Graphical Abstract

In the past few years there has been an explosive growth in the development of chemically diverse libraries of molecules. Combinatorial libraries have proven to be a powerful method to examine structure—function relationships, and the synthesis and screening of small molecule libraries is emerging as an important strategy for drug discovery.1 Several strategies have been employed to create and characterize these linear- and core-structured combinatorial libraries including: (i) solid phase and solution phase synthesis and (ii) grid, deconvolution, and tagging methods to identify compounds.1 We describe herein the synthesis and characterization of directed combinatorial libraries of meso-tetraphenylporphyrin (H2TPP) derivatives3—core-structured libraries—where the largest contains 1540 compounds (including isomers) (Figure 1). The derivitization of entire libraries to make them amphipathic (Figure 1) and the iterative selection of winning compounds by DNA binding are described. As a demonstration of the methodology, we find the dipositively charged porphyrins bearing at least one hydroxyl group to be the most efficacious in the photoinitiated cleavage of plasmid DNA.
Figure 1.
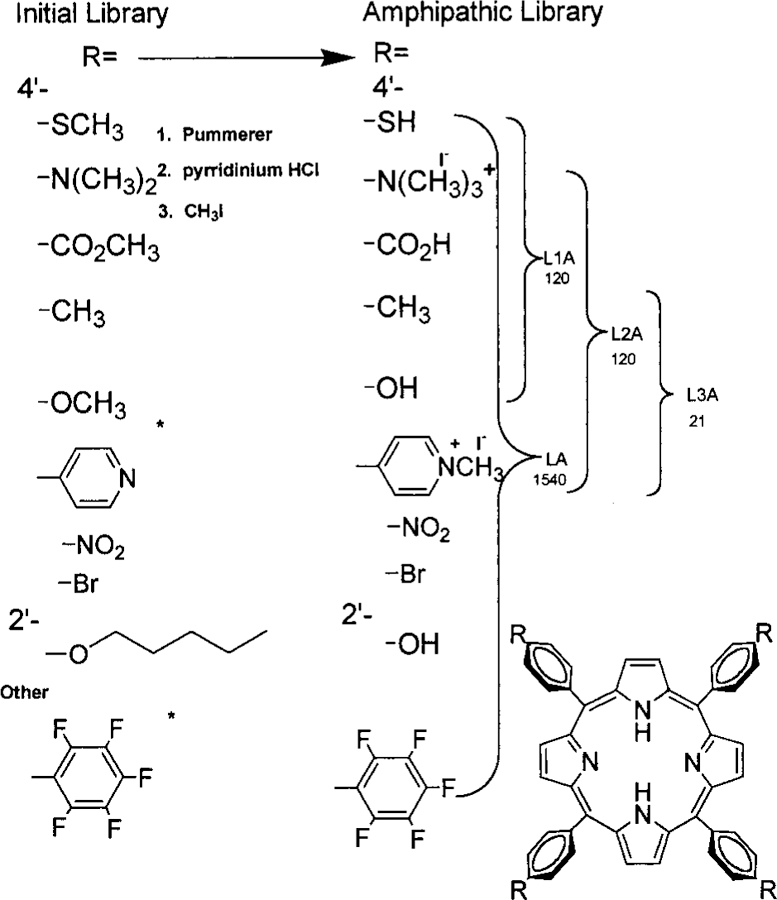
Porphyrin libraries bearing small functional groups, with the number of compounds in the library indicated under the library name. Polar functional groups are protected as the methyl derivative for efficient combinatorial synthesis and characterization of the initial hydrophobic libraries (L). These are then deprotected and the amines quaternized to make the amphiphilic libraries (LA). All libraries, L, LA, L1, L1A, L2, L2A, L3, and L3A, are fully characterized by ESI-MS, 1H NMR, and UV—vis. The asterisk (⁎) denotes groups attached directly to the meso positions of the porphyrin.
