Abstract
Previous studies in psychosis patients have shown hippocampal volume deficits across anterior and posterior regions or across subfields, but subfield specific changes in volume along the hippocampal long axis have not been examined. Here, we tested the hypothesis that volume changes exist across the hippocampus in chronic psychosis but only the anterior CA region is affected in early psychosis patients. We analyzed structural MRI data from 179 patients with a non-affective psychotic disorder (94 chronic psychosis; 85 early psychosis) and 167 heathy individuals demographically matched to the chronic and early psychosis samples respectively (82 matched to chronic patients; 85 matched to early patients). We measured hippocampal volumes using Freesurfer 6-derived automated segmentation of both anterior and posterior regions and the CA, dentate gyrus, and subiculum subfields. We found a hippocampal volume deficit in both anterior and posterior regions in chronic psychosis, but this deficit was limited to the anterior hippocampus in early psychosis patients. This volume change was more pronounced in the anterior CA subfield of early psychosis patients than in the dentate gyrus or subiculum. Our findings support existing models of psychosis implicating initial CA dysfunction with later progression to other hippocampal regions and suggest that the anterior hippocampus may be an important target for early interventions.
Keywords: Schizophrenia, Hippocampus, First episode psychosis, Volume, Subfields.
Highlights
-
•
Anterior and posterior hippocampal volumes are decreased in chronic psychosis
-
•
Only anterior hippocampal volume is reduced in early psychosis
-
•
Volume reduction in early psychosis is limited to the anterior cornu ammonis region
1. Introduction
Schizophrenia is a neurodevelopmental disorder associated with significant morbidity and mortality (De Hert et al., 2011; Olfson et al., 2015), yet the nature and timing of brain changes remain poorly understood. Abnormal brain structure is present in early and chronic stages of the illness, and some of these alterations appear to be progressive (Fusar-Poli et al., 2013; Haijma et al., 2013; Olabi et al., 2011). Smaller hippocampal volume is one of the most commonly replicated findings in schizophrenia (Adriano et al., 2012; Van Erp et al., 2016), with roughly an 8% volume deficit in the chronic stage relative to demographically similar healthy individuals (Velakoulis et al., 2006). However, it is currently unclear whether specific hippocampal subregions are affected early in the illness or if volume changes occur across the structure in a widespread, non-specific manner.
The hippocampus is not a unitary structure. A tripartite division of the hippocampus into head, body, and tail regions dates back to the earliest description of the structure by Arantius in 1587 as a ‘little seahorse’ (Rosene and Van Hoesen, 1987). A simpler bipartite parcellation of the human hippocampus into anterior (head) and posterior (body + tail) segments is based on an anatomical landmark, the uncus (Weiss et al., 2005). The anterior and posterior segments have largely divergent patterns of extra-hippocampal anatomical connectivity and functional properties (Poppenk et al., 2013). Despite the early recognition that there may be differential volume changes related to psychosis in the anterior and posterior hippocampal segments, no clear findings emerged from the initial literature on this topic (Becker et al., 1996; Bogerts et al., 1993; Bogerts et al., 1990; DeLisi et al., 1988; Hirayasu et al., 1998; Lieberman et al., 2001; Rajarethinam et al., 2001; Weinberger et al., 1992). This was likely due to low resolution imaging data, the inclusion of the amygdala in hippocampal volume estimates, or measurement of only specific parts of the hippocampus or temporal lobe. In more recent studies that delineated hippocampal volume separately from surrounding structures, the finding of decreased anterior hippocampal volume in chronic psychosis is more consistent (Goldman et al., 2007; Pegues et al., 2003; Schobel et al., 2009; Thoma et al., 2009), but not universal (Maller et al., 2012; Rametti et al., 2007; Weiss et al., 2005). In the limited number of studies examining this question in early psychosis, two have found evidence of an anterior volume deficit, without observing a similar decrease in posterior hippocampus (Kalmady et al., 2017; Szeszko et al., 2003); but see (Williams et al., 2012) for an exception. Decreased gray matter volume within the anterior hippocampus has been found using a voxel-based method in a meta-analysis of individuals at high risk for developing a psychotic disorder (Fusar-Poli et al., 2011) and in a large cohort of psychosis spectrum youth (Satterthwaite et al., 2016). In summary, there is burgeoning evidence of an anterior hippocampal deficit in early psychosis that may extend to the posterior hippocampus in the chronic illness stage. However, the studies used to examine this question have important methodological variations that necessitate further study. For example, studies differ in the particular boundary used to separate the anterior from posterior hippocampus (uncus vs. dividing slices in half vs. voxel based localization) and the method used to control for differences in intracranial volume (Voevodskaya, 2014). Critically, many studies did not explicitly test for a group by hippocampal region interaction, leaving open the possibility that anterior and posterior volumes in a patient cohort do not differ meaningfully (Strange et al., 2014).
In addition to a simple anterior/posterior dichotomy, the hippocampus has multiple subfields defined in coronal sections along its transverse axis, including the cytoarchitecturally defined cornu ammonis (CA) fields 1–4, dentate gyrus, and subiculum (Duvernoy et al., 2013). Manual segmentation of subfields on high resolution images is time and labor intensive (Winterburn et al., 2015), but automated segmentation software has been developed recently (Iglesias et al., 2015; Yushkevich et al., 2015a). Consequently, there are only a small number of high quality studies examining subfield specific changes in early and chronic psychosis. One investigation using automated segmentation of high resolution T2 images in chronic schizophrenia found decreased volume in the cornu ammonis and dentate gyrus subfields (Ota et al., 2017). An important study by Ho et al. using automated segmentation of T1 images suggests that CA1 volume deficits in the first 5 years of psychosis evolve to affect all subfields in chronic illness (Ho et al., 2017a). Another study by the same group indicates that CA1 changes may even be present in ultra-high-risk individuals as they transition to clinical psychosis (Ho et al., 2017b). Taken together, these studies support a role for CA1 in the early stages of psychosis. However, the cohorts studied by Ho et al. leave an intriguing gap between a relatively small group of ultra-high-risk individuals transitioning to psychosis and a larger cohort of patients who are at a somewhat later illness stage (~2 years). The first 2 years of a psychotic illness may represent a “critical period” in which pathological changes have not fully emerged and the potential opportunities for intervention and treatment during this illness phase warrant careful study of the neurobiological changes occurring during this period (Birchwood et al., 1998; Crumlish et al., 2009).
