. Phase 1 – formative research
Formative research will be conducted during the first six months of the study and will include focus groups and in-depth interviews with breast cancer patients, oncologists, nurses and key stakeholders (family members, Susan G. Komen local affiliate members, and community breast cancer advocates) to assess common barriers and motivators to EHT adherence, and identify intervention content and key features. This phase will guide the development and initial designs of the phone app. Additional focus groups will be conducted to review app mockups and to conduct a beta test of the phone application, before the start of the intervention, to ensure all features are working properly and solve any technical issues.
2.2.1. Focus groups
Four focus groups with a total of 16–24 breast cancer patients will be conducted (two in English and two Spanish) to assess the most common barriers and facilitators to treatment adherence that will be overcome through the intervention. Focus groups will assess: 1) patients' psychosocial factors (such as information preferences, language, social support, anxiety, attitudes and beliefs toward treatment), understanding of the importance of treatment adherence (risks/benefits), outcome expectations and perceived self-efficacy to adhere to oral hormone therapy, and potential motivators to follow treatment as prescribed; 2) patients' healthcare provider interactions and communication; 3) issues with treatment-related cost, complexity, side effects and delay in treatment benefits; and 4) reaction to key features and content of a mobile smartphone app and navigation intervention to promote adherence to treatment. Patients will receive a $25 gift-card as a compensation for their time and participation. Focus groups will be recorded and transcribed verbatim.
2.2.2. In-depth interviews
In-depth interviews will be conducted in person with 5–8 oncology physicians and nurses who will advise on content for key app components: education, symptoms monitoring and reporting, cues/reminders, communication with the oncology team, common questions and answers, etc. We will use a semi-structured interview guide that will include topics related to breast cancer concerns by patients, EHT information, importance of adherence, side effects, managing side effects, adherence barriers and facilitators, and general mobile phone app features. Interviews will last between 30 and 45 min and will be recorded and transcribed verbatim. No compensation will be provided to this group.
2.2.3. Mobile app design/mockups
Based on the qualitative information collected, the research team will work with oncologists, nurses, breast cancer patients and stakeholders to design and adjust the mobile smartphone app. Intervention mockups will be developed with support from our research team of iPhone and Android app developers at UT San Antonio (UTSA). In addition, an app companion brochure will be developed with graphic and text step-by-step information on how to download the app, description of key features, trouble shooting and contact information for assistance and technical support. To assess app mockups, interactive features, content, usability, clarity, etc., we will conduct three additional focus groups: two focus groups with 10–16 breast cancer patients and one focus group with 8 key stakeholders (family members, oncology nurses, Susan G. Komen local affiliate members, community breast cancer advocates). Based on their feedback, modifications will be made as needed to develop a functional phone app for beta testing.
2.2.4. Beta testing
Once fully developed based on focus group feedback and in-depth interviews, the mobile smartphone app will be tested in real time with 5 breast cancer patients to identify any potential issues prior to the start of the intervention. This will include a period of one week to use and test all the app components. The patient navigator will be trained to provide education on the importance of medication adherence, risks and benefits, identification of side effects, and management of symptoms. The patient navigator will also be trained to give hands-on information on how to use the app and general support and technical assistance as needed.
2.2.5. Medical record review
As part of the formative research, we will also conduct a retrospective medical record review to serve as baseline data to assess breast cancer patients' adherence rates prior to the beginning of the study. This retrospective review will cover 120 medical records from patients diagnosed with breast cancer within a year before the beginning of the intervention, following the same eligibility criteria as those for the patients randomized into this study.
The following variables will be collected during the medical record review: Age, education, marital status, number of children, health insurance, income, zip code of residence, country of birth, race/ethnicity, language preference, hormone receptor-positive breast cancer diagnosis, stage at diagnosis, and comorbidities. In addition, information on prescription of EHT, adherence to EHT and reasons for non-adherence, will be extracted from physician notes included in the electronic medical records.
