Abstract
Background
The initial roll-out of the English Bowel (Colorectal) Cancer Screening programme, during 2006 and 2009, found uptake to be low (54%) and socially graded. The current analysis used data from 2010 to 2015 to test whether uptake is increasing and becoming less socially graded over time.
Methods
Postcode-derived area-level uptake of 4.4 million first-time invitees, stratified by gender and the year of the first invitation (2010–2015), was generated using the National Bowel Cancer Screening System. Data were limited to people aged 60–64 years. Binomial regression tested for variations in uptake by the year of invitation, gender, region, area-based socio-economic deprivation and area-based ethnic diversity.
Results
Overall, the first-time colorectal cancer (CRC) screening uptake across 6 years was 52% (n = 2,285,996/4,423,734) with a decline between 2010 and 2015 (53%, 54%, 52%, 50%, 49%, 49% respectively). Uptake continued to be socially graded between the most and the least deprived area-level socio-economic deprivation quintiles (43% vs 57%), the most and the least area-based ethnic diversity quintiles (41% vs 56%) and men and women (47% vs 56%). Multivariate analysis demonstrated the effects of year, deprivation, ethnicity and gender on uptake. The effect of deprivation was more pronounced in the most deprived area quintile between men and women (40% vs 47%) than the least deprived area quintile (52% vs 62% respectively).
Conclusion
We did not find evidence of change in uptake patterns in CRC screening since its initial launch 10 years ago. The programme is unlikely to realise its full public health benefits and is en route to widening inequalities in CRC outcomes.
Keywords: Cancer screening uptake, Inequalities, Diffusion of innovation
Highlights
-
•
Colorectal cancer screening uptake among first-time invitees remains low at 52%.
-
•
There is a worrying reduction in colorectal cancer screening uptake between 2010 and 2015.
-
•
There is no evidence that the social inequalities in uptake have reduced over time.
-
•
There is no evidence of diffusion of innovation in colorectal cancer screening uptake in England.
1. Background
Colorectal cancer (CRC, also known as bowel cancer) is the fourth most common cancer and the second leading cause of cancer-related deaths in England [1]. The English National Health Service (NHS) Bowel Cancer Screening Programme (BCSP) provides organised, population-based screening for all adults aged 60–74 years since 2006. The screening test offered, the guaiac-based faecal occult blood test (gFOBT), has the potential to reduce CRC mortality by 25% [2], [3]. However, the success of the programme largely depends on uptake of the test among the invited population.
A review of the initial roll-out between 2006 and 2009 indicated overall screening uptake of 54% with an independent effect of deprivation: 35% in the most deprived to 61% in the least deprived area-based quintile [4]. Since 2009, CRC and the screening programme have received significant emphasis on the media and have been the focus of initiatives across Clinical Commissioning Groups [5] and public health campaigns [6]. In addition, major research projects aiming to facilitate uptake and reduce inequalities in CRC screening have been conducted, e.g. ASCEND trials [7].
When interventions and new technologies are implemented in real-life settings, their impact may differ, dissipate or enhance over time. Diffusion of innovation theory suggests that the adoption of new technologies will happen over time through social interactions and effective dissemination of health promotion and behaviour change interventions [8]. Thus, over time, an increase in FOBt uptake and reduction in inequalities would also be expected in cancer screening. The Scottish CRC screening programme is a case in point whereby the uptake has steadily increased since its conception [9].
Besides self-reported uptake from survey studies and snapshots of objective uptake from several large randomised controlled trials (RCTs) to reduce socio-economic inequalities, first-time uptake of CRC screening in England has not been updated since the 2006–2009 evaluation of the programme. Uptake of the first-time invitations has emerged as a critical performance factor in light of evidence that people who completed their test kit at least once continued to do so in subsequent screening rounds [10]. Indeed, many recent interventions to promote uptake were only seen to be effective among this ‘first-time’ group as opposed to those receiving repeat invitations [11], [12].
The aim of this article was to review the uptake trends and changes in inequalities among the first-time invitees in the English CRC screening programme between 2010 and 2015.