Both naturally occurring and synthetic porphyrins have long been known to exist in a large variety of isomers. For example, there are 60 possible isomers for protoporphyrin due to the four methyl, two vinyl, and two propionate groups on the eight pyrrole positions, and there are 420 possible isomers of the heme a prosthetic group in cytochrome a.4 Similarly, we and others have exploited the six possible compounds and isomers when two different arylaldehydes are employed in the synthesis of meso-substituted porphyrins5–7 and libraries of chemically inert, lipophilic, alkyl-substituted H2TPPs have been made.8,9 The Photo Dynamic Therapeutic (PDT) Photofrin (QLT Phototherapeutics, Canada) is a mixture of monomers and ester-linked multimers of protoporphyrin IX. The advantages and drawbacks of PDT are extensively reviewed.10–13 Although the binding mode is still controversial,14–19 the ability of 5,10,15,20-tetrakis(4′-N-methypyridinium)porphyrin to strongly bind DNA has been known for over 20 years, but negatively charged tetrabenzoate porphyrins do not bind strongly. Similarly, the mechanism of action of these compounds is also under debate, but it probably arises from the formation of singlet oxygen and subsequent damage to lipids, proteins, and nucleic acids, as well as hypoxia.10–13,20–23 The purpose of this study is to identify new combinations of functional groups that enhance porphyrin binding to biomolecules relative to the homosubstituted parent molecules, not to get embroiled in mechanistic debates. Since porphyrins have been shown to congregate in a variety of cell structures,20,24,25 it is likely that different substitution patterns will target different tissues or cellular components. In vivo experiments show that the greater the amphipathicity the greater the selectivity toward tumor cells.20–25 The assays chosen herein (DNA binding and cleavage, and water—octanol partition coefficient) are currently standards used to identify potential new PDT agents.10–25 Therefore, a directed porphyrin library is made in order to bind or cross the cell membrane (which generally contain negatively charged lipids), to be reasonably soluble under physiological conditions, and to bind nucleic acids. Thus, the members of the libraries should be amphipathic with some positive charge.
The standard Adler,28 Lindsey,29 and gas phase30 methods of porphyrin synthesis result in low or erratic yields when the aldehyde bears highly polar or charged groups such as alcohols, thiols, carboxylic acids, pyridinium, and quaternary ammonium moieties. Therefore the starting aryl aldehydes bearing the methyl protected precursors of the above groups were used in the Adler synthesis of combinatorial libraries L, L1, L2, L3—libraries of 1540, 120, 120, and 21 porphyrins, respectively. Subsequent removal of the methyl groups from the ether, thioether, and ester, followed by careful methylation of the amino groups resulted in the desired amphipathic porphyrin libraries, LA, L1A, L2A, and L3A (Figure 1, Scheme 1). This is a directed library since the rationale for choosing the aldehydes for this study includes the following: (i) the substituted aldehyde must yield the tetra derivative in normal yields, (ii) cleavage of the protecting group must proceed near quantitatively, (iii) the resulting functional group should impart some amphipathic character to the macrocycle,20,24,25 (iv) positively charged moieties are preferable to negatively charged ones for membrane, polysaccharide, and nucleic acid binding, (v) the number and kinds of derivatives should be maximized. Functional groups were chosen for their hydrophobic, electrostatic, and hydrogen-bonding potentials. The resulting libraries contain a variety of amphipathic, hydrophobic, and hydrophilic porphyrins. After partitioning the 1540-member LA into water, ethanol, and ethyl acetate fractions, the water and ethanol fractions were screened for DNA binding by elution through a calf-thymus (CT)-DNA column. Electrosspray ionization mass spectra (ESI-MS), 1H NMR, and UV—vis spectra were used to characterized the last fractions off the column. The results from this first screening showed a paucity of 2′-hydroxy, 4′-nitro, 4′-bromo, perfluoro, and 4′-thio groups. To further identify those porphyrins that bind strongest to DNA,32 it was decided to make smaller libraries, L1 and L2 (Scheme 1).
Scheme 1.