2.3. Phase 2 – intervention
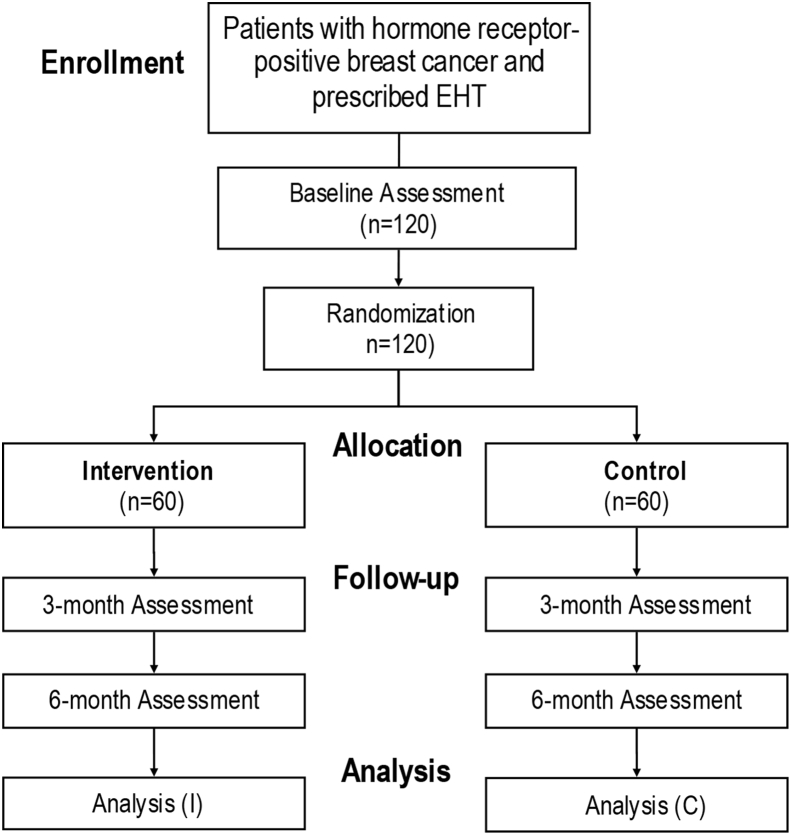
The study will enroll 120 breast cancer patients who are prescribed EHT at the breast clinic at the Mays Cancer Center (MCC) at UT Health San Antonio. Patients assigned to the intervention group (60) will: 1) use the personalized mobile phone app in their preferred language for a duration of 6 months; and 2) receive assistance from a patient navigator. They will also continue to receive the usual EHT care provided at MCC's breast clinic. Patients assigned to the control group (60) will receive the usual EHT care and materials offered at MCC's breast clinic (see Fig. 1).
Fig. 1.
CONSORT flow diagram.
2.3.1. Patient inclusion criteria
This will include: diagnosis with endocrine hormone receptor positive breast cancer, treatment with oral EHT, patient access to a smartphone that meets the app requirements, and access to Internet data connection.
2.3.2. Patient exclusion criteria
This will include: diagnosis with an endocrine hormone receptor negative breast cancer, use of a mobile phone that cannot support the app, or cannot access internet data connection.
2.3.3. Recruitment process
Participants will be drawn from breast cancer patients attending the MCC breast clinic and who have been prescribed EHT. On a weekly basis, the patient navigator (PN) will identify patients who have been prescribed EHT by reviewing new patients' medical records or by direct referral from the oncology team. The PN will approach eligible patients either by phone or in person and invite them to participate in the study. If the patients are interested, the PN will schedule an enrollment visit or will enroll them at the breast clinic after their doctor's appointment. Patients who agree will be consented and asked to respond to the baseline survey (using an iPad in their preferred language), then immediately randomized to the intervention or usual care control group. The iPad will be encrypted following UT Health San Antonio's strict data safety procedures to ensure protection of data and confidentiality. Patients randomized to the intervention group will receive support from the PN who will provide general information about EHT, hands-on instructions on how to download and use the mobile smartphone app, and the app user guide brochure.
Patients who refuse to participate in the study will be asked to complete a short refusal survey regarding main reason for not participating and basic attitudes and demographic information to assess potential differences with participants.
2.3.4. Bilingual mobile smartphone app
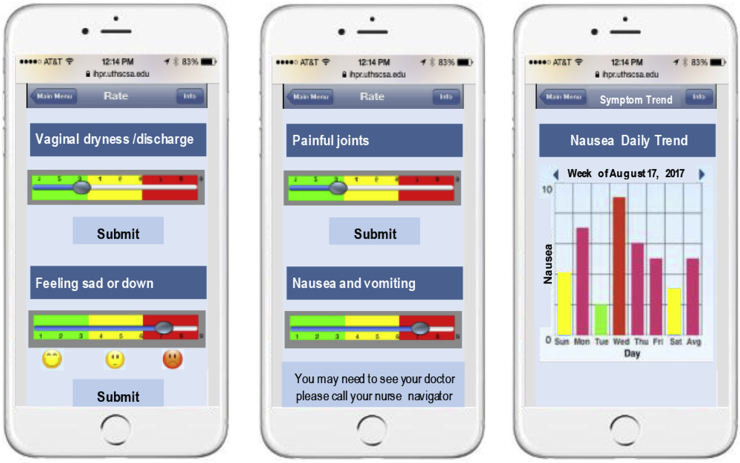
To improve EHT adherence, we will design a bilingual mobile smartphone app with tabs to provide educational content covering issues like hormone receptor positive breast cancer, importance of EHT adherence and potential side-effects, information on how to prevent or treat common side effects, self-monitoring/management of symptoms (self-efficacy), copying and self-care skills, etc. (see Fig. 2). The acquisition of skills and performance of new actions can be facilitated by peer modeling, in which participants are able to observe other breast cancer patients like themselves modeling those skills and performing the promoted behavior. Peer modeling serves two functions: 1) it shows the learner exactly how a behavior can be performed; and 2) it increases the peer modeling observer's self-efficacy for performing the behavior. The study app will include embedded links to role model videos (i.e., YouTube) to provide peer modeling of key skills for self-management. YouTube is already a widely used channel for peer modeling in instructional videos of all kinds, and offers a simple and easy way to create and upload new role model videos from study participants once the intervention has started.
