Highlights
-
•
Microbial and environmental signals set tonic activation status of barrier tissues.
-
•
Signaling from barrier tissues licenses dendritic cells to induce T helper 2 cells.
-
•
Pulmonary immune system in early-life prone to asthma development.
-
•
Mechanistic understanding needed to translate epidemiological findings in therapies.
Abstract
The hygiene hypothesis was initially proposed as an explanation for the alarming rise in allergy prevalence in the last century. The immunological idea behind this hypothesis was a lack of infections associated with a Western lifestyle and a consequential reduction in type 1 immune responses. It is now understood that the development of tolerance to allergens depends on microbial colonization and immunostimulatory environmental signals during early-life or passed on by the mother. These environmental cues are sensed and integrated by barrier epithelial cells of the lungs and possibly skin, which in turn instruct dendritic cells to regulate or impede adaptive T cell responses. Recent reports also implicate immunoregulatory macrophages as powerful suppressors of allergy by the microbiome. We propose that loss of adequate microbial stimulation due to a Western lifestyle may result in hypersensitive barrier tissues and the observed rise in type 2 allergic disease.
Current Opinion in Immunology 2018, 54:102–108
This review comes from a themed issue on Allergy and hypersensitivity
Edited by Onur Boyman, Alexander Eggel and Mario Noti
For a complete overview see the Issue and the Editorial
Available online 7th July 2018
https://doi.org/10.1016/j.coi.2018.06.007
0952-7915/© 2018 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Allergic sensitization is characterized by the presence of allergen-specific immunoglobulin E (IgE) in serum. Exposure to allergens via inhalation, ingestion or contact with the skin can lead to diseases such as asthma, hay fever, eczema and, in some cases, to systemic anaphylaxis. During the last 150 years, allergies have emerged in a very rapid way and their prevalence is still on the rise. Nowadays, more than 30% of children are allergic, up to 10% of children suffer from asthma and allergic rhinitis, and 5–7% of children have developed food allergy. It is still not entirely clear why asthma prevalence is so high, but the rapid time frame of its origination and expansion suggests that environmental or behavioral changes in Western lifestyle are involved.
A modern lifestyle is associated with dysbiosis
An important evolution of the last 150 years is a successful decrease of infectious disease burden, due to the massive introduction of hygiene measures, antibiotics, and vaccines. In 1989, Strachan observed that growing up in large families with more older siblings decreased the chances of developing hay fever or eczema [1]. He postulated that the recent increase in allergy incidence was a result of `declining family size, improvements in household amenities, and higher standards of personal cleanliness’, which had reduced `the opportunity for cross infection in young families’. The original ‘hygiene hypothesis’ was thus introduced. Since then, this hypothesis has been supported by numerous studies, especially in murine models, showing that exposure to bacteria, viruses, helminths or microbe-derived products could protect from allergy (reviewed in [2], [3••]). However, it should be kept in mind that not all pathogens are protective; for instance, respiratory syncytial virus (RSV) or rhinovirus are associated with a higher risk to develop wheeze and asthma up to adulthood [4].
Changes in lifestyle can also heavily influence the composition and diversity of the microbiome at several mucosal surfaces. These microbial communities have co-evolved with and within the human body for millions of years, and, consequently, the human immune system has been calibrated and fine-tuned so to maintain and shape symbiotic relationships with them (reviewed in [5]). Two theories, the ‘Old friends’ and the ‘Biodiversity’ hypotheses, have been proposed by Rook and by Haahtela as a more accurate, or at least complementary, explanation for the recent allergy pandemic [6,7]. They stipulate that the reason for the increased incidence in allergic disorders is a reduced exposure to such beneficial symbiotic bacteria or parasites. Indeed, several studies have reported that alterations in the composition of the skin, the nose or the gut microbiome are associated with eczema, asthma and food allergy [8, 9, 10]. These changes do not affect a single commensal, but rather reflect a reduced total microbial diversity [11], and they may be caused by several factors, including sibling order in the family [12], exposure to animals [13], and other early-life events [14]. The importance of a healthy microbiome in controlling allergies was further substantiated in mice, with germ-free mice being especially prone to develop overt allergic (airway) disease, a phenotype reverted by microbial recolonization [15,16]. However, other studies showed that germ-free mice are not universally more susceptible to house dust mite driven asthma, and that only selected strains of lung microbiota seem to suppress asthma [17]. During the last 30 years, the body of correlative epidemiological studies has expanded vastly, and is the subject of many excellent reviews. Here, we will zoom in on recent advances in the search for the underlying immunological mechanisms explaining the observed effects.