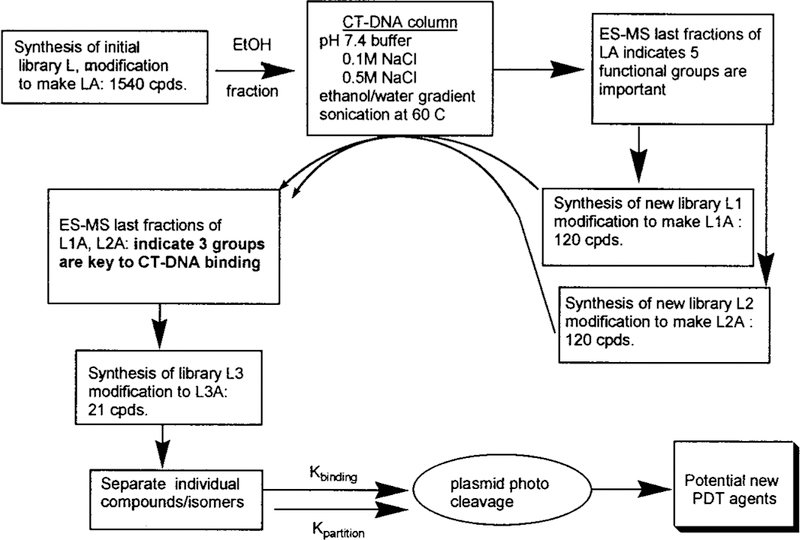
Characterization of the libraries and their diversity is accomplished by a variety of spectroscopic techniques.31 Characterization of the initial library, L, and that of the modified, amphipathic library, LA, follow the same course. A clean UV—visible spectrum indicates the lack of chlorins, polypyrromethanes, and other side products of porphyrin synthesis. 1H NMR spectra also demonstrate the lack of side products and starting materials. The methyl resonances can be used as a handle to characterize the library since they should represent a statistical distribution of compounds and therefore exhibit a broader peak width. Most importantly, the ESI-MS can be used to identify the number of isobaric species for small libraries of <60 components and provide a highly sensitive fingerprint in the characterization of all libraries.8,9,31,32
The characterization of the largest libraries, L and LA, is described and is generally applicable to the smaller libraries as well. A statistical mixture of 1540 porphyrins in L is clearly demonstrated by the UV—vis, ESI-MS (L has 715 isobaric species, L1 and L2 have 70, and L3 has 15 isobaric species), and 1H NMR spectra. The absorption spectra shows a substantially broadened Soret peak and that there are no porphyrin side products such as chlorins or dipyrromethanes.31 The 1H NMR spectra of L shows no starting aldehydes or pyrrole, all five of the different methyl group resonances between 2 and 5 ppm (there are 5 for L1, and 4 for L2), a highly complex aromatic region, and the characteristic absorption of the pyrrole NH at about −2.75 ppm. The half-widths of the methyl resonances of L are about 2.5 times as broad as those of the individual tetrasubstituted porphyrins and have a much broader base.31 There is a substantial increase in the 1H NMR methyl peak half-widths upon going from 1-, 6-, 21-, 55-, 120-, to 1540-member libraries.32 Metalating the libraries with high spin cobalt (II) expands the methyl resonances in the 1H NMR such that they are ∼3 times broader than those for the individual Co(II) porphyrins. NMR spectra of the free-base and Co(II) L1 and L2 are also consistent with fully diverse libraries. The ESI-MS of L31 (Figure 2) is remarkably similar to the calculated spectra. The calculated and observed ESI-MS spectra of the Co(III) metalated library L-Co [Co(III) imparts one positive charge on each porphyrin] ensures the results of the free-base L. Since these are core-structured libraries, the range of molecular weights is limited. Thus, L was chromato-graphed on a silica gel column, and the ESI-MS and 1H NMR of each fraction were taken. In this way, all 715 of the isobaric compounds are identified, and >93% of the com pounds/isomers are found based on relative intensities compared to known unique m/z peaks. There is a random distribution of functional groups on the porphyrins not identified by these procedures—ensuring the reactivities of the aldehydes are similar under the synthetic conditions. The 70 isobaric compounds/isomers in L1 and L2 are readily observed by ESI-MS using the same variety of instrumental methods,31 including the Co(III) metalated libraries. Taken together, these results indicate that the expected diversity of the libraries is maintained throughout the synthesis and purification.