Previous investigations have studied hippocampal volume either along the transverse or the longitudinal axes of the hippocampus, as described above. But the hippocampus might be described best as a series of multiple discrete subdomains superimposed on a longitudinal gradient, as defined by structural connectivity, functional properties, and gene expression patterns (Strange et al., 2014). To our knowledge, no studies have considered whether there are subfield volume differences along the anterior-posterior extent of the hippocampus in psychosis. In this study, we focus on identifying in early and chronic psychosis patients the pattern of volume changes observed along the longitudinal axis of the hippocampal formation, within the cornu ammonis, dentate gyrus, and subiculum subfields (Rosene and Van Hoesen, 1987). These allocortical regions are posited by different models to play a role in the pathophysiology of psychosis, including CA1 hyperactivity (Lieberman et al., 2018), GABAergic dysfunction in CA2/3 (Benes, 1999), altered glutamate transmission in the dentate gyrus (Tamminga et al., 2010), and subiculum hyperactivity (Grace, 2010). Lieberman et al. (2018) have proposed a neuroprogressive model in which early glutamate dysfunction in CA1 leads to neuronal hyperactivity, metabolic dysregulation, and ultimately a decrease in volume that spreads from CA1 to other subfields and eventually prefrontal areas. However, the data used to validate this model come from a limited number of clinical high-risk individuals who transitioned to psychosis and from mouse models of schizophrenia. As these authors point out, schizophrenia may develop from multiple underlying etiologies that share a common final pathway to phenotypic expression and it is unclear whether the origin and progressive pattern of structural changes predicted by the model will hold in a larger, more heterogeneous sample of psychosis patients in both the early and chronic illness stages.
Here, we test the hypothesis that volume deficits in chronic psychosis are present throughout the hippocampus, but are limited to the anterior CA region in early psychosis. We first examine whether the anterior hippocampus is preferentially affected in chronic and early psychosis by testing for volume deficits in the anterior vs. posterior regions of the hippocampus at each illness stage. Next, we test whether there are subfield specific changes along the hippocampal long axis at each illness stage by comparing CA, DG, and subiculum volumes in the hippocampal head and body.
2. Material and methods
2.1. Participants
We analyzed data from 346 individuals (179 patients with a non-affective psychotic disorder and 167 healthy controls: Table 1). Patients were recruited from the psychiatric inpatient and outpatient clinics of the Vanderbilt University Medical Center Psychotic Disorders Program as part of an ongoing data repository, the Psychiatric Phenotype/Genotype Project (PGPP). Healthy controls were recruited from the surrounding community through email advertisements. The study was approved by the Vanderbilt University Institutional Review Board and all participants provided written informed consent and received monetary compensation for their time. Diagnoses were assessed with the Structured Clinical Interview for DSM-IV (First et al., 2002). Clinical symptoms were further assessed with the Positive and Negative Syndrome Scale (PANSS, N = 176; Kay et al., 1987), the Young Mania Rating Scale (YMRS, N = 166; Young et al., 1978), and the Hamilton Depression Rating Scale (HAMD, N = 176; Hamilton, 1960). Premorbid IQ was estimated with the Wechsler Test of Adult Reading (WTAR, N = 345; Wechsler, 2001). Patient medication load was assessed with the structured interview and medical record review and is reported in terms of chlorpromazine (CPZ) equivalents (N = 157; Gardner et al., 2010). Participants were excluded for significant medical or neurological illness, head injury, pregnancy, age <16 or over 65, estimated premorbid IQ < 70, or meeting criteria for substance abuse or dependence within the past month. Healthy controls were excluded if they had current or past psychiatric illness, psychotropic drug use, or a first degree relative with a psychotic illness. Participants were selected from the PGPP repository for the present study if they had an MRI with a T1-weighted structural scan without motion artifacts and had a diagnosis of a non-affective psychotic disorder (Schizophrenia N = 85; Schizoaffective Disorder = 45; Schizophreniform Disorder = 77; Brief Psychotic Disorder N = 2) or were healthy controls (N = 190).
Table 1.
Participant demographics and clinical characteristics.
| Chronic sample |
Early sample |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC |
PSY |
HC > PSY |
HC |
PSY |
HC > PSY |
|||||||
|
n = 82 |
n = 94 |
n = 85 |
n = 85 |
|||||||||
| Mean | SD | Mean | SD | Statistic | p | Mean | SD | Mean | SD | Statistic | p | |
| Age (years) | 35.87 | 10.60 | 37.14 | 11.41 | t = −0.77 | 0.40 | 22.20 | 3.55 | 21.93 | 3.63 | t = 0.49 | 0.62 |
| Sex (M/F) | 44/38 | 52/42 | χ2 = 0.05 | 0.83 | 62/23 | 70/15 | χ2 = 2.17 | 0.14 | ||||
| Race (W/B/O) | 53/20/9 | 56/32/6 | χ2 = 2.65 | 0.27 | 66/19/0 | 63/21/1 | χ2 = 1.17 | 0.56 | ||||
| Participant educationa | 15.86 | 2.05 | 13.03 | 2.31 | t = 8.49 | <0.001 | 14.15 | 1.88 | 13.36 | 2.26 | t = 2.46 | 0.01 |
| Parental education | 14.17 | 2.16 | 13.66 | 2.89 | t = 1.21 | 0.23 | 14.67 | 2.49 | 14.90 | 2.58 | t = −0.57 | 0.57 |
| WTAR | 110.46 | 12.00 | 96.34 | 16.52 | 6.52 | <0.001 | 110.60 | 11.02 | 101.16 | 15.33 | t = 4.61 | <0.001 |
| ICV (mL) | 1501.30 | 147.67 | 1472.95 | 181.31 | 1.13 | 0.26 | 1547.00 | 161.92 | 1558.81 | 165.10 | t = −0.47 | 0.64 |
| Diagnosis | ||||||||||||
| Schizophrenia | 60 | 17 | ||||||||||
| Schizoaffective | 34 | 5 | ||||||||||
| Schizophreniform | 62 | |||||||||||
| Brief Psychotic Disorder | 1 | |||||||||||
| Duration of Illness (years)a | 16.09 | 11.50 | 0.56 | 0.82 | ||||||||
| PANSS | ||||||||||||
| Positive | 19.59 | 7.94 | 17.93 | 7.11 | ||||||||
| Negative | 14.59 | 6.50 | 17.39 | 8.10 | ||||||||
| General | 31.72 | 8.50 | 33.35 | 8.44 | ||||||||
| YMRS | 6.48 | 7.81 | 3.44 | 5.26 | ||||||||
| HAMD | 8.87 | 6.59 | 8.63 | 6.01 | ||||||||
| CPZ equivalents | 507.05 | 312.18 | 258.07 | 184.03 | ||||||||
HC = healthy controls; PSY = patients with psychosis.