Fig. 2.
Phone App Mockup
Bilingual YouTube videos will be produced in-house by the research team. They will feature doctors and breast cancer patients matching socio-cultural characteristics of Latina and non-Hispanic White patients. Video content will include doctors talking about the importance of adherence and reporting bothersome side effects to the medical team for timely management/control, as well as tips to manage common symptoms. Patients will provide testimonials of how they manage common symptoms, by communicating with the medical team, using natural approaches, and how they have been able to remain adherent. A role model who resembles the audience is more likely to empower them to imitate the behavior by conveying the idea that they are just as capable as the model is. Enhancing performance expectations through role modeling is a key element of Albert Bandura's self-efficacy theory [32]. People are more likely to imitate others who they feel are doing a little better than them. In this regard, a role model should be similar to the audience and appealing enough to match some of their aspirations [34]. In addition, social support, praise and encouragement—considered the most important forms of social reinforcement—will be provided by the study PN and by intermittent text push notifications to enhance self-efficacy and improve patients' adherence to EHT.
The app will be tailored to the language and socio-cultural characteristics of Latina and non-Hispanic white patients, these two groups comprise 85% of the patients attending the breast clinic at MCC (49% and 36% respectively). Cultural values, beliefs and behaviors will guide the content, motivational messages and educational videos, i.e., culture-specific role model and educational videos, motivational messages, emoticons, and images.
Patients will have the option to personalize the app settings and receive push notifications or text messages with tips and motivational content at their desired frequency. They will be able also to personalize cue/reminders to take the medication, attend doctor's appointments and respond to monthly adherence questions. Reporting of side effects will include a flag system to alert patients to contact their physician based on symptom severity. The app also will send notifications to the MCC team alerting them when severe side effects occur, and prompting them to call patients to determine if a follow-up visit is required and/or provide advice.
We will provide a simple app user guide brochure that will include clear text and graphic step-by-step information on how to download the app, description of key features, trouble shooting and contact information for assistance and technical support. The app will have embedded a brief questionnaire for patients' self-report of EHT adherence, reasons for not taking the medication and a section for additional comments. Both intervention and control groups will receive a notification with links to the REDCap follow-up surveys at three and six months. Additional features may be included based on formative research results to ensure the app is clinically sound and optimally useful for breast cancer patients. All intervention components will be in English and Spanish and designed with direct input from breast cancer patients, the oncology care team and key stakeholders.
2.3.5. Patient navigator
A bilingual patient navigator (PN) with good electronic and communication skills, who shares relevant age, socioeconomic status and cultural characteristics with the targeted patient population, will be hired as the study PN.
The MCC's breast clinic serves a large number of Latina breast cancer patients, so the PN will be a bilingual Latina within the mean age range of study participants. The PN will receive training in: motivational interviewing to facilitate and engage intrinsic motivation within patients to promote EHT adherence; answering patients' questions regarding their treatment; educating patients on the importance of EHT adherence, risks and benefits, adverse effects identification and management, self-care skills, social support; and app functionality. PN training also will include use and management of the mobile phone app, survey administration, barriers faced by patients regarding adherence to EHT, and will consist of a one-day course of instruction and role-playing recruitment of participants. The PN will be required to obtain HIPAA and Human Subjects certifications issued by UT Health San Antonio and an additional certification by the University Health System to access electronic medical records.
Potential study participants will be identified via weekly review of medical records or by direct referral from the oncology care team. The PN will approach eligible patients either by phone or in person and invite them to participate in the study. If the patients are interested, the PN will schedule an enrollment visit or will enroll them at the breast clinic after their doctor's appointment. Patients who agree will be consented, asked to respond to the baseline survey, and immediately randomized to the intervention or usual care comparison group. The PN will conduct monthly follow-ups with patients via phone, text or email to encourage use of the app, provide motivation, social support/reinforcement and respond to any questions patients may have. In addition, she will address patients' common barriers and facilitate their interaction with the medical team.
The PN will review the study participants' medical records to extract data needed to assess the effectiveness of the intervention on improving adherence to EHT.
2.3.6. Usual care control group
The usual care control group will be provided with the usual EHT care and information materials offered by the MCC to patients undergoing EHT, including information by the physician about the EHT treatment, importance of adherence and reporting of side-effects, and specific materials related to the medication prescribed.
2.4. Phase 3. evaluation, sample size, data analysis and reporting
This study's third phase will include evaluation, data analysis and results reporting.
Formative Evaluation will include focus groups and in-depth interviews with breast cancer patients, oncologists, nurses and key stakeholders and will be audio recorded with prior consent of participants. All audio recordings will be transcribed verbatim and analyzed using qualitative content analysis [35]. Anonymized transcripts will be reviewed by two bilingual team members line by line and paragraph by paragraph, looking for significant statements and codes. Researchers will meet in person to compare the various codes based on differences and similarities and sort them into categories and finally the categories will be formulated into themes.