2. Methods
2.1. The English BCSP
Since 2006, men and women within the screening age range (60–74) who are registered with a general practitioner (GP) and resident in England are eligible for screening. Invitations are sent out biennially to all eligible people (who have not explicitly opted out of screening) by their local programme hub. The gFOBT kit and instructions follow a week later. The individual is asked to collect samples from three separate bowel motions and return the completed kit to the hub in a prepaid envelope for processing. Repeat gFOBT kits are sent out following a ‘spoilt kit’, ‘technical failure’ or an ‘unclear result’. A reminder letter is sent after 4 weeks of non-response. If there is no response after a further 13 weeks, the ‘screening episode’ is closed. After a definitive abnormal result, a referral is made to the local screening centre for diagnostic investigations.
2.1.1. Sample
An aggregate postcode district-level data of 10,392,878 first-time BCSP invitees between 2006 and 2015 were extracted from the Bowel Cancer Screening System. Data included the number of unique invitations, the number of adequately screened and the number of positive test results at each postcode district. The data were received in three age bands (60–64, 65–69, 70–74) for each year to ensure anonymity of the individuals at postcode districts that could have identifiable number of households (n < 10). Data between 2006 and 2009 were excluded to limit the analysis to the full roll-out from 2010 (n = 4,646,079). Furthermore, late entrants to the screening programme because of the age extension from 69 to 74 from 2010, relocation from other countries or medical reasons and all first-time invitees aged 65 to 74 years were excluded from the final analysis (n = 1,323,065). In total, the final sample to be analysed comprised 4,423,734 adults who were invited to complete a gFOBT for the first time between 2010 and 2015.
2.1.2. Measures
A composite indicator of area-based socio-economic deprivation for each postcode district was derived using the 2011 Index of Multiple Deprivation (IMD) [13]. The IMD uses census-derived seven deprivation domains of income, education, employment, environment, health, crime and housing at a small-area level to generate a scale from 4.07 (least deprived) to 68.34 (most deprived) and is defined at the level of Lower Super Output Area (LSOA). We thus mapped the IMD at the geographic resolution of postcode districts and then applied the predicted value to the units in our sample. We used the area-level proportion of ethnicity to derive an area-level index of ethnic diversity based on the proportion of ‘non-white’ residents in each postcode districts (defined as all ethnic groups self-described as other than ‘white British’, ‘white Irish’ and ‘white other’).
Postcode districts were also mapped to five English Regions, i.e. South West and South East, London, East of England and East Midlands, Yorkshire and North East and West Midlands and North West. The original data were grouped by gender and the year of invitation from 2010, allowing an investigation of trends up to the end of 2015. CRC screening uptake at each postcode district was calculated by dividing the number adequately complete screening test kits by the number of invitees. Positivity rate was calculated by dividing the number of abnormal test results at each postcode district by the number of adequately complete test kits.
2.2. Statistical analysis
First, unadjusted screening uptake and positivity rates were presented by each sociodemographic, temporal and spatial determinant. The statistical analyses consists of several multivariate logistic regressions that looked at the effect of gender, deprivation score, ethnicity, geographical region and time on gFOBT uptake and percentage of abnormal test results in England between 2010 and 2015. We also conducted exploratory subgroup analysis of the same models by each geographical region to test for the effect of gender, deprivation score, ethnicity, geographical region and time on CRC uptake (see Supplementary Online Materials).
The adjusted logistic regression analyses were weighted by the number of invitations sent out in each postcode district. Similar to the 2006–2009 data analyses [4], we have tested different model specifications, including linear and non-linear trends. The latter did show difference in the results; thus, we present the linear models for simpler interpretation (see Supplementary Online Materials). The final models included interaction terms for gender by IMD, gender by area-level ethnic diversity, gender by year, the year by IMD and the year by area-level ethnic diversity.
3. Results
Of the 4, 423, 734 gFOBT kits sent out to men and women aged 60–64 years for the first-time, between 2010 and 2015, 51.68% were returned (Table 1). Uptake among women (56.08%) was higher than that among men (47.30%). Uptake ranged from 43.03% in the most deprived quintile to 56.96% in the least deprived quintile. Similarly, uptake varied by area-level ethnic diversity from 40.53% in the most ethnically diverse quintile of areas to 56.31 in the least diverse quintile of areas.
Table 1.