Exact duration of illness unavailable for 1 early stage patient.
2.2. Structural MRI data acquisition
Structural imaging data was acquired on a 3 T Philips Intera Achieva scanner at the Vanderbilt University Institute of Imaging Science (Philips Healthcare, Inc.). We acquired a 3D T1-weighted scan (voxel size = 1 mm3; field of view = 2562; number of slices = 170; TE = 3.7 ms; TR = 8.0 ms). Each image was visually inspected and determined to be free from motion or other artifacts prior to inclusion in the analysis.
2.3. Hippocampal segmentation
Each T1 image was processed using the Freesurfer 6 image analysis suite with standard parameters (http://surfer.nmr.mgh.harvard.edu/; Dale et al., 1999; Fischl et al., 2002) and the hippocampus subfield segmentation module (development version 20180220; Iglesias et al., 2015). This module performs an automated segmentation of hippocampal subfields based on a high-resolution probabilistic atlas generated from ex vivo MRI data. The subfield segmentation for each participant was visually inspected for errors and as a consequence, no manual editing of segmentations was performed. Segmentations that included tissue outside the hippocampus or that failed to include portions of the hippocampus were excluded. This led to the removal of data from 53 participants (Schizophrenia N = 8; Schizoaffective Disorder = 6; Schizophreniform Disorder = 15; Brief Psychotic Disorder N = 1; Healthy Controls N = 23). The excluded participants did not differ from included participants on age, sex, race, parental education, or ICV. Excluded patients also did not differ from those included on clinical symptom measures or medication load.
We created composite measures of hippocampal regions and subfields from the detailed segmentations carried out by Freesurfer. This approach has three conceptual and methodological advantages: 1) Our hypotheses concern volume differences across three allocortical regions of the hippocampal formation: the hippocampus proper (cornu ammonis fields), the dentate gyrus, and the subiculum. Investigation of these regions requires aggregation of the default Freesurfer regions; 2) Combination of subfields in this manner allows for easier comparison with data generated from other commonly used automated segmentation routines, e.g., ASHS (Mueller et al., 2018; Yushkevich et al., 2015b); 3) Our segmentations are based on the T1 image alone, rather than a multispectral segmentation of T1 and T2 data, and boundary definitions for smaller subregions (e.g., CA3, GC-DG, molecular layer) may be less reliable with only 1 mm T1 data. Freesurfer segments the hippocampal formation into 12 subregions. We constructed composite measures of anterior/posterior and subfield regions from these 12 subregions for each hemisphere separately (Fig. 1). Because our questions centered around the cornu ammonis, dentate gyrus, and subiculum, we excluded the hippocampal-amygdala transition area, parasubiculum, and fimbria from our analyses.
Fig. 1.
Description of composite measures of hippocampal subregions for volume. A. Default Freesurfer subdivisions of the hippocampus. B. Depiction of Freesurfer segmentation of head, body, and tail regions on a representative subject. For our analysis of volume differences along the hippocampal long axis, we defined the anterior and posterior regions as shown. C. To test for subfield specific volume differences in the head and body of the hippocampus, we defined composite subfields for the CA, DG, and subiculum separately in the head and body.
To examine volumetric differences along the longitudinal axis of the hippocampus, we created composite measures of anterior (head) and posterior (body + tail) volume (Fig. 1B). We defined the anterior hippocampus as the sum of the volumes for the following subfields within the hippocampal head: CA1, CA3, CA4, molecular layer, GC/DG, subiculum, and presubiculum. The posterior hippocampus was defined as the sum of these same subfields within the hippocampal body plus the hippocampal tail.
We defined composite regions for the cornu ammonis (CA), dentate gyrus (DG), and subiculum separately in the head and body of the hippocampus of each hemisphere (Fig. 1C). A full anterior/posterior definition of these subfields was not possible because Freesurfer does not separate subfields in the tail region. Delineation of subfields within the hippocampal tail can be difficult even with higher resolution images (Yushkevich et al., 2010). We defined the CA composite region as the sum of the volumes for CA1, CA3, subiculum, and the molecular layer. We constructed the DG region from the sum of the CA4 and GC/DG subfields. Finally, we defined the subiculum as Freesurfer's presubiculum subfield. The CA/subiculum boundary is not visible on in vivo MR images and is instead typically determined from geometric rules. We chose to define the subiculum in this manner because it is more consistent with the majority of common segmentation protocols and should permit easier comparison with other studies (Fig. 3 in Yushkevich et al., 2015b). Analysis of the data using the default Freesurfer definitions of the CA and subiculum did not alter the results for these subfields (Supplementary Fig. 1).
Fig. 3.
Volume of subfields in the hippocampal head and body by group. A. In patients with chronic psychosis, hippocampal volume is selectively reduced in the CA subfields of the head and body relative to healthy controls. B. In contrast, early psychosis patients exhibit a trend for decreased volume in the CA subfields of only the hippocampal head region compared to healthy controls. (*) indicates significant contrast testing for the effect of group within each subfield at p < .05, following Bonferroni correction for multiple comparisons. Error bars indicate 95% confidence intervals of the estimated marginal mean volumes.
2.4. Selection of chronic and early psychosis samples
The 346 subjects with good hippocampal segmentations were divided into 4 groups on the basis of illness stage and demographic factor (age, sex, race) propensity score matching of healthy controls with the MatchIt package (Ho et al., 2011) in R (version 3.4.3; R Core Team 2017). First, patients were divided into chronic (duration of illness ≥ 2 years, N = 94) and early (duration of illness <2 years, N = 85) samples, consistent with critical period hypotheses of psychosis (Birchwood et al., 1998). Next, a group of 85 healthy controls was selected by propensity score analysis to match the early stage patient sample based on age, sex, and race using the nearest neighbor method. The remaining 82 healthy controls did not significantly differ from the chronic patient sample on age, sex, or race (Table 1). The patient and control samples were also matched on mean parental education (N = 308), but differed in their own education level (N = 344).
2.5. Statistical analysis
We analyzed volume data using linear mixed models with the R packages lme4 (Bates et al., 2015), emmeans (Lenth, 2018), and car (Fox and Weisberg, 2011). First, to test for psychosis related differences in volume along the longitudinal axis of the hippocampus, we fit a linear mixed model predicting volume from the fixed effects of group (psychosis, control), hemisphere (left, right), region (anterior, posterior), and their interactions, adjusting for estimated total intracranial volume, age, sex, and race, with participant as a random effect. Second, we tested for subfield specific hippocampal volume deficits in psychosis along the longitudinal axis by fitting a linear mixed model predicting volume from the fixed effects of group (psychosis, control), hemisphere (left, right), region (head, body), subfield (CA, DG, subiculum) and their interactions, adjusting for estimated total intracranial volume, age, sex, and race with participant as a random effect.
