Abstract
Managing the nonlethal effects of disturbance on wildlife populations has been a long‐term goal for decision makers, managers, and ecologists, and assessment of these effects is currently required by European Union and United States legislation. However, robust assessment of these effects is challenging. The management of human activities that have nonlethal effects on wildlife is a specific example of a fundamental ecological problem: how to understand the population‐level consequences of changes in the behavior or physiology of individual animals that are caused by external stressors. In this study, we review recent applications of a conceptual framework for assessing and predicting these consequences for marine mammal populations. We explore the range of models that can be used to formalize the approach and we identify critical research gaps. We also provide a decision tree that can be used to select the most appropriate model structure given the available data. Synthesis and applications: The implementation of this framework has moved the focus of discussion of the management of nonlethal disturbances on marine mammal populations away from a rhetorical debate about defining negligible impact and toward a quantitative understanding of long‐term population‐level effects. Here we demonstrate the framework's general applicability to other marine and terrestrial systems and show how it can support integrated modeling of the proximate and ultimate mechanisms that regulate trait‐mediated, indirect interactions in ecological communities, that is, the nonconsumptive effects of a predator or stressor on a species' behavior, physiology, or life history.
Keywords: anthropogenic disturbance, environmental impact assessments, marine mammals, nonconsumptive effects, population consequences, trait‐mediated indirect interactions, uncertainty
1. INTRODUCTION
The nonlethal effects of disturbance, which we define as a deviation in an animal's physiology or behavior from patterns occurring in the absence of predators or humans (Frid & Dill, 2002), can strongly affect wildlife populations (Lima, 1998). Understanding the population‐level repercussions of these changes in individual behavior and physiology is part of a more comprehensive ecological challenge: the quantification of trait‐mediated indirect effects (Werner & Peacor, 2003), also referred to as nonconsumptive effects (Peckarsky et al., 2008), on ecological interactions. Under this paradigm, a predator or other stressor can affect a species directly via the removal of individuals and alteration of population density (the consumptive or lethal effects), and indirectly by inducing changes in morphology, physiology, behavior, or life history (i.e., the species' traits) that reduce the risk of predation (Schmitz, Krivan, & Ovadia, 2004). Trait‐mediated indirect interactions can, in turn, lead to cascading effects on other components of the ecological community (Peckarsky et al., 2008; Ripple & Beschta, 2012). Characterizing these processes requires an integrative approach that can scale the responses of individual animals to demographic effects in the context of their energy balance and the species' life history (Middleton et al., 2013).
Because the responses of animals to many anthropogenic stimuli are similar to their responses to predation risk (Beale & Monaghan, 2004; Frid & Dill, 2002), the evaluation and management of human activities that have nonlethal effects on wildlife can be framed in this wider context. Assessing the population consequences of disturbance (PCoD) has been a long‐term goal for ecologists, decision makers, and managers and is currently a requirement for most environmental impact assessments under European Union (European Habitats Directive 92/43/EEC) and United States (Marine Mammal Protection Act, 16 U.S.C. §§ 1361 et seq.) legislation (King et al., 2015). However, comprehensive assessments of the effects of disturbance are rarely undertaken because of a lack of relevant data and because permit and policy decisions about disturbance must be made within strict timelines. In addition to these constraints, the theoretical understanding and the empirical and analytical methods needed to evaluate these long‐term consequences are often not available. As a result, management decisions have been generally based on evidence of behavioral responses to disturbance, although such responses may have no population‐level effect (Christiansen & Lusseau, 2015). Conversely, the absence of an obvious behavioral response does not rule out a population‐level effect (Gill, Norris, & Sutherland, 2001). Given the increasing expansion of activities that can disturb wildlife, quantitatively linking disturbance to population dynamics is a major objective for modern conservation (Gill et al., 2001). A mechanistic understanding of the processes by which disturbance affects populations is especially useful for long‐lived, wide‐ranging species, for which empirical data are often collected over relatively small spatial and temporal scales (National Research Council, 2005).