Microbes induce protective regulatory DCs and T cells
Allergies are generally aberrant immune reactions to innocuous antigens, orchestrated by T helper 2 (Th2) cells and type 2 innate lymphoid cells (ILC2s). In the case of asthma, this type 2 cell activity leads to mucus hypersecretion, goblet cell hyperplasia, smooth muscle cell hyperreactivity, and the infiltration and/or activation of eosinophils, mast cells and basophils, ultimately culminating in breathing difficulties and airway remodeling [18]. Dendritic cells (DCs) are always found at the body’s barriers, and because they express a wide range of pattern recognition receptors (PRRs), they can sense the environment for the presence of danger signals [19]. Our group has shown that Th2 responses to house dust mite (HDM) allergens were induced by IRF4-dependent cDC2s in the lungs and in the skin [20,21•] (Figure 1). These cDC2s capture the HDM allergens in the airways and migrate to the draining lymph nodes, requiring ILC2-derived IL-13, where they present the allergens to naïve T cells [22]. It is easy to imagine that environmental changes sensed at the level of the lungs, the skin but also of the gut will modify the context of allergen recognition by DCs, and either protect against or enhance allergic responses.
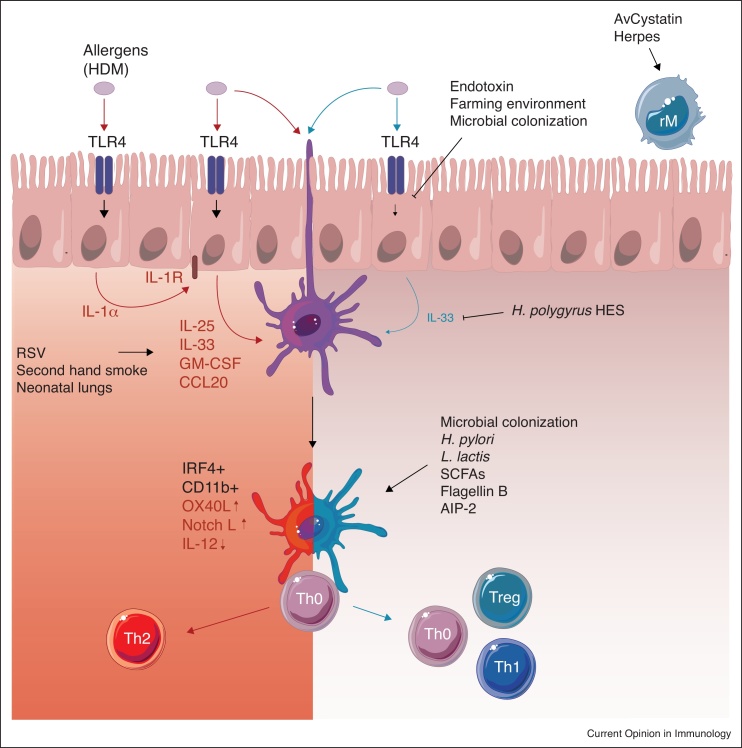
Figure 1.
Proposed model of airway tolerance. In the absence of immunoregulatory pathways, epithelial barrier cells readily respond to allergen binding on their pattern recognition receptors, among which TLR4, by the secretion of inflammatory mediators (IL-1α, IL-25, IL-33, GM-CSF, CCL20, and others). These mediators license antigen-bearing IRF4+ CD11b+ conventional dendritic cells (cDC2s) to polarize naïve T cells to T helper 2 (Th2) cells in the lung-draining lymph nodes. Neonatal and germ-free mice are especially prone to develop such Th2 responses. Respiratory syncytial virus (RSV) infection and second hand cigarette smoke, two known asthma risk factors, increase IL-33 secretion and may thereby stimulate this pathway. Exposure to endotoxin, farm dust or microbial colonization blunts the epithelial response by increasing the expression of negative regulators. Epithelial IL-33 release is also inhibited by helminth-derived excreted and secreted products (HES). DCs devoid of epithelial activation signals do not induce T cell activation (Th0). Other protective factors impede T cell activity by influencing the maturation, antigen presentation, or phagocytic capacity of DCs. Some protective factors induce DCs that provoke regulatory T cell (Treg) activity or T helper 1 (Th1) activity. Regulatory macrophages (rM) can also induce Tregs, or block DC-mediated Th2-polarization. Abbreviations: HDM, house dust mite; TLR4, Toll like receptor 4; IL-1R, IL-1 receptor; H. pylori, Helicobacter pylori; L. lactis, Lactococcus lactis; SCFA, short chain fatty acids; AIP-2, anti-inflammatory protein 2.