Outcome Evaluation will include data derived from prescription refills, self-report surveys, and medical records reviews. Validated and study specific questionnaires will be used to assess the study primary and secondary outcomes at three time points over the 6-month intervention (baseline, three and six months). Patients will receive $25 gift-certificate per survey as an incentive for their participation. Adherence rates one year prior to the intervention will be derived by retrospective medical record review. Data provided at baseline and follow-up surveys will be collected via REDCap, a secure web application with de-identified data export mechanisms to common statistical packages, and hosted on an encrypted, high-security server at UT Health San Antonio. Network transmissions (data entry, survey submission) in REDCap are protected via Secure Sockets Layer (SSL) encryption. In the same way, data extracted from medical records will be collected via REDCap and de-identified before exported for analysis.
Randomization. A randomization schedule prepared using SAS© PROC PLAN will be uploaded into the REDCap randomization module. Once patients respond to the baseline pre-intervention survey, they will be automatically assigned into the intervention or control group. An allocation ratio of 1:1 with random permuted blocks will be used to ensure close balance between groups and to reduce potential bias by inadvertently predicting the upcoming assignment on the part of staff conducting enrollment [36].
2.5. Outcome measures and data collection
Basic demographic information, disease and treatment related variables included in the medical record review will be also collected directly from the patients or via their medical records. The primary outcome is adherence to EHT, defined as the percentage of prescribed doses taken as prescribed, with optimal adherence considered at ≥80%. Currently there is no gold standard to measure adherence. However, refill history by pharmacy records is considered an accurate and inexpensive measure of overall adherence. We will review prescribing and refill records for EHT (tamoxifen or AIs) and the number of days covered by each prescription, the number of dispensed tablets and the daily dose, to calculate an adherence index across the 6-month intervention [13]. In addition, we will use monthly self-reporting data collected via the mobile app and a short patient self-report questionnaire (MMAS-4). This scale has shown to have concurrent and predictive validity of medication adherence [37].
Given that depression is a potential confounder in this study, we will assess depression with the Patient Health Questionnaire (PHQ-8), an 8-item self-administered screening tool that has been validated in large clinical and population studies [38]. If patients are identified with severe depression, based on this screener, they will be encouraged to talk with their doctors, and the PN will immediately notify the principal investigator and the participant's oncologist.
Self-efficacy will be measured by single items in which patients rate their confidence in identify and self-manage side effects, communicate with the medical team and adhere to the recommended EHT, on scales ranging from 1 to 10, making self-efficacy easily measureable in mobile-based research [39].
Social support will be measured by two questions about praise and encouragement with response options ranging from very rarely to very often. Given the centrality of social support and self-efficacy, we anticipate they will mediate intervention outcomes based on social cognitive theory.
Secondary outcomes include patient's quality of life, anxiety and usability and satisfaction with the personalized bilingual mobile smartphone app. Quality of life will be measured by the Functional Assessment of Cancer Therapy-General (FACT-G) questionnaire [40]. This well-validated questionnaire has been used in multiple studies around the world. To assess anxiety, we will use the Generalized Anxiety Disorder 7-item scale (GAD-7), a validated tool used in clinical practice and research studies to assess the severity of anxiety [41]. Lastly, we will assess usability and satisfaction of the app by including a small set of questions developed by the research team with response options ranging from completely agree to completely disagree.
2.5.1. Sample size
A sample size of 96 subjects, 48 in each group, is needed to detect a 6-point difference in medication adherence rates (measured by prescription refill records and self-report) between the two groups using a two-sided Mann-Whitney test with 80% power and Type I error rate of 5%. Considering a dropout rate of 20%, the sample size required is 120 (60 subjects per group). The assumptions used in our sample size calculations are based on similar studies, especially those that used the prescription refills and self-report data as the primary measures of medication adherence [[42], [43], [44], [45]].
2.5.2. Data analysis
The intention-to-treat approach will be used for all analysis. Descriptive statistics with means and percentages for continuous and categorical variables, respectively, will be used to summarize baseline demographics on the overall sample and by study group.
Between-group differences in the primary outcome, percentage of prescribed doses taken as prescribed, will be assessed by t-test or by the non-parametric Mann-Whitney U test if the data are not normally distributed.
For secondary outcomes, follow-up scores will be compared between groups using repeated measures analysis of covariance (ANCOVA) models adjusting by baseline scores.
Generalized mixed effects models with logit link for proportions and identity link for means will be used to model the study outcomes over time with fixed effects for baseline measurement, group, and time and random effects for participant.
We will conduct a mediation analysis following the causal inference framework approach [46] to investigate the indirect (mediating) effects of self-efficacy and social support on the effect of the intervention on the adherence to EHT. Disease- and treatment-related variables and demographics will be evaluated as potential confounders in the modeling process. The alpha level for significance will be set at 0.05, and p-values will be based on 2-sided tests.