Demographic variation in screening uptake.
| Demographic factors | Non-adjusted uptake rate (%) | Non-adjusted rate of abnormal test result (%) |
|---|---|---|
| Overall | 51.68 | 1.93 |
| Gender | ||
| Men | 47.30 | 2.43 |
| Women | 56.08 | 1.51 |
| Area-based deprivation quintiles (IMD score) | ||
| Quintile 1 (4.07–11.26) | 56.67 | 1.63 |
| Quintile 2 (11.27–15.33) | 56.19 | 1.68 |
| Quintile 3 (15.34–20.16) | 53.78 | 1.84 |
| Quintile 4 (20.17–28.26) | 49.52 | 2.13 |
| Quintile 5 (28.27–68.34) | 43.03 | 2.48 |
| Area-based ethnic diversity (% of residents with a Black and Minority Ethnic (BME) background) | ||
| Quintile 1 (1.31–4.04) | 56.31 | 1.75 |
| Quintile 2 (4.05–6.17) | 56.37 | 1.69 |
| Quintile 3 (6.17–11.40) | 54.14 | 1.78 |
| Quintile 4 (11.41–28.33) | 50.78 | 2.00 |
| Quintile 5 (28.34–92.39) | 40.53 | 2.80 |
| Regions | ||
| South West and South East | 54.23 | 1.88 |
| London | 42.33 | 2.55 |
| East of England and East Midlands | 54.48 | 2.05 |
| Yorkshire and North East | 53.42 | 1.79 |
| West Midlands and North West | 50.26 | 1.71 |
| Years | ||
| Year 2010 | 53.03 | 2.05 |
| Year 2011 | 54.40 | 2.00 |
| Year 2012 | 52.25 | 1.85 |
| Year 2013 | 50.17 | 1.80 |
| Year 2014 | 49.26 | 1.82 |
| Year 2015 | 48.80 | 2.00 |
IMD, Index of Multiple Deprivation.
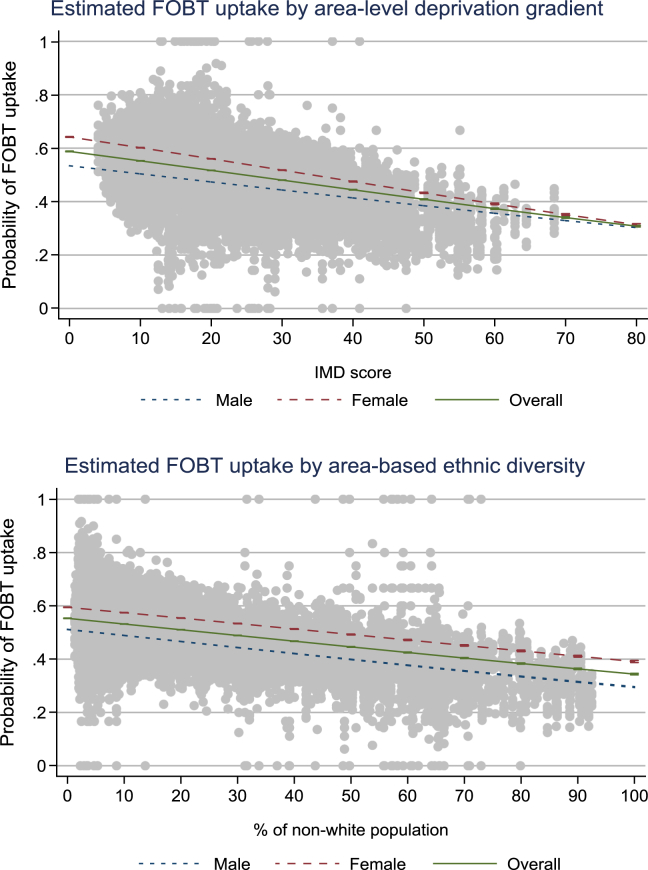
Fig. 1 illustrates that with every unit increase in the deprivation score, the probability of a test kit return reduced by 0.36%. Similarly, the probability decreased by 0.21% for every unit increase in area-based ethnic diversity.
Fig. 1.
gFOBT uptake as a function of deprivation and ethnic diversity. IMD, Index of Multiple Deprivation; gFOBT, guaiac-based faecal occult blood test.