Chronic Helicobacter pylori infection has been inversely linked to asthma in humans and can effectively protect mice from OVA-induced asthma [23,24]. In mice, H. pylori infection induced the accumulation of CD103+ cDCs in the lungs, which were required for the protection, as was their IL-10 production [24]. In a recent study, semi-therapeutic H. pylori extract treatment also reduced airway allergy, shifted the CD11b+/CD103+ DC ratio in the lungs, and reduced the antigen processing by lung and lymph node DCs [25]. Other studies demonstrated protective modulation of in vitro bone-marrow derived DC cultures (BMDCs). A synthetic TLR1/TLR2-agonist induced LPS-tolerance and IL-10 production in BMDCs, whereas the cowshed Lactococcus lactis instigated a Th1-polarizing program, both rendering the BMDCs unable to sensitize mice to OVA-allergen upon adoptive transfer [26,27•].
Trompette et al. recently found that feeding mice a fiber-rich diet changed the composition of the lung and gut microbiome, the latter metabolizing the fiber into circulating short-chain fatty acids (SCFA’s) [28]. The increased SCFA levels protected the mice from allergic lung inflammation. Mechanistically, the SCFA’s altered DC precursor generation in the bone marrow, and the DCs subsequently seeding the lungs had a higher phagocytic capacity and were impaired in polarizing Th2 cells. Additional studies have supported the protective effect of dietary fiber supplementation on allergic asthma development in mice [29], and on wheeze in human infants when the fiber was given to the pregnant mother [30].
One mechanism by which the DCs in microbe-exposed animals can confer protection, is by inducing the generation of regulatory T cells (Tregs). Microbial colonization in 2-week old mice was shown to be necessary for the transient upregulation of PD-L1 on lung CD11b + DCs, and the expansion of a specific pulmonary Treg subset [31]. PD-L1 blockade in neonates resulted in exaggerated responsiveness to HDM through adulthood, suggesting a crucial role for this microbial-induced DC–Treg axis for immunological tolerance. In another mouse model of H. pylori-mediated asthma protection, the Helicobacter infection inhibited TLR-induced DC maturation and reprogrammed the DCs towards a FoxP3+ Treg-polarizing phenotype [32]. The bacterial component flagellin B, given semi-therapeutically together with allergen, could also inhibit murine allergic asthma symptoms in a DC− and CD25+ Treg-dependent manner [33•].
Although helminths are prototypical inducers of type 2 immunity, they have been correlated with reduced allergen skin prick test reactivity, and to some degree with asthma protection (reviewed in [34]). A general explanation for this non-intuitive association is that helminths induce a so-called ‘modified Th2′ response, with immunoregulatory cells such as Tregs complementing the Th2-arm of immunity, and regulating the response to bystander antigens such as aeroallergens. Therefore, several groups have tried to find helminth-derived products with immunomodulatory properties that could be used to suppress Th2 immunity. For instance, an anti-inflammatory protein (-2; AIP-2) from the parasitic hookworm was identified to suppress murine airway allergy in a DC-dependent and Treg-dependent manner [35]. In another study, the helminth-derived immunomodulator AvCystatin was demonstrated to induce regulatory macrophages that protected against experimental asthma upon adoptive transfer [36]. Regulatory alveolar macrophages from bone marrow origin were recently also implicated in long-lasting protection conferred by a latent murine gammaherpesvirus infection, a model for Epstein–Barr virus infection in mice [37•]. The regulatory macrophages induced by the infection replaced the long-lived and self-replenishing alveolar macrophages that are generated shortly after birth, and became long-lived as well. This potentially explains the long-lasting effects of microbial stimuli in the lungs on allergy suppression.
Allergic asthma is initiated by aberrant immune responses at barrier tissues
To initiate an allergen-specific Th2 response, cDC2s need to be instructed by barrier epithelial cells (ECs) lining the airways. Barrier ECs are permanently exposed to environmental insults or innocuous signals and, like DCs, they are well-equipped to integrate these signals via a range of PRRs (reviewed in [38]). Activation of PRRs on ECs by allergens induces NF-κB activation and ROS production, resulting in the secretion of a wide range of inflammatory mediators, among which the cytokines IL-33, IL-25 and TSLP. DCs react to these cytokines by OX40L and Notch ligand upregulation, and downregulation of IL-12 production, an activation state that favors Th2 polarization in the lung-draining lymph node [39,40]. Interestingly, the barrier tissue of the skin also constitutes a possible entry route for aeroallergens [21•]. Thus, barrier cells act very upstream in the inflammatory cascade of events leading to allergic sensitization (Figure 1).