3. Discussion
Our randomized controlled trial will assess the effectiveness of a smartphone application in combination with patient navigation to improve adherence to EHT among breast cancer patients, while enhancing self-efficacy, and skill development for self-monitoring and management of symptoms. The potential physical and psychosocial symptoms associated with endocrine hormone therapy (EHT), including musculoskeletal symptoms, sexual dysfunction, vasomotor symptoms, urinary symptoms, cognitive dysfunction and other psychosocial distress, insomnia and fatigue, can become persistent and bothersome for some patients, affecting their quality of life, ability to function and compromising their adherence to treatment [3,[47], [48], [49], [50]]. Not adhering to prescribed EHT treatment may reduce the chances of receiving the benefits of EHT, including increased overall survival, distant disease-free survival, reduced breast cancer specific mortality, decreased risk of recurrence and contralateral breast cancer [3,11].
The mobile smartphone app + PN intervention proposed here addresses a very important need to improve the reported suboptimal adherence to EHT among breast cancer patients, and empower them to play an active role in their healthcare. If proven effective, this study will advance our knowledge about the use of mobile technology as an evidence-based strategy to promote medication adherence among breast cancer patients and improve health outcomes. The rapid proliferation of smartphones to access mobile apps and the Internet among all age groups (and especially among Latinos), offers unprecedented opportunities to improve population health and deliver interventions that can be tailored to the age, gender and ethnicity of patients, with special features to support medication adherence and promote communication with the medical team. Information, reminders, educational content and support can be provided wherever the person is located. Mobile technology also provides the anonymity and convenience people like, can be interactive, and can include links to short videos and other content to provide modeling of adherence behaviors while addressing common barriers.
In addition to the phone app specific features, the study's bicultural and bilingual patient navigator will offer instrumental and psychosocial support, including scheduling appointments, transportation, offering educational, psychosocial support and reinforcement, facilitating interactions with the medical team, managing cancer-related distress, translating and addressing common barriers to care and treatment adherence. Patient navigation have been proven effective in increasing participation in cancer screening, supporting timely access to cancer care, and improving cancer outcomes and patient satisfaction with the care received [19,20,[51], [52], [53]].
The proposed phone app and patient navigation intervention will empower self-monitoring and management by facilitating patient education, self-efficacy, early identification and reporting of side effects, delivery of self-care advice, and timely feedback through direct communication between the patient and the oncology team. The ultimate goal of this innovative multi-communication intervention is to improve overall survival and life expectancy, enhance quality of life, reduce recurrence, and decrease healthcare cost. The anticipated outcome is a scalable, evidence-based, and easily disseminated intervention with potentially broad use to patients using EHT and other oral anticancer agents.
Ethics approval and consent to participate
This study was approved by the UT Health San Antonio Institutional Review Board (HSC20160245H). Informed consent is required for participation.
Declaration of interests
We declare no competing interests.
Clinical trials registration
ClinicalTrials.gov: NCT02850939.
Authors' contributions
All authors made substantial contributions to conception and design of the study, protocol development and drafting of the article. They have reviewed and approved this submission and agree to be held accountable for all aspects of the work.
Acknowledgements
This study is funded by Susan G. Komen (Award No. SAB160005), the Mays Cancer Center (Grant No. P30 CA054174) and Redes En Acción (Grant No. U54 CA153511). The authors wish to thank Cliff Despres for his support editing the manuscript.
References
- 1.American Cancer Society . American Cancer Society, Inc; Atlanta: 2017. Breast Cancer Facts & Figures, 2017-2018.https://www.cancer.org/content/cancer/en/research/cancer-facts-statistics.html [Google Scholar]
- 2.American Cancer Society . American Cancer Society; Atlanta: 2016. Cancer Treatment and Survivorship Facts & Figures 2016-2017.https://www.cancer.org/content/cancer/en/research/cancer-facts-statistics.html [Google Scholar]