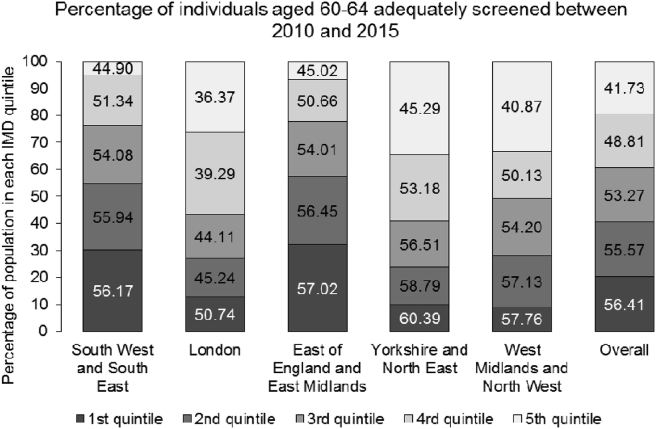
Among all other regions, London had the lowest uptake rate (42.33%) (See Fig. 2). However, each region showed a significant socio-economic gradient in uptake (see supplementary appendix for subgroup analysis).
Fig. 2.
Share of adequately screened (%) by the quintile of Index of Multiple Deprivation (IMD) score and geographic regions (2010–2015).
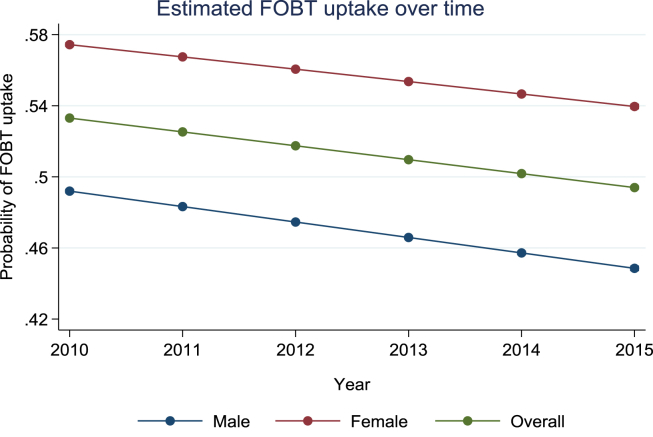
Our analysis of differences over time showed a reduction in CRC screening uptake between 2010 and 2015 (53%, 54%, 52%, 50%, 49% and 49% respectively). Multivariate analysis with linear terms indicated an association between uptake and year of invitation which is moderated by gender, IMD and area-level ethnic diversity (Table 2). Fig. 3 shows that for each successive year (measured as a continuous variable), the probability to return the test kit reduces by 0.78%.
Table 2.
Multivariate logistic regression models with linear time trends and time interaction with gender, IMD score and ethnicity separately.
| Demographic factors | Adequately screened |
Abnormal test result |
||
|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Gender (female) | 1.4844 | 1.4590–1.5102** | 0.5443 | 0.4996–0.5931** |
| Area-based deprivation (IMD score: 4.6–68.34) | 0.9853 | 0.9844–0.9862** | 1.0051 | 1.0006–1.0096* |
| Deprivation by gender | 0.9950 | 0.9946–0.9954** | 0.9988 | 0.9967–1.0009 |
| Area-based ethnic diversity (1.31–92.40) | 0.9880 | 0.9875–0.9885** | 1.0112 | 1.0089–1.0135** |
| Ethnic diversity by gender | 1.0008 | 1.0006–1.0010** | 1.0033 | 1.0023–1.0044** |
| Regions (compared with London) | ||||
| South West and South East | 1.0465 | 1.0381–1.0551** | 1.0682 | 1.0263–1.1117** |
| East of England and East Midlands | 1.0894 | 1.0804–1.0984** | 1.1316 | 1.0875–1.1775** |
| Yorkshire and North East | 1.1376 | 1.1270–1.1483** | 0.9035 | 0.8628–0.9461** |
| West Midlands and North West | 1.0435 | 1.0347–1.0523** | 0.8365 | 0.8022–0.8723** |
| Time trends | ||||
| Year (linear trend) | 0.9519 | 0.9493–0.9544** | 0.9522 | 0.9397–0.9648** |
| Year by gender | 1.0063 | 1.0041–1.0084** | 1.0134 | 1.0023–1.0246* |
| Deprivation by year | 1.0004 | 1.0002–1.0005** | 1.0015 | 1.0009–1.0021** |
| Ethnicity by year | 1.0004 | 1.0003–1.0005** | 0.9993 | 0.9990–0.9996** |
| N | 4,423,734 | 2,285,996 | ||
IMD, Index of Multiple Deprivation; CI, confidence interval.