Figure 2.
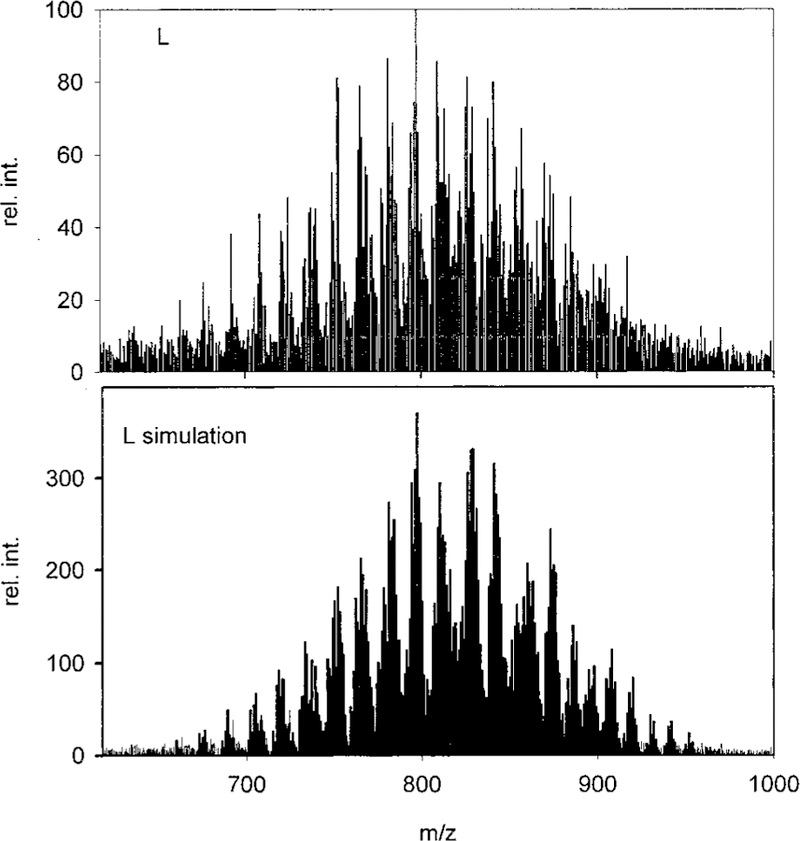
ESI-MS of 1540-member library L and its simulation. The sample (~10−6 M) was dissolved in acetonitrile/water (75/25) containing 1% trifluoroacetic acid. The spectrum was taken in positive ion mode and the fragmenter voltage ramped from 25 to 150 V.32
Upon completion of the quaternization and cleavage reactions, the 1H NMR exhibits the ammonium and tolyl methyl groups and the thiol, alcohol, and acid protons, in addition to the resonances due to the macrocycle. Integration of these resonances yielded the expected ratios and indicated that each modification reaction proceeds with >90% efficiency. Unreacted groups may be considered to further diversify the library. ESI-MS of amphipathic libraries LA, L1A, L2A, and L3A are essentially similar to their calculated spectra. Thus 6-, 21-, 120-, and 1540-member libraries have been constructed and characterized by 1H and 13C NMR, UV—vis, and ESI-MS and indicate that each library’s diversity is maintained upon modification.
We initially differentiated the resultant amphipathic porphyrin libraries by solubility: by exhaustively extracting solid LA, or L1A, or L2A with water, the remaining solid with ethanol, and finally with ethyl acetate. This yields three “fractions” (hydrophilic, amphipathic, hydrophobic) of which the ethanol fraction is by far the largest of all three. ESI-MS of all three fractions of the three libraries, using methods described above, further assist in characterization of the libraries, with the water and ethyl acetate fractions yielding members that would be expected or are known to be hydrophilic and hydrophobic, respectively.