In order to examine differences in hippocampal volume between patients and controls in different illness stages, the 2 models described above were fit separately for the early and chronic samples. For all models, we conducted significance tests on the fixed effects using an analysis of variance (ANOVA) on the model output. Significant effects were followed up with pairwise contrasts adjusted for multiple comparisons with Bonferroni correction.
3. Results
3.1. Volume deficits along the hippocampal long axis in psychosis
We tested for group differences in volume across the anterior and posterior regions along the hippocampal long axis. In chronic psychosis patients relative to controls, we observed a significant main effect of group (F(1,170) = 10.52, p = .001) and no significant interactions of group with region or hemisphere (all p's > 0.05; Fig. 2A). This finding is consistent with the large body of existing work demonstrating smaller hippocampal volume in chronic psychosis patients and confirms that deficits exist across the long axis in later stages of the illness.
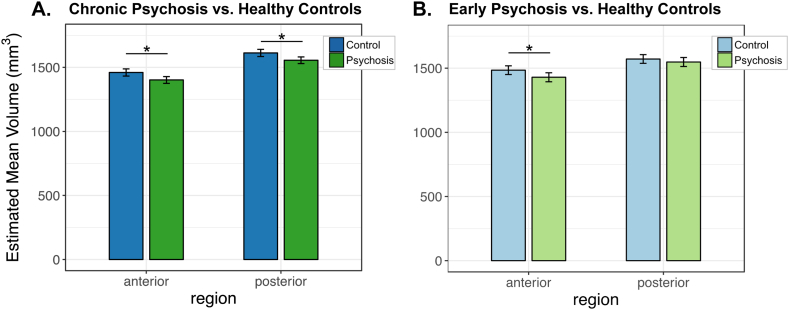
Fig. 2.
Volume of hippocampal region by group. A. In patients with chronic psychosis, hippocampal volume is reduced in both anterior and posterior regions relative to healthy controls. B. In contrast, anterior hippocampal volume was selectively reduced in early psychosis patients compared to healthy controls. (*) indicates significant contrast for the effect of group within each region at p < .05, following Bonferroni correction for multiple comparisons. Error bars indicate 95% confidence intervals of the estimated marginal mean volumes.
In contrast, comparing early psychosis patients with matched controls revealed a volume deficit in only the anterior hippocampus (Fig. 2B). This was demonstrated by a significant group by region interaction (F(1,504) = 4.74, p = .030) in the context of a significant main effect of group (F(1,164) = 4.65, p = .033). Follow up tests confirmed that hippocampal volume was smaller in the anterior (t(217.09) = 2.80, p = .005) but not posterior (t(217.09) = 1.21, p = .228) region in early psychosis patients relative to controls. The anterior volume deficit did not significantly differ by hemisphere (group by hemisphere by region interaction: F(1,504) = 2.92, p = .088).
3.2. Subfield specific volume analysis in hippocampal head and body
We next examined the volume of specific subfields within the head and body of the hippocampus in psychosis. Compared to healthy controls, chronic psychosis patients exhibited smaller volume of the CA subfield (Fig. 3A; group by subfield interaction: F(2,1914) = 28.98; p < .001). This was confirmed by a significant follow up test for the CA region (t(339.53) = 6.90, p < .001), but not the DG or subiculum (p's > 0.05). We also detected a significant group by region interaction (F(1,1914) = 4.43, p = .035) and main effect of group (F(1,170) = 10.92, p = .001). The group by region by subfield interaction was only present at trend level (F(2, 1914) = 2.36, p = .095). Because of our a priori hypothesis regarding the role of the CA in psychosis, we conducted planned comparisons investigating whether there were differences in volume across subfields within the head and body. We found significantly lower volume in chronic psychosis in both the head (t(656.60) = 7.39, p < .001) and body of the CA (t(656.60) = 4.07, p < .001), but not in the head or body of the subiculum or DG (all p's > 0.05). In the context of the significant group by region and group by subfield interactions, the presence of a trend level 3-way interaction suggests that variation in volume across the CA, DG, and subiculum along the long axis may reflect heterogeneity within the patient group.
When we examined subfield specific volumes in the early psychosis sample, once again we found a more constrained pattern of volume deficits than in the chronic cohort. As in the chronic cohort, we observed a significant main effect of group (F(1,164) = 5.36, p = .022), significant interactions of group by region (F(1,1848) = 6.08, p = .014), and trend level interactions of group by subfield (F(2,1848) = 2.49, p = .083) and group by region by subfield (F(2, 1848) = 2.62, p = .073). Planned comparisons showed that early psychosis patients displayed a volume deficit only in the CA subfield of the hippocampal head compared to healthy controls (t(680.21) = 4.38, p < .001; all other p's > 0.05).
4. Discussion
Competing models of hippocampal dysfunction in schizophrenia implicate different subfields (Benes, 1999; Grace, 2010; Lieberman et al., 2018; Tamminga et al., 2010) and emphasize either anterior (Goldman and Mitchell, 2004) or posterior (Ragland et al., 2017) pathology. In this study, we found only anterior hippocampal deficits in early psychosis patients, but volume deficits in both anterior and posterior regions in chronic psychosis patients. Additionally, we observed for the first time that this pattern of anterior volume changes in early psychosis and subsequent posterior involvement in chronic psychosis was more prominent in the CA subfields than in the dentate gyrus or subiculum.
The data obtained in this study support a model in which early dysfunction in the anterior CA subfields leads to volume changes that spread from the anterior CA to associated hippocampal regions as the psychotic illness progresses (Lieberman et al., 2018). Our finding of a more prominent volume deficit in the anterior hippocampus is consistent with the majority of the studies that have examined anterior and posterior volume differences in early psychosis (Kalmady et al., 2017; Szeszko et al., 2003). This finding also adds to the growing body of literature suggesting early deficits in the CA subfield both from volumetric (Ho et al., 2017a, Ho et al., 2017b) and shape analyses (Narr et al., 2004; Schobel et al., 2013). Our data are consistent with an early critical period in which pathological changes are ongoing (Birchwood et al., 1998; Crumlish et al., 2009) and highlight the importance of identifying illness stages to guide intervention and prevention efforts (McGorry et al., 2018; Wood et al., 2011). Ongoing longitudinal work with the early psychosis patients described here will help clarify the role of the hippocampus in progression from early stage psychosis to chronic schizophrenia.