Groups established by the National Research Council of the US National Academies and the US Office of Naval Research have addressed ways of modeling the population‐level effects of disturbance on marine mammals (National Academies, 2017; National Research Council, 2005; New et al., 2014). Their efforts led to the development of a conceptual framework that summarizes the functional links among processes (Figure 1). The underlying concept is that disturbance‐induced changes in behavior or physiology affect fitness through individuals' health and vital rates (survival, reproductive success, and growth rate, the latter affecting age at first breeding). The population‐level consequences of changes in individual fitness depend on what proportion of the population is affected, which in turn determines the distribution of fitness‐related traits in the population. The framework provides a way to quantify four phenomena: (a) the physiological and behavioral changes that occur as the result of exposure to a particular stressor, (b) the acute effects of these physiological and behavioral responses on individual vital rates, and their chronic effects via individual health, defined by New et al. (2014) as all internal factors that affect fitness or homeostasis, (c) the way in which changes in health may affect individuals' vital rates, and (d) how changes in individual vital rates may affect population dynamics.
Figure 1.
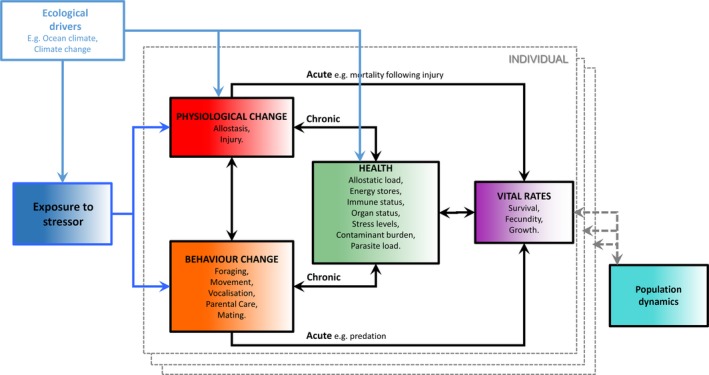
The Population Consequences of Disturbance (PCoD) conceptual framework, modified from National Academies (2017). The boxes within the dashed gray boundary line represent the effects of exposure to a stressor and a range of ecological drivers on the vital rates of an individual animal. The effects are then integrated across all individuals in the population to project their effects on the population's dynamics
Thirteen years after the first published formalization of this framework (National Research Council, 2005), we review its applications to marine mammal populations. We first discuss the assessment of the exposure levels of individuals in a population, and then review the approaches that have been used to model each of the functional links in the framework (Figure 2). Our synthesis will help reconcile the marine mammal literature with studies on other taxa that have quantified the effects of anthropogenic disturbance on vital rates and population dynamics (e.g., Broekhuis, 2018; Coetzee & Chown, 2016; Green, Johnson‐Ulrich, Couraud, & Holekamp, 2018; Kight & Swaddle, 2007; McClung, Seddon, Massaro, & Setiawan, 2004; Wood, Stillman, & Goss‐Custard, 2015). We also provide a decision tree that can guide the selection of the most applicable PCoD modeling approach, given the information available.
Figure 2.
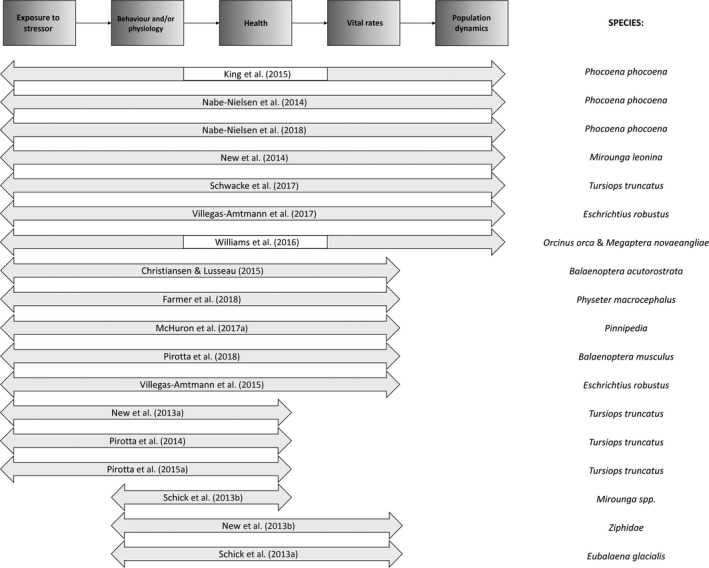
Studies investigating the Population Consequences of Disturbance (PCoD) on marine mammals, updated from Nowacek, Christiansen, Bejder, Goldbogen, and Friedlaender (2016). The arrows indicate the functional steps of the framework (simplified on top) that were included in each study. White gaps in the arrows indicate studies that evaluated the link between behavior and vital rates directly, without estimating health
2. ESTIMATING LEVELS OF EXPOSURE IN THE POPULATION
Estimating the population‐level consequences of individuals' responses to disturbance requires information on the proportion of the population that is exposed to the stressor, and the aggregate exposure of each individual (i.e., the total duration and intensity of exposure to the stressor during a given period). In this context, the stressor corresponds to an anthropogenic source of disturbance. The spatial and temporal overlap between the stressor and the focal animals determines the probability of exposure. This overlap is influenced by the patterns by which the stressor is produced at the source and propagates through the environment (Merchant, Faulkner, & Martinez, 2018) and the animals' residence time in the area where exposure may occur (Costa, Hückstädt, et al., 2016). Residence time is determined inter alia by the size of individual home ranges, the motivation underlying the use of the area of interest (e.g., whether the area contains foraging patches or is used solely for transit), and any migratory behavior. In some cases, these factors may result in the lack of any geographical or temporal overlap between a population and a stressor, obviating the need to assess population effects.