Elution of the ethanol and water soluble fractions, from LA, over a column of calf-thymus DNA absorbed onto glass wool further differentiates the porphyrins and selects those that tightly bind DNA.31,32 The eluent is a salt gradient followed by a water-to-ethanol gradient. In control experiments, we find that the tetracationic species are the predominant ones that bind to the glass wool, and since these are known compounds they are not used in further analysis. The last two salt and last two ethanol fractions from L showed very little or no thio, nitro, bromo, or perfluoro moieties by ESI-MS. Therefore L1 and L2 were constructed. ESI-MS analysis finds about eight porphyrins from L2A constitute the major part of the fraction that binds most strongly to the DNA (see Table 1), although we can detect small amounts of approximately eight others. While the pyridinium groups were expected, there are a surprising number of methyl and hydroxy groups present.
Table 1.
Characterization of Selected Porphyrin Compounds (from L3A)a
| porphyrin | R | R′ | R′′ | R′′′ | KB1 (× 10−6 M) | KB2 (× 10−6 M) | KOct/Water | ϕX174 photocleavage |
|---|---|---|---|---|---|---|---|---|
| 1 | OH | Me | Me | PyMe+ | 0.3 | 179 | >2500 | poor |
| 2 | OH | Me | PyMe+ | PyMe+ | 1.5 | 46 | 7.23 | excellent |
| 3 | OH | PyMe+ | Me | PyMe+ | 2.3 | 55 | 16.5 | good |
| 4 | OH | OH | PyMe+ | PyMe+ | 0.8 | 86 | 4.02 | excellent |
| 5 | OH | PyMe+ | OH | PyMe+ | 0.8 | 75 | 6.25 | poor |
| 6 | OH | OH | OH | PyMe+ | 1.7 | 245 | >2500 | good |
| 7 | OH | PyMe+ | PyMe+ | PyMe+ | good | |||
| 8 | OH | OH | Me | N(Me)3+ | - | - | - | |
| 9 | PyMe+ | PyMe+ | PyMe+ | PyMe+ | 12 | >1000 | <0.0004 | fair |
| 10 | Me | Me | PyMe+ | PyMe+ | 0.8 | 237 | >100 | fair |
| 11 | Me | PyMe+ | Me | PyMe+ | 1.8 | 453 | 38.5 | poor |
All compounds had satisfactory 1H NMR, UV—vis, and ESI-MS. Assays are all compared to the extensively studied tetrapyridinium-porphyrin no. 9. Though the mode of binding and mechanism of action for even extremely well studied porphyrins, no. 9 and Photofrin, are still controversial, a well accepted indicator of the potential efficacy is the rough binding constants measured by changes in the visible spectra23 of the porphyrin upon DNA addition. The octanol-water partition coefficient is an indicator of the amphiphilicity of the compound and of lipid bilayer binding. The cleavage reactions on X174 or PUC-19 supercoiled plasmids follow standard literature procedures.20–25 Changes in the appearance of the plasmid on an agarose gel occur after incubation, irradiation, and treatment with S1 nuclease (degrades single-strand DNA). Poor = only small changes, fair = noticeable changes only after S1 nuclease amplification, good = can be visualized without S1 nuclease treatment, excellent = complete degradation without S1 nuclease treatment; see Figure 3.
These results led to the construction of L3 and L3A (bearing only hydroxy, methyl, and methylpyridinium) to further delineate the members of the library with the desired properties. Several MPLC columns accomplish chromatographic separation of all 21 members of this library. Each compound is then fully characterized by ESI-MS, 1H NMR, and UV—vis. The DNA binding constant and the octanol—water partition coefficient are then measured for each compound (Table 1). The best of these are used in DNA photocleavage experiments.
As an indicator of bioactivity,10–16,19–25 the eight novel porphyrins thus found were incubated with a supercoiled plasmid DNA (ϕ-X174) for 2–12 h in the dark at room temperature and then irradiated for up to 90 min at 35 °C with continuous 50 foot-candles white light with a 450 nm cutoff filter that eliminates blue light. To test for sequence specificity of the resultant single strand nicks (and amplify the signal), some samples were treated with S1 nuclease. Gel electrophoresis shows that the plasmid DNA treated with the selected porphyrins derivatives has been degraded, Figure 3. Further studies are underway to examine the mode of binding of these selected porphyrins to small DNA fragments.