Our group has previously reported that the PRR toll-like receptor 4 (TLR4) on airway ECs was critically necessary to mount a Th2-mediated asthmatic response to HDM [41]. Strikingly, several HDM allergens have the intrinsic capacity to facilitate or amplify TLR4 signaling by binding directly to proteins of the TLR4 signaling complex or to its ligands [42,43]. However, TLR4 is best known as the receptor for LPS, also termed endotoxin, a component of gram-negative bacteria. It is difficult to reconcile how a receptor specialized in bacterial sensing can contribute to Th2 immunity and allergy, especially given the fact that high endotoxin levels in children’s mattresses are protective against atopic sensitization and asthma in humans and mice [44, 45, 46]. Another study also associated house dust endotoxin levels with a significantly reduced risk of allergic sensitization or eczema, specifically in children with a polymorphism in the CD14 gene [47]. In fact, a body of epidemiological studies have convincingly correlated a traditional farming environment, where endotoxin levels are high, with protection against hay fever, allergic sensitization, and asthma (reviewed in [48]). In a hallmark study, children growing up on agricultural Hutterite and Amish farms in the US were compared, and the latter were found to have six times less chance of developing atopy and asthma [49••]. These two farming populations share a similar genetic ancestry and lifestyle. Farming practices, however, differ, and the Amish house dust contained almost 7 times more endotoxin than the house dust from Hutterite farms. Only the transfer of the Amish dust intranasally to mice inhibited subsequent experimental asthma development. We have recently confirmed that farm dust collected from Bavarian farms (in which farming practices resemble the Amish’s ones), and LPS, conferred protection against experimental asthma. This protection was mediated by an increased epithelial expression of TNFAIP3 (better known as A20), a negative regulator of the NF-κB pathway, which blunted the epithelial cell response to HDM and downstream DC activity [50]. A similar tolerance to LPS mediated by A20 induction was demonstrated in intestinal ECs [51]. Interestingly, A20 expression was very low in neonatal rats, spontaneously increased shortly after birth coinciding with microbial colonization, and could be downregulated by treatment with antibiotics. It remains to be investigated if the gut or lung microbiota can similarly influence expression of A20 and other negative regulators in airway ECs. EC modulation has recently been demonstrated for Heligmosomoides polygyrus, a helminth often confirmed to protect against murine allergy [52]. Secreted and excreted products (HES) of this parasite inhibited IL-33 release by ECs and thereby suppressed Alternaria-induced airway allergy [3••]). Mechanistically, the H. polygyrus alarmin release inhibitor (HpARI), a 26 kDa protein, binds to activated IL-33 and at the same time tethers IL-33 to the DNA of necrotic cells, thus inhibiting IL-33 action in a dual manner and inhibiting innate eosinophilic airway inflammation [53••]. Other molecules secreted by the parasite are more related to TGFβ and can induce a Foxp3+ Treg population with immunoregulatory potential [54].
A dysregulated immune response at the skin can cause atopic dermatitis (AD), which in itself is a risk factor to accumulate more allergies later in life, among which asthma, a process known as ‘the atopic march’. In fact, AD and asthma share several risk factors. AD is strongly correlated with changes in the skin microbiome, the most well-known being pertinent Staphylococcus aureus colonization of allergic skin. In a recent study, IL-17Rα−/− mice spontaneously developed AD with naturally occurring skin dysbiosis and a compromised skin barrier, and antibiotic treatment ameliorated skin inflammation [55]. A similar dysbiosis–AD axis has also been demonstrated in ADAM17−/− mice [56]. Topical treatment with non-pathogenic bacteria, on the other hand, can alleviate cutaneous inflammation in murine AD [57]. It has become clear in recent years that tonic sensing of skin commensals heavily shapes host DC and T cell functions [58, 59, 60]. It remains to be investigated what the relative importance is of passive barrier integrity and active signaling through keratinocyte PRRs, also poised to rapidly respond to innate immune ligands, in this microbe-immune cell cross-talk [61]. It will also be of great interest to study how environmental exposures influence the skin microbiome and/or the immune threshold of skin epithelium.
One intriguing observation is that allergies tend to develop early in life. In the same time window, and even in utero, protective effects of environmental factors and the microbiome are also the strongest [62,63]. We recently demonstrated that the lung environment in neonatal mice is strongly type 2-skewed, with a gradual increase in IL-33 release by lung ECs, and with the spontaneous recruitment of several Th2-associated innate immune cells, peaking 2 weeks after birth [64••]. This spontaneous wave of early type 2 immunity is likely to be caused by the mechanical stress induced by the breathing patterns [65], but also by the constant remodeling necessary to build up new lung structures. Interestingly, this period is prone to favor stronger Th2 sensitization to inhaled allergens [64••], but also to favor lower immunity to bacteria [65]. Many environmental factors, like second hand smoking or RSV infection are known to facilitate Th2 sensitization in children. These triggers have in common that they induce high levels of IL-33 [66,67]. It is tempting to speculate that these risk factors act by prolonging or amplifying the epithelial cytokine response to allergens during early-life, and that combined early-life exposures thus define the final threshold for EC activation.