*p < 0.05; **p < 0.01.
Fig. 3.
Estimated uptake over time (2010–2015). FOBT, faecal occult blood test.
Since 2010, the reduction in men's uptake from 48.79% to 44.28% has been more pronounced than the reduction in women's uptake from 57.31% to 53.93% (Table 3). Similarly, there has been a larger reduction in uptake in the least deprived IMD quintile from 57.61% to 53.95%, whereas the difference is minimal in the most deprived IMD quintile from 43.85% to 41.09%. Finally, the reduction in uptake from 2010 to 2015 is more apparent in the least ethnically diverse quintile (from 57.60% to 53.54%) than in the most diverse quintile (from 40.69% to 38.79%).
Table 3.
Screening uptake across the years for different sociodemographic groups.
| Demographic factors | Uptake across the years |
|||||
|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
| Gender | ||||||
| Male | 48.79% | 50.16% | 47.78% | 45.91% | 44.61% | 44.28% |
| Female | 57.31% | 58.67% | 56.73% | 54.65% | 54.46% | 53.93% |
| IMD quintiles | ||||||
| 1st quintile | 57.61% | 59.18% | 57.50% | 55.12% | 54.57% | 53.95% |
| 2nd quintile | 57.19% | 58.77% | 57.16% | 54.67% | 53.82% | 53.26% |
| 3rd quintile | 54.99% | 56.47% | 54.37% | 52.30% | 51.29% | 50.91% |
| 4th quintile | 50.60% | 52.26% | 50.20% | 48.14% | 47.21% | 46.85% |
| 5th quintile | 43.85% | 45.01% | 43.76% | 42.31% | 41.51% | 41.09% |
| Area-based ethnic diversity | ||||||
| 1st quintile | 57.60% | 58.71% | 57.33% | 54.77% | 54.06% | 53.54% |
| 2nd quintile | 57.69% | 58.77% | 56.81% | 54.97% | 53.74% | 53.87% |
| 3rd quintile | 55.23% | 56.48% | 54.86% | 52.38% | 51.93% | 51.43% |
| 4th quintile | 51.63% | 53.38% | 51.62% | 49.53% | 48.53% | 48.10% |
| 5th quintile | 40.69% | 42.79% | 41.59% | 40.03% | 39.15% | 38.79% |
IMD, Index of Multiple Deprivation.
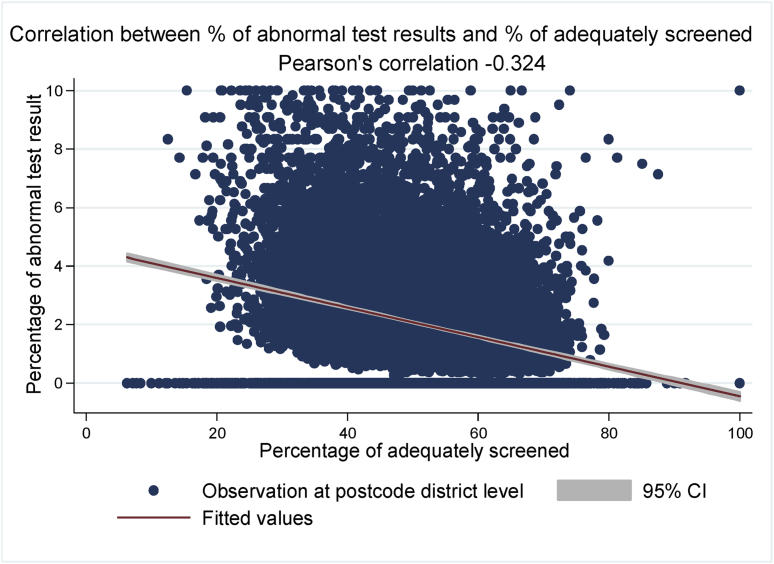
There was a negative correlation between CRC uptake and the proportion of abnormal test results (r = −0.324) (Fig. 4). Of the 2,285,995 people who were adequately screened, 1.93% (n = 44,208) had an abnormal test result. The proportion of individuals with abnormal results was higher among men (2.43%) than women (1.51%) and among people living in most deprived IMD quintile (2.48%) compared with those in the least deprived IMD quintile (1.63%). Additionally, there were regional differences in abnormality with London having the highest proportion of abnormal results compared with all other regions (Table 1). Having an abnormal test result was independently associated with gender, IMD, area-based ethnic diversity, region and year (Table 2).