- 3.Burstein H.J., Prestrud A.A., Seidenfeld J., Anderson H., Buchholz T.A., Davidson N.E. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer. J. Clin. Oncol. 2014;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. https://www.ncbi.nlm.nih.gov/pubmed/20625130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts M.C., Wheeler S.B., Reeder-Hayes K. Racial/ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am. J. Public Health. 2015 Jul;105(S3) doi: 10.2105/AJPH.2014.302490. https://www.ncbi.nlm.nih.gov/pubmed/25905855 e4-e15. Epub 2015 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCue D.A., Lohr L.K., Pick A.M. Improving adherence to oral cancer therapy in clinical practice. Pharmacotherapy. 2014 May;34(5):481–494. doi: 10.1002/phar.1399. https://www.ncbi.nlm.nih.gov/pubmed/24877187 [DOI] [PubMed] [Google Scholar]
- 6.Paolella G.A., Boyd A.D., Wirth S.M., Cuellar S., Venepalli N.K., Crawford S.Y. Adherence to oral anticancer medications: evolving interprofessional roles and pharmacist workforce considerations. Pharmacy. 2018 Mar 8;6(1):23. doi: 10.3390/pharmacy6010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Liew J.R., Christensen A.J., de Moor J.S. Psychosocial factors in adjuvant hormone therapy for breast cancer: an emerging context for adherence research. J Cancer Surviv. 2014 Sep;8(3):521–531. doi: 10.1007/s11764-014-0374-2. https://www.ncbi.nlm.nih.gov/pubmed/24986227 [DOI] [PubMed] [Google Scholar]
- 8.Berry D.A., Cronin K.A., Plevritis S.K., Fryback D.G., Clarke L., Zelen M. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. https://www.ncbi.nlm.nih.gov/pubmed/16251534 [DOI] [PubMed] [Google Scholar]
- 9.Davies C., Pan H., Godwin J., Gray R., Arriagada R., Raina V. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptorpositive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. https://www.ncbi.nlm.nih.gov/pubmed/23219286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque R., Ahmed S.A., Fisher A., Avila C.C., Shi J., Guo A. Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer. Cancer Med. 2012;1:318–327. doi: 10.1002/cam4.37. https://www.ncbi.nlm.nih.gov/pubmed/23342281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makubate B., Donnan P.T., Dewar J.A., Thompson A.M., McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br. J. Canc. 2013 Apr 16;108(7):1515–1524. doi: 10.1038/bjc.2013.116. https://www.ncbi.nlm.nih.gov/pubmed/23519057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banning M. Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. Eur. J. Canc. Care. 2012 Jan;21(1):10–19. doi: 10.1111/j.1365-2354.2011.01295.x. https://www.ncbi.nlm.nih.gov/pubmed/22004071 [DOI] [PubMed] [Google Scholar]
- 13.McCowan C., Wang S., Thompson A.M., Makubate B., Petrie D.J. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br. J. Canc. 2013 Sep 3;109(5):1172–1180. doi: 10.1038/bjc.2013.464. https://www.ncbi.nlm.nih.gov/pubmed/23949153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershman D.L., Shao T., Kushi L.H., Buono D., Tsai W.Y., Fehrenbacher L., Kwan M., Gomez S.L., Neugut A.I. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Canc. Res. Treat. 2011 Apr;126(2):529–537. doi: 10.1007/s10549-010-1132-4. https://www.ncbi.nlm.nih.gov/pubmed/20803066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunfeld E.A., Hunter M.S., Sikka P., Mittal S. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ. Counsel. 2005 Oct;59(1):97–102. doi: 10.1016/j.pec.2004.10.005. https://www.ncbi.nlm.nih.gov/pubmed/16198223 [DOI] [PubMed] [Google Scholar]
- 16.Miaskowski C., Shockney L., Chlebowski R.T. Adherence to oral endocrine therapy for breast cancer: a nursing perspective. Clin. J. Oncol. Nurs. 2008 Apr 1;12(2):213. doi: 10.1188/08.CJON.213-221. [DOI] [PubMed] [Google Scholar]
- 17.Mathes T., Pieper D., Antoine S.L., Eikermann M. Adherence influencing factors in patients taking oral anticancer agents: a systematic review. Cancer Epidemiol. 2014 Jun;38(3):214–226. doi: 10.1016/j.canep.2014.03.012. https://www.ncbi.nlm.nih.gov/pubmed/24768601 [DOI] [PubMed] [Google Scholar]
- 18.Gerber B.S., Cho Y.I., Arozullah A.M., Lee S.Y. Racial differences in medication adherence: a cross-sectional study of Medicare enrollees. Am. J. Geriatr. Pharmacother. 2010 Apr;8(2):136–145. doi: 10.1016/j.amjopharm.2010.03.002. https://www.ncbi.nlm.nih.gov/pubmed/20439063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez A.G., Pérez‐Stable E.J., Penedo F.J., Talavera G.A., Carrillo J.E., Fernandez M.E., Holden A.E., Munoz E., San Miguel S., Gallion K. Navigating Latinas with breast screen abnormalities to diagnosis. Cancer. 2013 Apr 1;119(7):1298–1305. doi: 10.1002/cncr.27912. https://www.ncbi.nlm.nih.gov/pubmed/23233265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez A.G., Pérez-Stable E.J., Talavera G.A., Penedo F.J., Carrillo J.E., Fernandez M.E., Muñoz E., Parma D.L., Holden A.E.C., De Majors S.S., Nápoles A., Castañeda S.F., Gallion K.J. Time to definitive diagnosis of breast cancer in Latina and non-Hispanic white women: the six cities study. SpringerPlus. 2013 Dec;2(1):84. doi: 10.1186/2193-1801-2-84. https://www.ncbi.nlm.nih.gov/pubmed/23233265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castaldi M., Safadjou S., Elrafei T., McNelis J. A Multidisciplinary Patient Navigation Program improves compliance with adjuvant breast cancer therapy in a Public Hospital. Am. J. Med. Qual. 2017 Jul;32(4):406–413. doi: 10.1177/1062860616656250. https://www.ncbi.nlm.nih.gov/pubmed/27357461 [DOI] [PubMed] [Google Scholar]