Conclusion
Effects of microbes on inducing Treg cells, Th1 cells and allergen cross-reactive antibody responses, are well-observed. In addition, we propose a model in which environmental and microbial stimuli are sensed and integrated by barrier tissues of the lung, the skin and the gut, resulting in a tonic DC activation status promoting either inflammatory or tolerogenic immunity. Together with direct effects on DCs and T cells, most protective stimuli thus seem to converge in the same central tolerogenic immune pathways. Fully understanding the fundamental immunological pathways underlying these protective triggers, their relative contribution, and how they interact, should hopefully allow us to pinpoint, modify or newly develop prophylactic or therapeutic therapies to cure asthma.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Research in the Lambrecht and Hammad lab is supported by an Advanced European Research Council (ERC) grant, an FWO Flanders Excellence of Science grant, and the World Without Asthma (AWWA) program of the Dutch Lung Foundation. Figure 1 includes derivative material from Servier Medical Art (https://smart.servier.com/) by Servier, available under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Contributor Information
Hamida Hammad, Email: Hamida.Hammad@UGent.be.
Bart N Lambrecht, Email: bart.lambrecht@ugent.be.
References
- 1.Strachan D.P. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smits H.H., Hiemstra P.S., Prazeres da Costa C., Ege M., Edwards M., Garn H. Microbes and asthma: opportunities for intervention. J Allergy Clin Immunol. 2016;137:690–697. doi: 10.1016/j.jaci.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 3••.McSorley H.J., Blair N.F., Smith K.A., McKenzie A.N., Maizels R.M. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7:1068–1078. doi: 10.1038/mi.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence of very early immunomodulation by a soluble parasite product.H. polygyrus excretory/secretory (HES) products were shown to block early Alternaria-induced IL-33 release by epithelial cells, and to strongly suppress the innate eosinophilia and ILC2 response. In an OVA asthma model using Alternaria as an adjuvant, HES also inhibited adaptive type 2 responses. The observation that parasites release products that block early epithelial IL-33 release, probably to inhibit the host’s anti-parasite immune responses, affirms the importance of this process in the development of type 2 immunity.
- 4.Feldman A.S., He Y., Moore M.L., Hershenson M.B., Hartert T.V. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191:34–44. doi: 10.1164/rccm.201405-0901PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid Y., Harrison O.J. 2017. Homeostatic Immunity and the Microbiota; pp. 562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rook G.A. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:5–15. doi: 10.1007/s12016-011-8285-8. [DOI] [PubMed] [Google Scholar]
- 7.Hanski I., von Hertzen L., Fyhrquist N., Koskinen K., Torppa K., Laatikainen T. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci U S A. 2012;109:8334–8339. doi: 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrahamsson T.R., Jakobsson H.E., Andersson A.F., Bjorksten B., Engstrand L., Jenmalm M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 9.Clausen M.L., Agner T., Lilje B., Edslev S.M., Johannesen T.B., Andersen P.S. Association of disease severity with skin microbiome and filaggrin gene mutations in adult atopic dermatitis. JAMA Dermatol. 2018;154:293–300. doi: 10.1001/jamadermatol.2017.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage J.H., Lee-Sarwar K.A., Sordillo J., Bunyavanich S., Zhou Y., O’Connor G. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy: Eur J Allergy Clin Immunol. 2018;73:145–152. doi: 10.1111/all.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depner M., Ege M.J., Cox M.J., Dwyer S., Walker A.W., Birzele L.T. Bacterial microbiota of the upper respiratory tract and childhood asthma. J Allergy Clin Immunol. 2017;139 doi: 10.1016/j.jaci.2016.05.050. 826–834.e13. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa K., Linnemann R.W., Mansbach J.M., Ajami N.J., Espinola J.A., Fiechtner L.G. Household siblings and nasal and fecal microbiota in infants. Pediatr Int. 2017;59:473–481. doi: 10.1111/ped.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tun H.M., Konya T., Takaro T.K., Brook J.R., Chari R., Field C.J. Exposure to household furry pets influences the gut microbiota of infant at 3–4 months following various birth scenarios. Microbiome. 2017;5:40. doi: 10.1186/s40168-017-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin R., Makino H., Cetinyurek Yavuz A., Ben-Amor K., Roelofs M., Ishikawa E. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS ONE. 2016;11:e0158498. doi: 10.1371/journal.pone.0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst T., Sichelstiel A., Schar C., Yadava K., Burki K., Cahenzli J. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184:198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 16.Atarashi K., Tanoue T., Oshima K., Suda W., Nagano Y., Nishikawa H. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 17.Remot A., Descamps D., Noordine M.L., Boukadiri A., Mathieu E., Robert V. Bacteria isolated from lung modulate asthma susceptibility in mice. ISME J. 2017;11:1061–1074. doi: 10.1038/ismej.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambrecht B.N., Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 19.Lambrecht B.N. Dendritic cells and the regulation of the allergic immune response. Allergy. 2005;60:271–282. doi: 10.1111/j.1398-9995.2005.00708.x. [DOI] [PubMed] [Google Scholar]
- 20.Plantinga M., Guilliams M., Vanheerswynghels M., Deswarte K., Branco-Madeira F., Toussaint W. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 21•.Deckers J., Sichien D., Plantinga M., Van Moorleghem J., Vanheerswynghels M., Hoste E. Epicutaneous sensitization to house dust mite allergen requires interferon regulatory factor 4-dependent dermal dendritic cells. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2016.12.970. [DOI] [PubMed] [Google Scholar]; This study reveals that unmanipulated skin is a possible entry route for HDM allergens, with IRF4-dependent skin cDC2s priming sensitization to subsequent airway allergen challenges. This implicates that perturbations or exposures at the level of the skin may be relevant for the ‘hygiene effect’ for asthma.