Fig. 4.
Correlation between the percentage of abnormal test results and percentage of adequately screened. CI, confidence interval.
4. Discussion
First-time gFOBT uptake remains low and socially graded. Unlike the Scottish programme [9] that found an overall increase (albeit not significant in the first prevalent round) (9), we have observed a small but significant decline particularly from 2011 to 2014.
The results, therefore, do not suggest that barriers to CRC screening have been reduced over time by the diffusion of innovation. However, after the recent evidence from pilot studies testing a new and improved type of home-based test, there is a suggestion that a structural change such as the implementation of the faecal immunochemical test (FIT) is more promising to eliminate at least some of the root causes of the low uptake [14], [15], [16]. The largest improvement in CRC screening by far was obtained by using FIT as the screening tool as opposed to gFOBT with an 11.4% increase in uptake among previous non-responders and with approximately 7% overall increase in uptake across all IMD quintiles [17].
Regional differences in uptake, in particular the persistent and strong social gradient in London, are very striking considering the vast majority of initiatives and interventions taken place there; for example, a text message trial [12], many community engagement initiatives with different ethnic groups [18] and national ASCEND trials [7]. Further research using spatial classification based on population density and location characteristics (urban vs. rural) may provide further insights into the better understanding of inequalities in London which are not usually a target of epidemiological research.
Furthermore, the social gradient in positivity has been observed previously in the initial analysis of the gFOBT pilot outcomes which adds further emphasis on the importance of reducing inequalities in CRC screening uptake [19], [20]. What was particularly interesting in the present study was the inverse association between uptake and positivity. Future studies should explore potential mechanisms for this relationship, e.g. probe whether in low uptake areas people use CRC screening to follow-up their existing symptoms.
There were some limitations to our analyses. The data were at postcode district level which meant that the average IMD scores for postcode districts may not be directly representative of the population living in those areas, e.g. a postcode district in London may include the least and the most deprived postcodes. Thus, it can be assumed that the area-level analysis is underestimating the actual impact of socio-economic deprivation.
While IMD scores are useful to understand inequalities, future research could benefit from using geo-segmentation tools such as those used in marketing and consumer science. Furthermore, we were limited to using annual rather than quarterly or monthly data extractions which limits our ability to observe seasonal fluctuations or the short-term versus long-term impact of interventions on CRC screening uptake.
In conclusion, this study provides an update on previously reported CRC screening uptake and confirms that the inequalities in CRC screening are persistent and could be widening. In light of the positive impact on uptake and inequalities associated with FIT which were observed in the recent pilot study [17], there is an urgent need to implement this change to avoid further exacerbation of social inequalities in screening uptake and its long-term consequences on CRC outcomes.
Author contributions
Y.H. and C.v.W. conceived the study. Y.H. facilitated the data extraction and Y.H., S.S., G.B. and C.v.W. contributed to the data analysis. Y.H. completed the first draft of the manuscript, and all other authors have contributed to the further documents. All authors have agreed to the final version of the manuscript.
Funding
This project was funded by the Cancer Research UK programme grant awarded to Professor Jane Wardle [C1418/A14134].
Conflict of interest statement
The authors have declared no competing interests.
Data sharing statement
Data for this study are based on the information collected and quality assured by the PHE Population Screening Programmes. The access to data was facilitated by the PHE Office for Data Release.
Acknowledgements
The authors thank Bowel Cancer Screening Research Advisory Committee, Claire Nickerson and Dr Suzanne Wright for their support during the study development and data extraction.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejca.2018.07.135.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Cancer Research UK. Cancer incidence for common cancers [Internet]. ]. [accessed 17 April 2018]. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/incidence/commoncancers/uk-cancer-incidence-statistics-for-common-cancers.
- 2.Hewitson P., Glasziou P., Watson E., Towler B., Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (Hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 3.Levin B., Lieberman D.A., McFarland B., Smith R.A., Brooks D., Andrews K.S. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American cancer society, the US multi-society task force on colorectal cancer, and the American College of Radiology. CA Cancer J. 2008;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 4.Wagner C. von, Baio G., Raine R., Snowball J., Morris S., Atkin W. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol. 2011;40(3):712–718. doi: 10.1093/ije/dyr008. [DOI] [PubMed] [Google Scholar]
- 5.Shankleman J., Massat N.J., Khagram L., Ariyanagam S., Garner A., Katoon S. Evaluation of a service intervention to improve awareness and uptake of bowel cancer screening in ethnically-diverse areas. Br J Canc. 2014;111(7):1440–1447. doi: 10.1038/bjc.2014.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Research UK. Be clear on cancer [Internet]. [accessed 17 April 2018]. Available from: http://www.cancerresearchuk.org/sites/default/files/cruk_bcoc16_bowel_brief_10.pdf.