- 22.Heitkemper E.M., Mamykina L., Travers J., Smaldone A. Do health information technology self-management interventions improve glycemic control in medically underserved adults with diabetes? A systematic review and meta-analysis. J. Am. Med. Inf. Assoc. 2017 Mar 31;24(5):1024–1035. doi: 10.1093/jamia/ocx025. https://www.ncbi.nlm.nih.gov/pubmed/28379397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmick G., Edmond S.N., Bosworth H.B., Peppercorn J., Marcom P.K., Blackwell K. Medication taking behaviors among breast cancer patients on adjuvant endocrine therapy. Breast. 2015 Oct 1;24(5):630–636. doi: 10.1016/j.breast.2015.06.010. https://www.ncbi.nlm.nih.gov/pubmed/26189978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert L.K., Balneaves L.G., Howard A.F., Gotay C.C. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Canc. Res. Treat. 2017 Nov 6:1–9. doi: 10.1007/s10549-017-4561-5. https://www.ncbi.nlm.nih.gov/pubmed/29110151 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Malin J.L., Diamant A.L., Thind A., Maly R.C. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider–patient communication. Breast Canc. Res. Treat. 2013 Feb 1;137(3):829–836. doi: 10.1007/s10549-012-2387-8. https://www.ncbi.nlm.nih.gov/pubmed/23263740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wouters H., Stiggelbout A.M., Bouvy M.L., Maatman G.A., Van Geffen E.C., Vree R. Endocrine therapy for breast cancer: assessing an array of women's treatment experiences and perceptions, their perceived self-efficacy and nonadherence. Clin. Breast Canc. 2014 Dec 1;14(6):460–467. doi: 10.1016/j.clbc.2014.04.005. https://www.ncbi.nlm.nih.gov/pubmed/24981234 [DOI] [PubMed] [Google Scholar]
- 27.Wuensch P., Hahne A., Haidinger R., Meissler K., Tenter B., Stoll C. Discontinuation and non-adherence to endocrine therapy in breast cancer patients: is lack of communication the decisive factor? J. Canc. Res. Clin. Oncol. 2015 Jan 1;141(1):55–60. doi: 10.1007/s00432-014-1779-z. https://www.ncbi.nlm.nih.gov/pubmed/25085010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiMatteo M.R. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004 Mar;23(2):207. doi: 10.1037/0278-6133.23.2.207. https://www.ncbi.nlm.nih.gov/pubmed/15008666 [DOI] [PubMed] [Google Scholar]
- 29.Cluze C., Rey D., Huiart L., BenDiane M.K., Bouhnik A.D., Berenger C. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann. Oncol. 2011 Jul 25;23(4):882–890. doi: 10.1093/annonc/mdr330. https://www.ncbi.nlm.nih.gov/pubmed/21788360 [DOI] [PubMed] [Google Scholar]
- 30.Huiart L., Bouhnik A.D., Rey D., Tarpin C., Cluze C., Bendiane M.K. Early discontinuation of tamoxifen intake in younger women with breast cancer: is it time to rethink the way it is prescribed? Eur. J. Canc. 2012 Sep 1;48(13):1939–1946. doi: 10.1016/j.ejca.2012.03.004. https://www.ncbi.nlm.nih.gov/pubmed/22464016 [DOI] [PubMed] [Google Scholar]
- 31.Quinn E.M., Fleming C., O'Sullivan M.J. Endocrine therapy adherence: a cross-sectional study of factors affecting adherence and discontinuation of therapy. Ir. J. Med. Sci. 2016 May 1;185(2):383–392. doi: 10.1007/s11845-015-1307-4. https://www.ncbi.nlm.nih.gov/pubmed/25971465 [DOI] [PubMed] [Google Scholar]
- 32.Bandura A. Prentice-Hall; Englewood Cliffs, NJ: 1986. Social Foundations of Thought and Action: a Social Cognitive Theory. [Google Scholar]
- 33.Teeter B.S., Kavookjian J. Telephone-based motivational interviewing for medication adherence: a systematic review. Transl. Behav. Med. 2014;4(4):372–381. doi: 10.1007/s13142-014-0270-3. https://www.ncbi.nlm.nih.gov/pubmed/25584086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez A.G., Vélez L.F., Chalela P., Gallion K., McAlister A.L. Diffusion Acceleration: a model for behavior change and social mobilization. In: Huff R.M., Kline M.V., Peterson D.V., editors. Health Promotion in Multicultural Populations: a Handbook for Practitioners and Students. third ed. SAGE Publications; Thousand Oaks, CA: 2015. [Google Scholar]
- 35.Taylor S.J., Bogdan R., DeVault M.L. John Wiley & Sons; New Jersey: 2016. Introduction to Qualitative Research Methods: a Guidebook and Resource. [Google Scholar]