Figure 3.

Lane 1: α1Kb molecular marker; lane 2: ϕ-X174 plasmid DNA; lane 3: plasmid with ~4 µM porphyrin 3; lanes 4, 5, 6, 7: irradiation with 50 Lux light (450–800 nm) for 15, 30, 60, 90 min, respectively; lane 8: with ~0.8 µM of porphyrin 4 irradiated for 15 min.
This report demonstrates that (i) a large porphyrin library, containing 1540 different members, may be formed and subsequently modified to yield libraries with a wide range of solubilities and functionalities; (ii) the libraries may be differentiated into hydrophilic, amphipathic, and hydrophobic fractions; and (iii) the resultant fractions can be screened for DNA binding. Since the mode of DNA binding (electrostatic, hydrogen bond, intercalation, etc.) of even the simplest porphyrins is controversial and these multifunctional porphyrins can potentially bind in a variety of ways, we cannot as yet speculate on how the tight-binding porphyrins bind to DNA. Since the mode of action and the localization of PDT agents is also under intense investigation,1–25 these libraries may serve as a means to probe these questions. However, the optical spectra of the selected porphyrin(s) exhibit a 3–10 nm red shift23,33 and a substantial broadening with a concomitant decrease in of the porphyrin Soret bands upon addition of CT-DNA. The fluorescence emission and excitation spectra are dramatically altered and there is a substantial fluorescence anisotropy observed upon DNA addition (all at ~1 µM). Upon excitation in the nucleotide region at 270 nm where the absorbance of DNA is at least 10 times that of the porphyrin, the porphyrin strongly fluoresces. These observed spectral changes are consistent with those observed for most other porphyrin—DNA complexes.10–25Together with the photocleavage experiments, the evidence that this small cadre of porphyrins (~0.5% of the parent library) bind to DNA is unequivocal.31 Since the mechanism, the mode of binding, and the localization of PDT agents is under intense investigation, libraries such as these may aid in the determination of what factors direct porphyrins to certain cellular structures and tissues. Once compounds have been identified or selected, they may be readily reduced to the corresponding chlorin to enhance their absorption in the >700 nm region where biofluids are more transparent.27 We find that a specific set of motifs or substituents bind quite strongly to CT-DNA and cause strand scission upon illumination with white light. Notably, these substituents are a combination of polar (alcohol), nonpolar (methyl), and cationic (pyridininium) moieties, indicating that a combination of hydrophilic, hydrophobic, and electrostatic effects are key considerations in the binding of porphyrins to biomolecules such as lipids34,35 and nucleic acids. The powerful analytical techniques based on mass spectrometry allow for the characterization of exquisitely small amounts of material obtained from these selection methods.
Last, we and others8 have developed a simple computer program that makes a table of all the possible derivatives and their associated molecular weights for use as a simulation of the mass spectra. This invaluable aid complements the deconvolution in identifying which compounds (from libraries with 7260 members for a core with 4 positions and as many as 15 functionalities) in a library are selected by an assay. This program will be placed on our home page for public use.36
Supplementary Material
Acknowledgment.
This work was supported by N.S.F. CAREER-9732950 and PSC-CUNY 668331 grants to C.M.D. The Hunter College mass-spec facility is supported by NIH RCMI grant RR-03037 and NSF 9708881. V.R. is supported by the MBRS & MARC programs to Hunter College. The authors thank Ms. Xin Chen and Mr. Lincoln Roland for their assistance in several photocleavage experiments.