When considered in light of current neurobiological models of hippocampal functional organization, our volume findings suggest a characteristic pattern of functional deficits. The anterior and posterior hippocampus have distinct afferent and efferent connections, with the anterior region projecting preferentially to areas more involved in emotion and higher order cognition (including the amygdala, orbital and medial prefrontal cortex, and midline subcortical areas) and the posterior region connecting to more posterior multimodal cortical regions associated with vision and spatial processing (Poppenk et al., 2013). The anterior hippocampus is posited to be involved in the formation of higher order associations among stimuli (Strange et al., 2014), the encoding of “gist-like” stimulus representations (Poppenk et al., 2013), and the learning of and abstraction of concepts (Mack et al., 2017). Consistent with such models, schizophrenia patients exhibit deficits in relational memory (Armstrong et al., 2012; Williams et al., 2010) and transitive inference (Titone et al., 2004) linked to anterior hippocampal dysfunction (Öngür et al., 2006). It is possible that when applied to concepts, the binding and inference errors observed in schizophrenia contribute to either positive illness symptoms, such as delusions, or disorganized thinking, speech, and behavior. Future studies will need to determine whether the types of conceptual learning proposed to be under the purview of the anterior hippocampus are affected in early psychosis (e.g., Mack et al., 2016).
Animal models of hippocampal organization commonly emphasize a role for the ventral (anterior) hippocampus in anxiety-related behaviors (Bannerman et al., 2004). Forty-two patients in our study had a comorbid anxiety disorder, and this may have contributed to the observed volumetric deficits. However, distinct hippocampal subfields within the anterior hippocampus appear to be affected in psychosis and anxiety. For example, while we have found that the CA subfields are preferentially affected in psychosis, extant data suggest that the dentate gyrus may be more associated with anxiety (Kheirbek et al., 2013; Persson et al., 2014). Alternatively, anxiety disorders are more prevalent in patients with a schizophrenia spectrum disorder diagnosis than the general population (Achim et al., 2011), suggesting the non-independence of psychosis and anxiety. We believe that investigation of the impact of anxiety on hippocampal structure in the context of a psychotic disorder is an important direction for future studies.
Why are the anterior cornu ammonis subfields affected in the early stage of psychosis? There are several characteristic gradients along the hippocampal long axis that render this region especially vulnerable. Most importantly, a gradient of GABA-A, NMDA, and AMPA receptor expression along the ventral-dorsal axis of the rodent hippocampus (anterior-posterior axis in humans) (Pandis et al., 2006; Sarantis et al., 2008; Sotiriou et al., 2005) points to greater excitability of the ventral/anterior hippocampus (Papatheodoropoulos, 2018), particularly in the CA1 subfield (Dougherty et al., 2012). In addition, the anterior hippocampus receives relatively denser dopaminergic projections from the ventral tegmental area (Strange et al., 2014). Although the number of hippocampal neurons is not decreased in schizophrenia, there is a reduction in the number and density of GABAergic interneurons (Konradi et al., 2011). The gradients described above, when combined with genetic vulnerability (Skene et al., 2018), may lead to an excitation-inhibition imbalance, hippocampal hyperactivity, and subsequent volume changes in the anterior CA1 subfield (Heckers and Konradi, 2015; Lisman and Grace, 2005). Initial work suggests functional changes within the anterior CA in high risk individuals who convert to psychosis (Schobel et al., 2013). An important question for future research is whether this anterior hippocampal hyperactivity is present across a more heterogeneous sample of early psychosis patients and precedes the volumetric deficit we have observed here.
The strengths of our study include large, well-matched cohorts of early and chronic psychosis patients, use of automated hippocampal segmentation software, and definition of hippocampal regions in a consistent manner across illness stages. Our study has several limitations. The Freesurfer definition of hippocampal subfield volumes using 1 mm T1 images alone may be less accurate than definitions found using segmentation of higher resolution T2 or multimodal images. Although this approach is consistent with other recent studies of hippocampal structure in schizophrenia, we cannot specify whether specific CA regions are preferentially affected with the resolution of our data. Additionally, we were unable to investigate subfield specific changes in the hippocampal tail (Maller et al., 2012). Future studies using high resolution 7 T imaging or limited field-of-view hippocampal imaging at 3 T are needed to more precisely refine the location of these early CA volume changes. Additionally, patients were excluded for substance abuse or dependence only within the past month. It is possible that a history of substance use could contribute to some of the volume changes we have observed. Finally, we have separated patients into early and chronic psychosis based on an illness duration of 2 years in order to address gaps in the existing literature. However, the dichotomization of patient groups in this way could have reduced our power to detect smaller effects (Altman and Royston, 2006) and presumes an illness threshold that may not hold for all patients.
In summary, our study finds novel evidence for an anterior volume deficit in early psychosis that is more pronounced in the CA subfields. Future studies are needed to clarify the progression of this volume deficit and to determine how it links to functional deficits in psychosis.
Acknowledgements
This work was supported by the Charlotte and Donald Test Fund, NIMH grant R01-MH70560 (Heckers), VA MERIT grant CX001226 (Blackford), Jack Martin MD Research Professor in Psychopharmacology (Blackford), the Vanderbilt Psychiatric Genotype/Phenotype Project, the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/NIH) and the Advanced Computing Center for Research and Education at Vanderbilt University, Nashville, TN.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.10.021.