- 36.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. https://www.ncbi.nlm.nih.gov/pubmed/18929686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morisky D.E., Ang A., Krousel-Wood M., Ward H.J. Predictive validity of a medication adherence measure in an outpatient setting. J. Clin. Hypertens. 2008 May;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. https://www.ncbi.nlm.nih.gov/pubmed/18453793 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Kroenke K., Strine T.W., Spritzer R.L., Williams J.B., Berry J.T., Mokdad A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 39.McAlister A.L., Perry C.L., Parcel G.S. How individuals, environments and health behaviors interact. In: Glanz K., Rimer B.K., Viswanath K., editors. Health Behavior and Health Education: Theory, Research, and Practice. fourth ed. Jossey-Bass; San Francisco, CA: 2008. [Google Scholar]
- 40.Cella D.F., Tulsky D.S., Gray G. The functional assessment of cancer therapy scale: development and validation of the general measure. J. Clin. Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. https://www.ncbi.nlm.nih.gov/pubmed/8445433 [DOI] [PubMed] [Google Scholar]
- 41.Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006 May 22;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. https://www.ncbi.nlm.nih.gov/pubmed/16717171 [DOI] [PubMed] [Google Scholar]
- 42.Hershman D.L., Shao T., Kushi L.H., Buono D., Tsai W.Y., Fehrenbacher L., Kwan M., Gomez S.L., Neugut A.I. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Canc. Res. Treat. 2011 Apr;126(2):529–537. doi: 10.1007/s10549-010-1132-4. https://www.ncbi.nlm.nih.gov/pubmed/20803066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziller V., Kalder M., Albert U.S., Holzhauer W., Ziller M., Wagner U., Hadji P. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann. Oncol. 2009 Mar;20(3):431–436. doi: 10.1093/annonc/mdn646. https://www.ncbi.nlm.nih.gov/pubmed/19150950 an Epub 2009 Jan 15. [DOI] [PubMed] [Google Scholar]
- 44.Partridge A.H., LaFountain A., Mayer E., Taylor B.S., Winer E., Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J. Clin. Oncol. 2008 Feb 1;26(4):556–562. doi: 10.1200/JCO.2007.11.5451. https://www.ncbi.nlm.nih.gov/pubmed/18180462 [DOI] [PubMed] [Google Scholar]
- 45.Sedjo R.L., Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Canc. Res. Treat. 2011 Jan;125(1):191–200. doi: 10.1007/s10549-010-0952-6. https://www.ncbi.nlm.nih.gov/pubmed/20495864 [DOI] [PubMed] [Google Scholar]
- 46.Valeri L., VanderWeele T.J. Mediation analysis allowing for exposure–mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol. Methods. 2013 Jun;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aiello Bowles E.J., Boudreau D.M., Chubak J., Yu O., Fujii M., Chestnut J., Buist D.S. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract. 2012 Jul 17;8(6):e149–e157. doi: 10.1200/JOP.2012.000543. https://www.ncbi.nlm.nih.gov/pubmed/23598850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henry N.L., Azzouz F., Desta Z., Li L., Nguyen A.T., Lemler S., Hayden J., Tarpinian K., Yakim E., Flockhart D.A., Stearns V. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J. Clin. Oncol. 2012 Feb 13;30(9):936–942. doi: 10.1200/JCO.2011.38.0261. https://www.ncbi.nlm.nih.gov/pubmed/22331951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ochayon L., Zelker R., Kaduri L., Kadmon I. Relationship between severity of symptoms and quality of life in patients with breast cancer receiving adjuvant hormonal therapy. Oncol. Nurs. Forum. 2010 Sep 1;37(5):E349–E358. doi: 10.1188/10.ONF.E349-E358. https://www.ncbi.nlm.nih.gov/pubmed/20797943 [DOI] [PubMed] [Google Scholar]
- 50.Van Londen G.J., Beckjord E.B., Dew M.A., Cooper K.L., Davidson N.E., Bovbjerg D.H., Donovan H.S., Thurston R.C., Morse J.Q., Nutt S., Rechis R. Associations between adjuvant endocrine therapy and onset of physical and emotional concerns among breast cancer survivors. Support. Care Canc. 2014 Apr 1;22(4):937–945. doi: 10.1007/s00520-013-2041-y. https://www.ncbi.nlm.nih.gov/pubmed/24271937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paskett E.D., Harrop J., Wells K.J. Patient navigation: an update on the state of the science. Ca - Cancer J. Clin. 2011 Jul 1;61(4):237–249. doi: 10.3322/caac.20111. https://www.ncbi.nlm.nih.gov/pubmed/21659419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudley D.J., Drake J., Quinlan J., Holden A., Saegert P., Karnad A., Ramirez A. Beneficial effects of a combined navigator/promotora approach for Hispanic women diagnosed with breast abnormalities. Cancer Epidemiol. Biomark. Prev. 2012 Oct;21(10):1639–1644. doi: 10.1158/1055-9965.EPI-12-0538. https://www.ncbi.nlm.nih.gov/pubmed/23045538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Percac-Lima S., Ashburner J.M., McCarthy A.M., Piawah S., Atlas S.J. Patient navigation to improve follow-up of abnormal mammograms among disadvantaged women. J. Wom. Health. 2015 Feb 1;24(2):138–143. doi: 10.1089/jwh.2014.4954. https://www.ncbi.nlm.nih.gov/pubmed/25522246 [DOI] [PMC free article] [PubMed] [Google Scholar]