References and Notes
- (1).Terrett NK Combinatorial Chemistry; Oxford University Press: New York, 1998. [Google Scholar]
- (2).Shipps GW; Pryor KE; Xian J; Skyler DA; Davidson EH; Rebek J Jr. Synthesis and screening of small molecule libraries active in binding to DNA. Proc. Natl. Acad. Sci. U.S.A 1997, 94, 11833–11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).A simple algorithm to calculate the molar masses and the number of isobaric species will be made available on the Internet. The number of compounds for meso-substituted porphyrins (not including possible rotameric forms) is found by the following formula: N = C1m + 4C2m + 6C3m + 3C4m where Cnm) m!/(n!(m − n)!), n = positions on the meso-porphyrin (n = 1 when all the substituents are the same to n = 4 when all are different), and m = the number of starting aldehydes. For 10 starting aldehydes there are 1540 compounds of which 715 are isobaric.
- (4).Tapscott RE; Marcovich D Enumeration of permutational isomers: the porphyrins. J. Chem. Educ 1978, 55, 446–447. [Google Scholar]
- (5).Drain CM; Lehn J-M Self-assembly of square multiporphyrin arrays by metal ion coordination. J. Chem. Soc., Chem. Commun 1994, 2313–2315.
- (6).Drain CM; Nifiatis F; Vasenko A; Batteas J Porphyrin tessellation by design: Metal mediated self-assembly of large arrays and tapes. Angew. Chem., Int. Ed 1998, 37, 2344–2347; Angew. Chem 1998, 110, 2474–2477. [DOI] [PubMed] [Google Scholar]
- (7).Fleischer EB; Shachter AM Coordination oligomers and a coordination polymer of zinc tetraarylporphyrins. Inorg. Chem 1991, 30, 3763–3769. [Google Scholar]
- (8).Berlin K; Jain RK; Tetziaff C; Steinbeck C; Richert C Spectrometrically monitored selection experiments: Quantitative laser desorption mass spectrometry of small chemical libraries. Chem. Biol 1997, 4, 63–77. [DOI] [PubMed] [Google Scholar]
- (9).Berlin K; Jani RK; Richert C Are porphyrin mixtures favorable photodynamic anticancer drugs? A model study with combinatorial libraries of tetraphenylporphyrins. Biotech. Bioeng 1998, 61, 107–118. [DOI] [PubMed] [Google Scholar]
- (10).Sternberg ED; Dolphin D; Brücker C Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar]
- (11).Armitage B Photocleavage of nucleic acids. Chem. Rev 1998, 98, 1171–1200. [DOI] [PubMed] [Google Scholar]
- (12).Bonnet R Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. ReV 1995, 19–32.
- (13).Rouhi AM Let there be light and let it heal. Chem. Eng. News 1998, November 2, 22–27. [Google Scholar]
- (14).Sari MA; Battioni JP; Dupre D; Mansuy D; Pecq JBL Interaction of Cationic Porphyrins with DNA: Importance of the number and position of the charges and minimum structural requirements for intercalation. Biochemistry 1990, 29, 4205–4215. [DOI] [PubMed] [Google Scholar]
- (15).Meng GG; James BR; Skov KA Porphyrin chemistry pertaining to the design of anti-cancer drugs; part 1, the synthesis of Porphyrins containing meso-pyridyl and meso-substituted phenyl functional groups. Can. J. Chem 1994, 72, 1894–1909. [Google Scholar]
- (16).Meng GG; James BR; Skov KA; Korbelik M Porphyrin chemistry pertaining to the design of anti-cancer drugs: part 2, the synthesis and in vitro testes of water-soluble porphyrins containing, in the meso positions, the functional groups: 4-methylpyridinium, or 4-sulfantophenyl, in combination with phenyl, 4-pyridyl, 4-nitro-phenyl, or 4-aminophenyl. Can. J. Chem 1994, 72, 2447–2457. [Google Scholar]
- (17).Uno T; Hamasaki K; Tanigawa M; Shimabayashi S Binding of meso-tetrakis(N-methylpyridinium-4-yl)porphyrin to double helical RNA and DNA-RNA hybrids Inorg. Chem 1997, 36, 1676–1683. [DOI] [PubMed] [Google Scholar]