We believe that evaluating the spatial and temporal overlap between a population's range and the distribution of stressors on the basis of density maps derived from the results of dedicated or historical surveys (Ellison et al., 2016; Hammond et al., 2002) should be a routine component of environmental impact assessments. Telemetry data can provide information on the patterns of repeated exposure for specific individuals (Costa et al., 2003; Falcone et al., 2017; Jones et al., 2017; Madsen et al., 2006; Pirotta, New, & Marcoux, 2018), and photographic identification (e.g., Calambokidis, Barlow, Ford, Chandler, & Douglas, 2009) can be used to estimate exposure risks for regularly monitored populations (Christiansen, Bertulli, Rasmussen, & Lusseau, 2015; Pirotta, Thompson, Cheney, Donovan, & Lusseau, 2015). In alternative, some studies examined the consequences of exposing all individuals in a population to the same amount of disturbance (Braithwaite, Meeuwig, & Hipsey, 2015; New et al., 2014; Villegas‐Amtmann, Schwarz, Gailey, Sychenko, & Costa, 2017; Villegas‐Amtmann, Schwarz, Sumich, & Costa, 2015).
3. EFFECT OF EXPOSURE ON PHYSIOLOGY AND BEHAVIOR
The initial step in implementing the PCoD framework (Figure 1) is the quantification of the physiological and behavioral responses of individuals to a known or potential stressor. Controlled exposure experiments (Harris et al., 2018) have used electronic loggers to assess changes in the movement and vocalizations of marine mammals exposed to military sonar and air guns used for seismic surveys (Dunlop et al., 2013; Wensveen et al., 2017). Loggers have also been applied to monitor marine mammal responses to actual disturbance events; for example, of Cuvier's beaked whales (Ziphius cavirostris) to sonar exercises (Falcone et al., 2017) as well as of harbor seals (Phoca vitulina) to pile driving for wind farm construction (Russell et al., 2016) and to pedestrian and vessel approaches at their haul‐outs (Andersen, Teilmann, Dietz, Schmidt, & Miller, 2014). Visual observations have been used to quantify activity budgets and estimate changes in behaviors such as resting or foraging in the presence of other human activities, such as whale watching (e.g., Christiansen, Rasmussen, & Lusseau, 2013; Lusseau, 2003; New et al., 2015; Williams, Trites, & Bain, 2002). Visual studies on pinnipeds have also monitored flushing response and return to haul‐out sites (e.g., Cowling, Kirkwood, Boren, Sutherland, & Scarpaci, 2015; Osterrieder, Salgado Kent, & Robinson, 2017). Passive acoustic monitoring techniques offer a more continuous alternative to visual sampling for collecting such data on cetaceans. They have been used to assess the responses of harbor porpoises (Phocoena phocoena) to wind farm developments (Brandt, Diederichs, Betke, & Nehls, 2011; Nabe‐Nielsen, Sibly, Tougaard, Teilmann, & Sveegaard, 2014; Nabe‐Nielsen et al., 2018), of Blainville's beaked whales (Mesoplodon densirostris) to sonar (Moretti et al., 2014; Tyack et al., 2011), and of bottlenose dolphins (Tursiops truncatus) to boat presence (Pirotta, Merchant, Thompson, Barton, & Lusseau, 2015). In the absence of empirical data, behavioral responses have been extrapolated from better‐studied species or assumed, often in terms of the number of lost foraging days (King et al., 2015; New et al., 2014; Villegas‐Amtmann et al., 2015, 2017). Most studies (e.g., Williams, Lusseau, & Hammond, 2006) have evaluated the decrease in energy intake due to the observed behavioral responses. However, there have been efforts to quantify the change in energy