Abstract
Evidence is presented to support a possible X-Y chromosome interchange etiology for the rare infertile 46,XX male syndrome. Cytogenetic studies including various chromosome banding techniques on lymphocytes and fibroblasts revealed the absence of a Y chromosome in the 400 early metaphase and midmetaphase chromosome plates analyzed. Measurements of the X chromosomes of this patient showed a significant difference in length between the terminal unstained bands on the short arms designated as p22 of the two X chromosomes. The difference was also significant when compared to the same type of measurements of the X chromosomes from normal females.
THE 46,XX male syndrome, which resembles the Klinefelter’s syndrome except for short stature, normal body-hair distribution and normal intelligence, has been a curious phenomenon since the first reported case by de la Chapelle in 1964.1 Since then, approximately 65 cases have appeared in the literature. In spite of the additional case reports, the etiology of this syndrome has not been clearly established.
Three theories have been proposed to explain the XX male syndrome. These include:
the autosomal mutation theory which suggests that male determining factors are located on chromosomes other than the Y;2
the X-Y chromosome interchange theory which hypothesizes the translocation of a portion of the Y chromosome to the X;3
and mosaicism with an undetected XXY cell line present in some tissue.4 Herein, evidence is presented to support the X-Y chromosome interchange theory of sex reversal in this unique syndrome.
Materials and methods
A 31 year-old male was referred to us for urological evaluation of infertility and underdeveloped testes. He was short but well-proportioned and had normal intelligence. Many of his features, such as the testicular histology of interstitial cell hyperplasia and tubular atrophy are common to the 46,XX male syndrome. He had male secondary sex characteristics, although the genitalia were small and bilateral gynecomastia was present.
Cytogenetic studies including the use of G-5, C-6, and Q-7 chromosome banding procedures as well as prometaphase banding techniques8 were performed on lymphocytes and on fibroblasts. A total of 275 early metaphase and mid-phase plates were analyzed from phytohemagglutin-stimulated lymphocytes and 115 metaphase plates were analyzed from fibroblast cultures. Buccal cells were stained with cresyl violet for X- and Y-chromatin determination.
Results and discussion
The initial studies showed a 46,XX chromosome complement confirmed by G-, C-, and Q-chromosome banding but no Y chromosome could be positively identified. One metaphase plate with 46 chromosomes containing five small acrocentric chromosomes and one X chromosome with the rest of the chromosomes being normal was found. Four of the five small acrocentric chromosomes were thought to be normal chromosome pairs no. 21 and 22. The remaining acrocentric chromosome was shorter in length and did not have the typical morphology of a Y-chromosome. No fluorescense was detected with the Q-banding technique. It is doubtful that this acrocentric chromosome was a Y; therefore, we have no true evidence for XX/XY mosaicism. The buccal smear showed 28% X-chromatin positive cells with a normal female control of 30% and there was no evidence of Y-chromatin in the buccal cells.
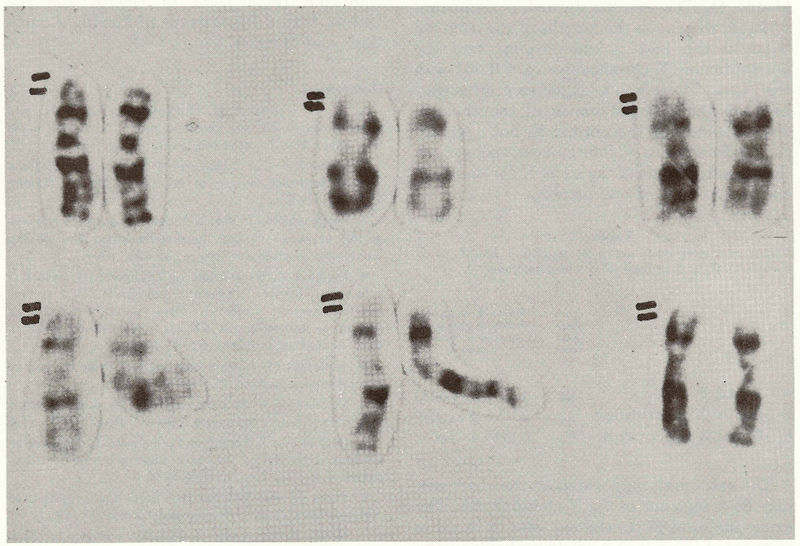
It was noted that the X chromosomes were mismatched in size in approximately 70% of the cells analyzed while the other chromosomes appeared normal. Visually, the short arms were not uniform in length. In particular, the terminal unstained band (p22) was longer in one than in the other X chromosome (Fig. 1). Similar X chromosome polymorphisms have been noted by others.9-12 Parental karyotypes could not be obtained to evaluate this phenomenon. To judge the statistical significance of this size variation, quantitative analysis was undertaken. 26 X chromosome pairs from the 46,XX patient were photographed, analyzed and measured.