- (18).Lipscomb LA; Zhou FX; Presnell SR; Woo RJ; Peek ME; Plaskon RR; Williams LD Structure of a DNA-porphyrin complex. Biochemistry 1996, 35, 2818–2823. [DOI] [PubMed] [Google Scholar]
- (19).Mettath S; Munsun BR; Pandey RK DNA interaction and photocleavage properties of porphyrins containing cationic substituents at the peripheral position. Bioconjugate Chem 1999, 10, 94–102. [DOI] [PubMed] [Google Scholar]
- (20).Takemura T; Ohta N; Nakajima S; Sakata I The mechanism of photosensitization in photodynamic therapy: chemiluminescence caused by photosensitization of porphyrins in saline containing human serum albumin. Photochem. Photobiol 1992, 55, 137–40. [DOI] [PubMed] [Google Scholar]
- (21).Oulmi D; Maillard P; Vever-Bizet C; Momenteau M; Brault D Glycosylated porphyrins: characterization of association in aqueous solutions by absorption and fluorescence spectroscopies and determination of singlet oxygen yield in organic media. Photochem. Photobiol 1998, 67, 511–518. [Google Scholar]
- (22).Valenzeno DP Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem. Photobiol 1987, 46, 147–160. [DOI] [PubMed] [Google Scholar]
- (23).Pasternack RF Garrity P; Ehrlich B; Davis CB; Gibbs EJ; Orloff G; Giartosio A; Turano C The influence of ionic strength on the binding of a water soluble porphyrin to nucleic acids. Nucl. Acids Res 1986, 14, 5919–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Giulio J Far-red-absorbing photosensitizers: their use in photodynamic therapy of tumors. J. Photochem. Photobiol., A 1992, 62, 371–378. [Google Scholar]
- (25).Woodburn KW; Vardaxis NJ; Hill JS; Kaye AH; Reiss JA Phillips DR Evaluation of porphyrin characteristics required for photodynamic therapy. Photochem. Photobiol 1992, 55, 697–704. [DOI] [PubMed] [Google Scholar]
- (26).Wainwright M Non-porphyrin photosensitizers in biomedicine. Chem. Soc. ReV 1996, 351–359.
- (27).Adams KR; Berenbaum MC; Bonnett R; Nizhnik AN; Salgado A; Valles MA Second generation tumor photo sensitizers: the synthetic and biological activity of octaalkyl chlorins and bacteriochlorins with graded amphiphilic character. J. Chem. Soc., Perkin Trans 1 1992, 1465–1470. [Google Scholar]
- (28).Adler AD; Longo FR; Finarelli JD; Goldmacher J; Assour J; Korsakoff L A mechanistic study of the synthesis of meso-tetraphenylporphin. J. Org. Chem 1967, 32, 476–484. [Google Scholar]
- (29).Lindsey JS; Schreiman IC; Hsu HC; Kearney PC; Marguerettaz AM Rothemund and Adler-Longo reactions revisited: Synthesis of tetraphenylporphyrins under equilibrium conditions. J Org. Chem 1987, 52, 827–836. [Google Scholar]
- (30).Drain CM; Gong X Efficient synthesis of meso-substituted porphyrins by reactions in the gas phase. Chem. Commun 1997, 2117–2118. [Google Scholar]
- (31).Drain CM; Soll CE; Gong X; Chicoineau PF Unpublished results
- (32).Supporting Information.
- (33).Pasternack RF; Gibbs EJ; Collings PJ; dePaula JC; Turzo CL; Terracina A A nonconventional approach to supramolecular formation dynamics. The kinetics of assembly of DNA-bound porphyrins. J. Am. Chem. Soc 1998, 120, 5873–5878. [Google Scholar]
- (34).Drain CM; Mauzerall D Photogating of ionic currents across the lipid bilayer: Hydrophobic ion conduction by an ion chain mechanism. Biophys. J 1992, 63, 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Mauzerall D; Drain CM Photogating of ionic currents across the lipid bilayer: Electrostatics of ions and dipoles inside the membrane. Biophys. J 1992, 63, 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36). http://patsy.hunter.cuny.edu:8001/FandS/CMD/drain.html.CC990011S.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