- 7.Wardle J., Wagner C. von, Kralj-Hans I., Halloran S.P., Smith S.G., McGregor L.M. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS Bowel Cancer Screening Programme (ASCEND): four cluster-randomised controlled trials. Lancet. 2016;387:751–759. doi: 10.1016/S0140-6736(15)01154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers E.M. Simon and Schuster; 2010 Jul 6. Diffusion of innovations. [Google Scholar]
- 9.Quyn A.J., Fraser C.G., Stanners G., Carey F.A., Carden C., Shaukat A. Uptake trends in the Scottish Bowel Screening Programme and the influences of age, sex, and deprivation. J Med Screen. 2018 Mar;25(1):24–31. doi: 10.1177/0969141317694065. [DOI] [PubMed] [Google Scholar]
- 10.Lo S.H., Halloran S., Snowball J., Seaman H., Wardle J., von Wagner C. Colorectal cancer screening uptake over three biennial invitation rounds in the English bowel cancer screening programme. Gut. 2014 May 7;64(2):282–291. doi: 10.1136/gutjnl-2013-306144. gutjnl-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White B., Power E., Ciurej M., Lo S.H., Nash K., Ormiston-Smith N. BioMed research international; 2015. Piloting the impact of three interventions on guaiac faecal occult blood test uptake within the NHS Bowel Cancer Screening Programme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirst Y., Skrobanski H., Kerrison R.S., Kobayashi L.C., Counsell N., Djedovic N. Text-message reminders in colorectal cancer screening (TRICCS): a randomised controlled trial. Br J Canc. 2017 May;116(11):1408. doi: 10.1038/bjc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.English indices of deprivation 2010 - GOV.UK [Internet]. [Accessed 11 May 2018]. Available from: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2010.
- 14.Davis M., Oaten M., Occhipinti S., Chambers S.K., Stevenson R.J. An investigation of the emotion of disgust as an affective barrier to intention to screen for colorectal cancer. Eur J Canc Care. 2017 Jul 1;26(4) doi: 10.1111/ecc.12582. [DOI] [PubMed] [Google Scholar]
- 15.Chambers J.A., Callander A.S., Grangeret R., O'Carroll R.E. Attitudes towards the faecal occult blood test (FOBT) versus the faecal immunochemical test (FIT) for colorectal cancer screening: perceived ease of completion and disgust. BMC Canc. 2016 Dec;16(1):96. doi: 10.1186/s12885-016-2133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Wagner C., Good A., Smith S.G., Wardle J. Responses to procedural information about colorectal cancer screening using faecal occult blood testing: the role of consideration of future consequences. Health Expect. 2012 Jun 1;15(2):176–186. doi: 10.1111/j.1369-7625.2011.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss S., Mathews C., Day T.J., Smith S., Seaman H.E., Snowball J. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut. 2016 Jun 7;66(9):1631–1644. doi: 10.1136/gutjnl-2015-310691. gutjnl-2015. [DOI] [PubMed] [Google Scholar]
- 18.Palmer C.K., Thomas M.C., McGregor L.M., von Wagner C., Raine R. Understanding low colorectal cancer screening uptake in South Asian faith communities in England–a qualitative study. BMC Publ Health. 2015 Dec;15(1):998. doi: 10.1186/s12889-015-2334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weller D., Coleman D., Robertson R., Butler P., Melia J., Campbell C. The UK colorectal cancer screening pilot: results of the second round of screening in England. Br J Canc. 2007 Dec;97(12):1601. doi: 10.1038/sj.bjc.6604089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steele R.J., Kostourou I., McClements P., Watling C., Libby G., Weiler D. Effect of gender, age and deprivation on key performance indicators in a FOBT-based colorectal screening programme. J Med Screen. 2010 Jun;17(2):68–74. doi: 10.1258/jms.2010.009120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.