- 22.Halim T.Y., Steer C.A., Matha L., Gold M.J., Martinez-Gonzalez I., McNagny K.M. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amberbir A., Medhin G., Erku W., Alem A., Simms R., Robinson K. Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy. 2011;41:1422–1430. doi: 10.1111/j.1365-2222.2011.03831.x. [DOI] [PubMed] [Google Scholar]
- 24.Engler D.B., Reuter S., van Wijck Y., Urban S., Kyburz A., Maxeiner J. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc Natl Acad Sci U S A. 2014;111:11810–11815. doi: 10.1073/pnas.1410579111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Wijck Y., De Kleijn S., John-Schuster G., Mertens T.C.J., Hiemstra P.S., Müller A. Therapeutic application of an extract of therapeutic application of an extract of Helicobacter pylori ameliorates the development of allergic airway disease. J Immunol. 2018;200:1570–1579. doi: 10.4049/jimmunol.1700987. [DOI] [PubMed] [Google Scholar]
- 26.Stiehm M., Peters K., Wiesmuller K.H., Bufe A., Peters M. A novel synthetic lipopeptide is allergy-protective by the induction of LPS-tolerance. Clin Exp Allergy. 2013;43:785–797. doi: 10.1111/cea.12116. [DOI] [PubMed] [Google Scholar]
- 27•.Stein K., Brand S., Jenckel A., Sigmund A., Chen Z.J., Kirschning C.J. Endosomal recognition of Lactococcus lactis G121 and its RNA by dendritic cells is key to its allergy-protective effects. J Allergy Clin Immunol. 2017;139 doi: 10.1016/j.jaci.2016.06.018. 667–678.e5. [DOI] [PubMed] [Google Scholar]; Study on direct protective DC modulation by the cowshedLactococcus lactis. Upon bacterial uptake, endosomal acidification, and TLR-mediated recognition of the released RNA, human moDCs or BMDCs secreted Th1 polarizing cytokines. In coculture, Lactococcus-pulsed DCs induced Th1 cells, and in vivo, they conferred protection against OVA-induced allergic asthma.
- 28.Trompette A., Gollwitzer E.S., Yadava K., Sichelstiel A.K., Sprenger N., Ngom-Bru C. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 29.Verheijden K.A., Willemsen L.E., Braber S., Leusink-Muis T., Jeurink P.V., Garssen J. The development of allergic inflammation in a murine house dust mite asthma model is suppressed by synbiotic mixtures of non-digestible oligosaccharides and Bifidobacterium breve M-16V. Eur J Nutr. 2016;55:1141–1151. doi: 10.1007/s00394-015-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorburn A.N., McKenzie C.I., Shen S., Stanley D., Macia L., Mason L.J. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6:7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 31.Gollwitzer E.S., Saglani S., Trompette A., Yadava K., Sherburn R., McCoy K.D. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014;20:642–647. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- 32.Oertli M., Sundquist M., Hitzler I., Engler D.B., Arnold I.C., Reuter S. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122:1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Shim J.U., Lee S.E., Hwang W., Lee C., Park J.W., Sohn J.H. Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. J Allergy Clin Immunol. 2016;137:426–435. doi: 10.1016/j.jaci.2015.07.010. [DOI] [PubMed] [Google Scholar]; Use of microbe-derived flagellin B to suppress OVA-allergic or HDM-allergic asthma in a semi-therapeutic setting. The protection was dependent on TLR5 and on the generation of regulatory DCs, which could actively suppress asthma development upon adoptive transfer. Flagellin B treatment of PBMCs from HDM-allergic asthma patients rendered the DCs more prone to induce Tregs in an IL-10 dependent manner, making this microbe-derived product a possible candidate for cell-based asthma therapy.