Figure 1.
Pairs of X chromosomes from a selection of cells representing the additional chromosome material.
The length of the terminal band (p22) on the short arm13 and the total length of the chromosomes were measured with vernier calipers. The ratios of the p22 to the total length of the chromosome were calculated for the long X chromosome and the short X chromosome of our patient plus X chromosomes from normal females. The means and standard errors of the various chromosomes were calculated and statistically analyzed (Table 1). The results showed a significant difference between the lengths of the terminal band of the long X chromosome and the normal female X chromosome (p < 0.001 with the t test). There was no significant difference between the terminal bands of the short X chromosome and the normal X, but a significant difference (p < 0.001 occurred between the terminal bands of the short X chromosome and the long X of our patient.
Table 1.
Means and standard errors of p22/total length of X chromosome in 3 groups of X chromosomes.
| # of Chromosomes | Means & standard errors of p22/total length of X chromosome | ||
|---|---|---|---|
| A. | Normal X | 64 | 0.1125 ± 0.0035 |
| B. | Shorter X of our patient | 26 | 0.1096 ± 0.0047 |
| C. | Longer X of our patient | 26 | 0.1941 ± 0.0054 |
The extra material located on the short arm may be a segment of the Y chromosome. The extra material is unstained and if it is a segment of the Y then it would probably be the region that does not stain intensely. The most likely segment of the Y that would fit this description would be the short arm. This is also the area that contains male determining genes.14 There is also evidence, presented by Pearson and Bobrow in 1970,15 that the short arms of the X and Y chromosomes associate end to end at meiosis. This could allow a translocation to occur. Other evidence to support the X-Y interchange theory would be the H-Y antigen studies by Noël and Tous in 1978.16
Therefore, the cytogenetic findings support the X-Y interchange theory as the etiology of the 46,XX male syndrome in this patient. We cannot rule out the other theories as being relevant in other cases of this unique syndrome.
It is our hope that additional cases of the 46,XX male syndrome will be carefully studied with the X-Y interchange theory in mind. By so doing, the relative frequency of X-Y interchange as the cause of this syndrome would be determined.
Down Memory Lane.
Thus, even before I studied medicine I had begun to form an opinion which was very much like Graham Steele’s aphorism, “Nobody ever dies of mitral insufficiency alone.”
The hospital has changed in twenty-five years from a boarding house for the sick with small bed capacity to an enormous institution rendering extensive detailed service.
Nothing should be done to hasten a normal labor.
Sixty per cent of hospital beds in the United States are owned by cities, states and the Federal government.
Curtis, a place of 1,000 people, seat of a Branch of the Nebraska School of Agriculture, is said to offer a good field for another physician.
A story was told the other day about a practitioner outstate in Michigan, who had the reputation of being a very busy man in the profession, who, when called on by an official of the state society, explained his crowded waiting room by saying that he was unusually busy that day because Thursday was Insulin Day — all the diabetics came in for their weekly dose of insulin!
A state journal can and should be the most cohesive thing in medical organization.
The community should take care of its indigent.
Under German State Medicine — Die Kranken-Kasse — the worst possible condition exists, physicians being no more than counter dispensers. Under the English system conditions are better, but leave much to be desired.