Appendix A. Supplementary data
Supplementary material
References
- Achim A.M., Maziade M., Raymond É., Olivier D., Mérette C., Roy M.A. How prevalent are anxiety disorders in schizophrenia? a meta-analysis and critical review on a significant association. Schizophr. Bull. 2011;37:811–821. doi: 10.1093/schbul/sbp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriano F., Caltagirone C., Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012 doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Altman D.G., Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong K., Kose S., Williams L., Woolard A., Heckers S. Impaired associative inference in patients with schizophrenia. Schizophr. Bull. 2012;38:622–629. doi: 10.1093/schbul/sbq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D.M., Rawlins J.N.P., McHugh S.B., Deacon R.M.J., Yee B.K., Bast T., Zhang W.N., Pothuizen H.H.J., Feldon J. Regional dissociations within the hippocampus - memory and anxiety. Neurosci. Biobehav. Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Becker T., Elmer K., Schneider F., Schneider M., Grodd W., Bartels M., Heckers S., Beckmann H. Confirmation of reduced temporal limbic structure volume on magnetic resonance imaging in male patients with schizophrenia. Psychiatry Res. 1996;67:135–143. doi: 10.1016/0925-4927(96)03002-8. [DOI] [PubMed] [Google Scholar]
- Benes F.M. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol. Psychiatry. 1999;46:589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Birchwood M., Todd P., Jackson C. Early-intervention in psychosis: the critical period hypothesis. Br. J. Psychiatry. 1998;13(suppl 1):S31–S40. [PubMed] [Google Scholar]
- Bogerts B., Ashtari M., Degreef G., Alvir J.M.J., Bilder R.M., Lieberman J.A. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. Neuroimaging. 1990;35:1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- Bogerts B., Lieberman J.A., Ashtari M., Bilder R.M., Degreef G., Lerner G., Johns C., Masiar S. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol. Psychiatry. 1993;33:236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- Crumlish N., Whitty P., Clarke M., Browne S., Kamali M., Gervin M., McTigue O., Kinsella A., Waddington J.L., Larkin C., O'Callaghan E. Beyond the critical period: longitudinal study of 8-year outcome in first-episode non-affective psychosis. Br. J. Psychiatry. 2009;194:18–24. doi: 10.1192/bjp.bp.107.048942. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Hert M., Correll C.U., Bobes J., Cetkovich-Bakmas M., Cohen D.A.N., Asai I., Detraux J., Gautam S., Möller H.J., Ndetei D.M., Newcomer J.W., Uwakwe R., Leucht S. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi L.E., Dauphinais I.D., Gershon E.S. Perinatal complications and reduced size of brain limbic structures in familial schizophrenia. Schizophr. Bull. 1988;14:185–191. doi: 10.1093/schbul/14.2.185. [DOI] [PubMed] [Google Scholar]
- Dougherty K.A., Islam T., Johnston D. Intrinsic excitability of CA1 pyramidal neurones from the rat dorsal and ventral hippocampus. J. Physiol. 2012;590:5707–5722. doi: 10.1113/jphysiol.2012.242693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy H.M., Cattin F., Risold P.-Y. 4th Ed. Springer-Verlag; Berlin: 2013. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. [Google Scholar]
- Van Erp T.G.M., Hibar D.P., Rasmussen J.M., Glahn D.C., Pearlson G.D., Andreassen O.A., Agartz I., Westlye L.T., Haukvik U.K., Van Erp T.G.M., Hibar D.P., Rasmussen J.M., Glahn D.C., Pearlson G.D., Andreassen O.A., Agartz I., Westlye L.T., Haukvik U.K., Dale A.M., Melle I., Hartberg C.B., Gruber O., Kraemer B., Zilles D., Donohoe G., Kelly S., McDonald C., Morris D.W., Cannon D.M., Corvin A., Machielsen M.W.J., Koenders L., De Haan L., Veltman D.J., Satterthwaite T.D., Wolf D.H., Gur R.E.C., Gur R.E.C., Potkin S.G., Mathalon D.H., Mueller B.A., Preda A., Macciardi F., Ehrlich S., Walton E., Hass J., Calhoun V.D., Bockholt H.J., Sponheim S.R., Shoemaker J.M., Van Haren N.E.M., Pol H.E.H., Ophoff R.A., Kahn R.S., Roiz-Santiaez R., Crespo-Facorro B., Wang L., Alpert K.I., Jönsson E.G., Dimitrova R., Bois C., Whalley H.C., McIntosh A.M., Lawrie S.M., Hashimoto R., Thompson P.M., Turner J.A. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry. 2016;21:547–553. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Miriam G., Williams J. 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. (Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fox J., Weisberg S. SAGE; 2011. An R Companion to Applied Regression. (2nd Ed.) [Google Scholar]
- Fusar-Poli P., Borgwardt S., Crescini A., Deste G., Kempton M.J., Lawrie S., Mc Guire P., Sacchetti E. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci. Biobehav. Rev. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Smieskova R., Kempton M.J., Ho B.C., Andreasen N.C., Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? a meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 2013;37:1680–1691. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D.M., Murphy A.L., O'Donnell H., Centorrino F., Baldessarini R.J. International consensus study of antipsychotic dosing. Am. J. Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Goldman M.B., Mitchell C.P. What is the functional significance of hippocampal pathology in schizophrenia? Schizophr. Bull. 2004 doi: 10.1093/oxfordjournals.schbul.a007086. [DOI] [PubMed] [Google Scholar]
- Goldman M.B., Torres I.J., Keedy S., Marlow-O'Connor M., Beenken B., Pilla R. Reduced anterior hippocampal formation volume in hyponatremic schizophrenic patients. Hippocampus. 2007;17:554–562. doi: 10.1002/hipo.20292. [DOI] [PubMed] [Google Scholar]
- Grace A.A. Ventral hippocampus, interneurons, and schizophrenia. Curr. Dir. Psychol. Sci. 2010;19:232–237. [Google Scholar]
- Haijma S.V., Van Haren N., Cahn W., Koolschijn P.C.M.P., Hulshoff Pol H.E., Kahn R.S. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr. Bull. 2013;39:1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S., Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr. Res. 2015;167:4–11. doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y., Shenton M.E., Salisbury D.F., Dickey C.C., Fischer I.A., Mazzoni P., Kisler T., Arakaki H., Kwon J.S., Anderson J.E., Yurgelun-Todd D., Tohen M., McCarley R.W. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am. J. Psychiatry. 1998;155:1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- Ho D.E., Imai K., King G., Stuart E.A. MatchIt: nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011;42:1–28. [Google Scholar]