- 34.Cruz A.A., Cooper P.J., Figueiredo C.A., Alcantara-Neves N.M., Rodrigues L.C., Barreto M.L. Global issues in allergy and immunology: parasitic infections and allergy. J Allergy Clin Immunol. 2017;140:1217–1228. doi: 10.1016/j.jaci.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Navarro S., Pickering D.A., Ferreira I.B., Jones L., Ryan S., Troy S. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aaf8807. 362ra. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler T., Rausch S., Steinfelder S., Klotz C., Hepworth M.R., Kühl A.a. A novel regulatory macrophage induced by a helminth molecule instructs IL-10 in CD4+ T cells and protects against mucosal inflammation. J Immunol. 2015;194:1555–1564. doi: 10.4049/jimmunol.1401217. [DOI] [PubMed] [Google Scholar]
- 37•.Machiels B., Dourcy M., Xiao X., Javaux J., Mesnil C., Sabatel C. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat Immunol. 2017;18:1310–1320. doi: 10.1038/ni.3857. [DOI] [PubMed] [Google Scholar]; This mouse study demonstrates that infection with the murid herpesvirus MuHV-4 induces the replacement of resident alveolar macrophages (AMs) with monocytes from bone marrow origin, which persist long term in the lungs and are sufficient to block HDM-induced airway allergy. Preincubation of HDM-pulsed BMDCs with AMs from infected animals rendered the BMDCs less efficient in sensitizing acceptor mice to HDM-mediated asthma.
- 38.Hammad H., Lambrecht B.N. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Kuipers H., Heirman C., Hijdra D., Muskens F., Willart M., van Meirvenne S. Dendritic cells retrovirally overexpressing IL-12 induce strong Th1 responses to inhaled antigen in the lung but fail to revert established Th2 sensitization. J Leukoc Biol. 2004;76:1028–1038. doi: 10.1189/jlb.0604325. [DOI] [PubMed] [Google Scholar]
- 40.Kuipers H., Lambrecht B.N. The interplay of dendritic cells, Th2 cells and regulatory T cells in asthma. Curr Opin Immunol. 2004;16:702–708. doi: 10.1016/j.coi.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Hammad H., Chieppa M., Perros F., Willart M.A., Germain R.N., Lambrecht B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trompette A., Divanovic S., Visintin A., Blanchard C., Hegde R.S., Madan R. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi H.J., Park S.Y., Cho J.H., Park J.W., Sohn J.H., Kim Y.J. The TLR4-associated phospholipase D1 activation is crucial for der f 2-induced IL-13 production. Allergy Eur J Allergy Clin Immunol. 2015;70:1569–1579. doi: 10.1111/all.12764. [DOI] [PubMed] [Google Scholar]
- 44.Weber J., Illi S., Nowak D., Schierl R., Holst O., von Mutius E. Asthma and the hygiene hypothesis. Does cleanliness matter? Am J Respir Crit Care Med. 2015;191:522–529. doi: 10.1164/rccm.201410-1899OC. [DOI] [PubMed] [Google Scholar]
- 45.Braun-Fahrlander C., Riedler J., Herz U., Eder W., Waser M., Grize L. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 46.Kuipers H., Hijdra D., De Vries V.C., Hammad H., Prins J.B., Coyle A.J. Lipopolysaccharide-induced suppression of airway Th2 responses does not require IL-12 production by dendritic cells. J Immunol. 2003;171:3645–3654. doi: 10.4049/jimmunol.171.7.3645. [DOI] [PubMed] [Google Scholar]
- 47.Simpson A., John S.L., Jury F., Niven R., Woodcock A., Ollier W.E. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006;174:386–392. doi: 10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]
- 48.von Mutius E., Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 49••.Stein M.M., Hrusch C.L., Gozdz J., Igartua C., Pivniouk V., Murray S.E. Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]; This hallmark study compared Amish and Hutterite children, which share a similar genetic ancestry and lifestyle, but have different farming practices. In the two groups of children, significant differences were found in the proportions, marker expression levels, and gene-expression profiles of peripheral-blood leukocytes. Importantly, Amish children had a decreased chance for allergy development, whereas median endotoxin content in their home dust samples was much higher. Only transfer of the Amish dust could inhibit HDM-induced asthma in a mouse model, and this was only the case in Myd88-sufficient and Trif-sufficient animals, consolidating the hypothesis that environmental innate immune signals shape asthma susceptibility.