Students do not practice as well as they are taught. They are too quick to acquire the haste and rapid delivery methods of older practitioners — forceps, pituitrin, Caesarean section, etc.
Acknowledgments
This work was partially supported by the Nebraska Department of Health and the United States Department of Health, Education and Welfare.
Contributor Information
MERLIN G. BUTLER, Center for Human Genetics, University of Nebraska Medical Center, Omaha, Nebraska
MYRON P. WALZAK, Department of Urology, Creighton Medical School, Omaha, Nebraska
WARREN G. SANGER, Center for Human Genetics, University of Nebraska Medical Center, Omaha, Nebraska
CURTIS T. TODD, Department of Obstetrics and Gynecology, University of Nebraska Medical Center, Omaha, Nebraska
References
- 1.de la Chapelle A, Hortling H, Niemi M and Wennstrom J: XX sex chromosomes in a human male: first case. Acta Med Scand Suppl 412:25, 1964. [DOI] [PubMed] [Google Scholar]
- 2.Hamerton JL: Significance of sex chromosome derived heterochromatin in mammals. Nature (Lond), 219:910, 1968. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson-Smith MA: X-Y chromosomal interchange in the aetiology of true hermaphroditism and of XX Klinefelter’s syndrome. Lancet II 475, 1966. [DOI] [PubMed] [Google Scholar]
- 4.Lindsten J, Bergstrand G, Tillinger K-G, Schwarzacher H-G, Tiepolo L, Mudal S and Hokfelt B: A clinical and cytogenetic study of three patients with male phenotype and apparent XX sex chromosomal constitution. Acta Endocr (Kbh), 52:91, 1966. [DOI] [PubMed] [Google Scholar]
- 5.Seabright M: A rapid banding technique for human chromosomes. Lancet, 971, 1971. [DOI] [PubMed] [Google Scholar]
- 6.Sumner A: A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304, 1972. [DOI] [PubMed] [Google Scholar]
- 7.Caspersson T, Zech L and Johansson C: Differential binding of alkylating fluorochromes in human chromosomes. Exp Cell Res 60:315, 1970. [DOI] [PubMed] [Google Scholar]
- 8.Yunis JJ and Sanchez O: The G-banded prophase chromosomes of man. Humangenetik 27:167, 1975. [DOI] [PubMed] [Google Scholar]
- 9.Maden K and Walker S: Possible evidence for Xp+ in an XX male. Lancet, I 1223, 1978. [DOI] [PubMed] [Google Scholar]
- 10.Wachtel SS, Koo GC, Breg WR, Thaler HT, Dillard GM, Rosenthal IM, Dosik H, Gerald PS, Saenger P, New M, Lieber E and Miller OJ: Serologic detection of a Y-linked gene in XX males and XX true hermaphrodites. NEJM 295:750, 1976. [DOI] [PubMed] [Google Scholar]
- 11.Pescia G and Jotterand M: Possible evidence of X-Y interchange in an XX male. Lancet, I 550, 1977. [DOI] [PubMed] [Google Scholar]
- 12.Evans HJ, Buckton KE, Spowart G and Carothers AD: Heteromorphic X chromosomes in 46,XX males: Evidence for the involvement of X-Y interchange. Hum. Genet, 49:11, 1979. [DOI] [PubMed] [Google Scholar]
- 13.Paris Conference (1971) Standardization in human cytogenetics. Birth Defects: Original Article Series VIII:7. The National Foundation, New York, 1972. [PubMed] [Google Scholar]
- 14.Rary JM, Cummings DK, Jones HW Jr and Rock JA: Assignment of the H-Y antigen gene to the short arm of chromosome Y. J Hered 70:78, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Pearson PL and Bobrow M: Definite evidence for the short arm of the Y chromosome associating with the X chromosome during meiosis in the human male. Nature (Lond), 226:959, 1970. [DOI] [PubMed] [Google Scholar]
- 16.Noël B and Tous J: Sexual determination of XX men. J Genet Hum 26(3):287, 1978. [PubMed] [Google Scholar]