- Ho N.F., Iglesias J.E., Sum M.Y., Kuswanto C.N., Sitoh Y.Y., De Souza J., Hong Z., Fischl B., Roffman J.L., Zhou J., Sim K., Holt D.J. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol. Psychiatry. 2017;22:142–152. doi: 10.1038/mp.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N.F., Holt D.J., Cheung M., Iglesias J.E., Goh A., Wang M., Lim J.K., De Souza J., Poh J.S., See Y.M., Adcock A.R., Wood S.J., Chee M.W., Lee J., Zhou J. Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacology. 2017;42:1361–1370. doi: 10.1038/npp.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M., Roy N., Frosch M.P., McKee A.C., Wald L.L., Fischl B., Van Leemput K. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. NeuroImage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmady S.V., Shivakumar V., Arasappa R., Subramaniam A., Gautham S., Venkatasubramanian G., Gangadhar B.N. Clinical correlates of hippocampus volume and shape in antipsychotic-naïve schizophrenia. Psychiatry Res. Neuroimaging. 2017;263:93–102. doi: 10.1016/j.pscychresns.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kheirbek M.A., Drew L.J., Burghardt N.S., Costantini D.O., Tannenholz L., Fenton A.A., Hen R. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77:955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C., Yang C.K., Zimmerman E.I., Lohmann K.M., Gresch P., Pantazopoulos H., Berretta S., Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr. Res. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R. WWW Document; 2018. Emmeans: Estimated Marginal Means, aka Least-Squares Means.https://cran.r-project.org/package=emmeans [Google Scholar]
- Lieberman J., Chakos M., Wu H., Alvir J., Hoffman E., Robinson D., Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol. Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- Lieberman J.A., Girgis R.R., Brucato G., Moore H., Provenzano F., Kegeles L., Javitt D., Kantrowitz J., Wall M.M., Corcoran C.M., Schobel S.A., Small S.A. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol. Psychiatry. 2018 doi: 10.1038/mp.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J.E., Grace A.A. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Mack M.L., Love B.C., Preston A.R. Dynamic updating of hippocampal object representations reflects new conceptual knowledge. Proc. Natl. Acad. Sci. U. S. A. 2016;113:13203–13208. doi: 10.1073/pnas.1614048113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M.L., Love B.C., Preston A.R. Building concepts one episode at a time: the hippocampus and concept formation. Neurosci. Lett. 2017 doi: 10.1016/j.neulet.2017.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J.J., Daskalakis Z.J., Thomson R.H.S., Daigle M., Barr M.S., Fitzgerald P.B. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: the devil's in De-Tail. Hippocampus. 2012;22:9–16. doi: 10.1002/hipo.20873. [DOI] [PubMed] [Google Scholar]
- McGorry P.D., Hartmann J.A., Spooner R., Nelson B. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry. 2018;17:133–142. doi: 10.1002/wps.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.G., Yushkevich P.A., Das S., Wang L., Van Leemput K., Iglesias J.E., Alpert K., Mezher A., Ng P., Paz K., Weiner M.W. Systematic comparison of different techniques to measure hippocampal subfield volumes in ADNI2. NeuroImage Clin. 2018;17:1006–1018. doi: 10.1016/j.nicl.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr K.L., Thompson P.M., Szeszko P., Robinson D., Jang S., Woods R.P., Kim S., Hayashi K.M., Asunction D., Toga A.W., Bilder R.M. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. NeuroImage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Olabi B., Ellison-Wright I., McIntosh A.M., Wood S.J., Bullmore E., Lawrie S.M. Are there progressive brain changes in schizophrenia? a meta-analysis of structural magnetic resonance imaging studies. Biol. Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Olfson M., Gerhard T., Huang C., Crystal S., Stroup T.S. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72:1172. doi: 10.1001/jamapsychiatry.2015.1737. [DOI] [PubMed] [Google Scholar]
- Öngür D., Cullen T.J., Wolf D.H., Rohan M., Barreira P., Zalesak M., Heckers S. The neural basis of relational memory deficits in schizophrenia. Arch. Gen. Psychiatry. 2006;63:356. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- Ota M., Sato N., Hidese S., Teraishi T., Maikusa N., Matsuda H., Hattori K., Kunugi H. Structural differences in hippocampal subfields among schizophrenia patients, major depressive disorder patients, and healthy subjects. Psychiatry Res. Neuroimaging. 2017;259:54–59. doi: 10.1016/j.pscychresns.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Pandis C., Sotiriou E., Kouvaras E., Asprodini E., Papatheodoropoulos C., Angelatou F. Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience. 2006;140:163–175. doi: 10.1016/j.neuroscience.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C. Electrophysiological evidence for long-axis intrinsic diversification of the hippocampus. Front. Biosci. (Landmark Ed.) 2018;23:109–145. doi: 10.2741/4584. [DOI] [PubMed] [Google Scholar]
- Pegues M.P., Rogers L.J., Amend D., Vinogradov S., Deicken R.F. Anterior hippocampal volume reduction in male patients with schizophrenia. Schizophr. Res. 2003;60:105–115. doi: 10.1016/s0920-9964(02)00288-8. [DOI] [PubMed] [Google Scholar]
- Persson A., Sim S.C., Virding S., Onishchenko N., Schulte G., Ingelman-Sundberg M. Decreased hippocampal volume and increased anxiety in a transgenic mouse model expressing the human CYP2C19 gene. Mol. Psychiatry. 2014 doi: 10.1038/mp.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H.R., Moscovitch M., Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Ragland J.D., Layher E., Hannula D.E., Niendam T.A., Lesh T.A., Solomon M., Carter C.S., Ranganath C. Impact of schizophrenia on anterior and posterior hippocampus during memory for complex scenes. NeuroImage Clin. 2017;13:82–88. doi: 10.1016/j.nicl.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarethinam R., DeQuardo J.R., Miedler J., Arndt S., Kirbat R.A., Brunberg J., Tandon R. Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psychiatry Res. Neuroimaging. 2001;108:79–87. doi: 10.1016/s0925-4927(01)00120-2. [DOI] [PubMed] [Google Scholar]
- Rametti G., Segarra N., Junqué C., Bargalló N., Caldú X., Ibarretxe N., Bernardo M. Left posterior hippocampal density reduction using VBM and stereological MRI procedures in schizophrenia. Schizophr. Res. 2007;96:62–71. doi: 10.1016/j.schres.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Rosene D.L., Van Hoesen G.W. Cerebral Cortex. Springer; Boston, MA: 1987. The hippocampal formation of the primate brain; pp. 345–456. [Google Scholar]