- 50.Schuijs M.J., Willart M.A., Vergote K., Gras D., Deswarte K., Ege M.J. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 51.Wang J., Ouyang Y., Guner Y., Ford H.R., Grishin A.V. Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes. J Immunol. 2009;183:1384–1392. doi: 10.4049/jimmunol.0803987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitagaki K., Businga T.R., Racila D., Elliott D.E., Weinstock J.V., Kline J.N. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177:1628–1635. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- 53••.Osbourn M., Soares D.C., Vacca F., Cohen E.S., Scott I.C., Gregory W.F. HpARI Protein secreted by a helminth parasite suppresses interleukin-33. Immunity. 2017;47 doi: 10.1016/j.immuni.2017.09.015. 739–751.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides a molecular mechanism of howH. polygyrus alarmin release inhibitor protein (HpARI) inhibits the biological activity of IL-33. HpARI binds to IL-33 and blocks its access to the IL-33R, while at the same time binding to DNA and inhibiting IL-33 release from necrotic cells as an alarmin.
- 54.Johnston C.J.C., Smyth D.J., Kodali R.B., White M.P.J., Harcus Y., Filbey K.J. A structurally distinct TGF-beta mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat Commun. 2017;8:1741. doi: 10.1038/s41467-017-01886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Floudas A., Saunders S.P., Moran T., Schwartz C., Hams E., Fitzgerald D.C. IL-17 receptor A maintains and protects the skin barrier to prevent allergic skin inflammation. J Immunol. 2017;199:707–717. doi: 10.4049/jimmunol.1602185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi T., Glatz M., Horiuchi K., Kawasaki H., Akiyama H., Kaplan D.H. Dysbiosis and Staphyloccus aureus colonization drives inflammation in atopic dermatitis. Immunity. 2015;42:756–766. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volz T., Skabytska Y., Guenova E., Chen K.M., Frick J.S., Kirschning C.J. Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J Investig Dermatol. 2014;134:96–104. doi: 10.1038/jid.2013.291. [DOI] [PubMed] [Google Scholar]
- 58.Naik S. Compartmentalized control of skin. Science. 2012;1115:1115–1120. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naik S., Bouladoux N., Linehan J.L., Han S.J., Harrison O.J., Wilhelm C. Commensal–dendritic–cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scharschmidt T.C., Vasquez K.S., Truong H.A., Gearty S.V., Pauli M.L., Nosbaum A. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity. 2015;43:1011–1021. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takai T., Chen X., Xie Y., Vu A.T., Le T.A., Kinoshita H. TSLP expression induced via Toll-like receptor pathways in human keratinocytes. Methods Enzymol. 2014;535:371–387. doi: 10.1016/B978-0-12-397925-4.00021-3. [DOI] [PubMed] [Google Scholar]
- 62.Arrieta M.C., Stiemsma L.T., Dimitriu P.A., Thorson L., Russell S., Yurist-Doutsch S. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 63.Lambrecht B.N., Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18:1076–1083. doi: 10.1038/ni.3829. [DOI] [PubMed] [Google Scholar]
- 64••.de Kleer I.M., Kool M., de Bruijn M.J., Willart M., van Moorleghem J., Schuijs M.J. Perinatal activation of the interleukin-33 pathway promotes type 2 Immunity in the developing lung. Immunity. 2016;45:1285–1298. doi: 10.1016/j.immuni.2016.10.031. [DOI] [PubMed] [Google Scholar]; Evidence for type 2 prone lung environment in neonatal mice and a decreased threshold in this period for innate immune responses to allergens. Developing lungs in the alveolarization phase spontaneously produced IL-33 and accumulated type 2 innate immune cells. Lung CD11b + cDCs in this period were scarce yet very efficient in presenting HDM allergen to T cells in draining lymph nodes and to promote Th2 polarization.
- 65.Saluzzo S., Gorki A.D., Rana B.M., Martins R., Scanlon S., Starkl P. First-breath-induced type 2 pathways shape the lung immune environment. Cell Rep. 2017;18:1893–1905. doi: 10.1016/j.celrep.2017.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis B.W., Sultana R., Sharma R., Noël A., Langohr I., Patial S. Early postnatal secondhand smoke exposure disrupts bacterial clearance and abolishes immune responses in muco-obstructive lung disease. J Immunol. 2017;199:1170–1183. doi: 10.4049/jimmunol.1700144. [DOI] [PubMed] [Google Scholar]
- 67.Saravia J., You D., Shrestha B., Jaligama S., Siefker D., Lee G.I. Respiratory syncytial virus disease is mediated by age-variable IL-33. PLoS Pathog. 2015;11:e1005217. doi: 10.1371/journal.ppat.1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]