- Sarantis K., Sotiriou E., Papatheodoropoulos C., Matsokis N., Angelatou F. Differential pharmacological properties of GABAA/benzodiazepine receptor complex in dorsal compared to ventral rat hippocampus. Neurochem. Int. 2008;52:1019–1029. doi: 10.1016/j.neuint.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Calkins M.E., Vandekar S.N., Erus G., Ruparel K., Roalf D.R., Linn K.A., Elliott M.A., Moore T.M., Hakonarson H., Shinohara R.T., Davatzikos C., Gur R.C., Gur R.E. Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2016;73:515–524. doi: 10.1001/jamapsychiatry.2015.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel S.A., Kelly M.A., Corcoran C.M., Van Heertum K., Seckinger R., Goetz R., Harkavy-Friedman J., Malaspina D. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr. Res. 2009;114:110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel S.A., Chaudhury N.H., Khan U.A., Paniagua B., Styner M.A., Asllani I., Inbar B.P., Corcoran C.M., Lieberman J.A., Moore H., Small S.A. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene N.G., Bryois J., Bakken T.E., Breen G., Crowley J.J., Gaspar H.A., Giusti-Rodriguez P., Hodge R.D., Miller J.A., Muñoz-Manchado A.B., O'Donovan M.C., Owen M.J., Pardiñas A.F., Ryge J., Walters J.T.R., Linnarsson S., Lein E.S., Sullivan P.F., Hjerling-Leffler J. Genetic identification of brain cell types underlying schizophrenia. Nat. Genet. 2018;50:825–833. doi: 10.1038/s41588-018-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou E., Papatheodoropoulos C., Angelatou F. Differential expression of γ-aminobutyric acid-A receptor subunits in rat dorsal and ventral hippocampus. J. Neurosci. Res. 2005;82:690–700. doi: 10.1002/jnr.20670. [DOI] [PubMed] [Google Scholar]
- Strange B.A., Witter M.P., Lein E.S., Moser E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Szeszko P.R., Goldberg E., Gunduz-Bruce H., Ashtari M., Robinson D., Malhotra A.K., Lencz T., Bates J., Crandall D.T., Kane J.M., Bilder R.M. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am. J. Psychiatry. 2003;160:2190–2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- Tamminga C.A., Stan A.D., Wagner A.D. The hippocampal formation in schizophrenia. Am. J. Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Thoma R.J., Monnig M., Hanlon F.M., Miller G.A., Petropoulos H., Mayer A.R., Yeo R., Euler M., Lysne P., Moses S.N., Cañive J.M. Hippocampus volume and episodic memory in schizophrenia. J. Int. Neuropsychol. Soc. 2009;15:182–195. doi: 10.1017/S1355617709090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titone D., Ditman T., Holzman P.S., Eichenbaum H., Levy D.L. Transitive inference in schizophrenia: impairments in relational memory organization. Schizophr. Res. 2004;68:235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- Velakoulis D., Wood S.J., Wong M.T.H., McGorry P.D., Yung A., Phillips L., Smith D., Brewer W., Proffitt T., Desmond P., Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis. Arch. Gen. Psychiatry. 2006;63:139. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Voevodskaya O. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer's disease. Front. Aging Neurosci. 2014;6:1–14. doi: 10.3389/fnagi.2014.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 2001. Wechsler Test of Adult Reading. [Google Scholar]
- Weinberger D.R., Berman K.F., Suddath R., Torrey E.F. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am. J. Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Weiss A.P., Dewitt I., Goff D., Ditman T., Heckers S. Anterior and posterior hippocampal volumes in schizophrenia. Schizophr. Res. 2005;73:103–112. doi: 10.1016/j.schres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Williams L.E., Must A., Avery S., Woolard A., Woodward N.D., Cohen N.J., Heckers S. Eye-movement behavior reveals relational memory impairment in schizophrenia. Biol. Psychiatry. 2010;68:617–624. doi: 10.1016/j.biopsych.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.E., Avery S.N., Woolard A.A., Heckers S. Intact relational memory and normal hippocampal structure in the early stage of psychosis. Biol. Psychiatry. 2012;71:105–113. doi: 10.1016/j.biopsych.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn J., Pruessner J.C., Sofia C., Schira M.M., Lobaugh N.J., Voineskos A.N., Chakravarty M.M. High-resolution in vivo manual segmentation protocol for human hippocampal subfields using 3T magnetic resonance imaging. J. Vis. Exp. 2015 doi: 10.3791/51861. (e51861–e51861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.J., Yung A.R., McGorry P.D., Pantelis C. Neuroimaging and treatment evidence for clinical staging in psychotic disorders: From the at-risk mental state to chronic schizophrenia. Biol. Psychiatry. 2011;70:619–625. doi: 10.1016/j.biopsych.2011.05.034. [DOI] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yushkevich P.A., Wang H., Pluta J., Das S.R., Craige C., Avants B.B., Weiner M.W., Mueller S. Nearly automatic segmentation of hippocampal subfields in in vivo focal T2-weighted MRI. NeuroImage. 2010;53:1208–1224. doi: 10.1016/j.neuroimage.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.A., Pluta J.B., Wang H., Xie L., Ding S.L., Gertje E.C., Mancuso L., Kliot D., Das S.R., Wolk D.A. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum. Brain Mapp. 2015;36:258–287. doi: 10.1002/hbm.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.A., Amaral R.S.C.C., Augustinack J.C., Bender A.R., Bernstein J.D., Boccardi M., Bocchetta M., Burggren A.C., Carr V.A., Chakravarty M.M., Chételat G., Daugherty A.M., Davachi L., Ding S.L., Ekstrom A., Geerlings M.I., Hassan A., Huang Y., Iglesias J.E., La R., Kerchner G.A., Larocque K.F., Libby L.A., Malykhin N., Mueller S.G., Olsen R.K., Palombo D.J., Parekh M.B., Pluta J.B., Preston A.R., Pruessner J.C., Ranganath C., Raz N., Schlichting M.L., Schoemaker D., Singh S., Stark C.E.L.L., Suthana N., Tompary A., Turowski M.M., Van Leemput K., Wagner A.D., Wang L., Winterburn J.L., Wisse L.E.M.M., Yassa M.A., Zeineh M.M., Sub H., Hsg G., La Joie R., Kerchner G.A., Larocque K.F., Libby L.A., Malykhin N., Mueller S.G., Olsen R.K., Palombo D.J., Parekh M.B., Pluta J.B., Preston A.R., Pruessner J.C., Ranganath C., Raz N., Schlichting M.L., Schoemaker D., Singh S., Stark C.E.L.L., Suthana N., Tompary A., Turowski M.M., Van Leemput K., Wagner A.D., Wang L., Winterburn J.L., Wisse L.E.M.M., Yassa M.A., Zeineh M.M., La R., Kerchner G.A., Larocque K.F., Libby L.A., Malykhin N., Mueller S.G., Olsen R.K., Palombo D.J., Parekh M.B., Pluta J.B., Preston A.R., Pruessner J.C., Ranganath C., Raz N., Schlichting M.L., Schoemaker D., Singh S., Stark C.E.L.L., Suthana N., Tompary A., Turowski M.M., Van Leemput K., Wagner A.D., Wang L., Winterburn J.L., Wisse L.E.M.M., Yassa M.A., Zeineh M.M., Sub H., Hsg G., La Joie R., Kerchner G.A., Larocque K.F., Libby L.A., Malykhin N., Mueller S.G., Olsen R.K., Palombo D.J., Parekh M.B., Pluta J.B., Preston A.R., Pruessner J.C., Ranganath C., Raz N., Schlichting M.L., Schoemaker D., Singh S., Stark C.E.L.L., Suthana N., Tompary A., Turowski M.M., Van Leemput K., Wagner A.D., Wang L., Winterburn J.L., Wisse L.E.M.M., Yassa M.A., Zeineh M.M. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: Towards a harmonized segmentation protocol. NeuroImage. 2015;111:526–541. doi: 10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material