expenditure associated with avoidance responses (Braithwaite et al., 2015; Christiansen, Rasmussen, & Lusseau, 2014; Miller et al., 2009; Williams, Blackwell, Richter, Sinding, & Heide‐Jørgensen, 2017; Williams, Kendall, et al., 2017). Measuring physiological responses to disturbance is more challenging than measuring behavioral responses, and may require the analysis of tissue, exhalations, or feces from wild animals (Hogg et al., 2009; Rolland et al., 2012), dedicated physiological tags (Karpovich, Skinner, Mondragon, & Blundell, 2015; Williams, Blackwell, et al., 2017; Wilson, Wikelski, Wilson, & Cooke, 2015), or experiments in captivity (Kvadsheim, Sevaldsen, Folkow, & Blix, 2010; Miksis et al., 2001; Thomas, Kastelein, & Awbrey, 1990). Due to these limitations, most applications of the PCoD framework have not modeled the physiological consequences of disturbance explicitly.
4. EFFECT OF BEHAVIORAL AND PHYSIOLOGICAL CHANGES ON HEALTH
Some behavioral or physiological changes can have acute effects on individuals' vital rates, for example, by changing their predation risk or because injury directly affects their survival probability (Hooker et al., 2012). However, such changes can also affect vital rates indirectly by impairing an individual's health. Modeling health explicitly provides the mechanistic link scaling individual responses to demographic effects that is required for the assessment of trait‐mediated indirect interactions (Middleton et al., 2013). Although an individual's health encompasses many aspects of its physiology (for example, immune status, stress levels, and contaminant and parasite load, Pettis et al., 2017), most PCoD applications have used an individual's energy stores (that is, its body condition) as the measure of health. For example, New et al. (2014) and Schick, New, et al. (2013) examined the relation between foraging activity and energy stores (estimated from changes in buoyancy) of female southern elephant seals (Mirounga leonina) over the course of a foraging trip. Other applications have inferred changes in energy stores from models of foraging activity that either treat energy explicitly using a bioenergetic approach (Beltran, Testa, & Burns, 2017; Christiansen & Lusseau, 2015; Farmer, Noren, Fougères, Machernis, & Baker, 2018; McHuron, Costa, Schwarz, & Mangel, 2017; McHuron, Mangel, Schwarz, & Costa, 2017; Noren, 2011; Pirotta, Mangel, et al., 2018; Villegas‐Amtmann et al., 2015, 2017) or use an arbitrarily scaled energy metric that represents an underlying motivational state (Nabe‐Nielsen et al., 2014, 2018; New, Harwood, et al., 2013; Pirotta, Harwood, et al., 2015; Pirotta, New, Harwood, & Lusseau, 2014). Although technologies that can measure the morphometrics of individuals remotely may make it easier to estimate changes in body condition directly (e.g., Christiansen, Dujon, Sprogis, Arnould, & Bejder, 2016; Miller, Best, Perryman, Baumgartner, & Moore, 2012), extensive health assessment in cetaceans will probably remain limited to a few closely monitored coastal populations, due to logistical constraints (Wells et al., 2004). In contrast, some pinniped populations can be regularly accessed to measure the variation in body condition and health among individuals (e.g., McDonald, Crocker, Burns, & Costa, 2008; McMahon, Harcourt, Burton, Daniel, & Hindell, 2017; Shero, Krotz, Costa, Avery, & Burns, 2015; Wheatley, Bradshaw, Davis, Harcourt, & Hindell, 2006). However, even when such assessments are possible, establishing the cause of observed changes in health is challenging.
5. EFFECT OF VARIATIONS IN HEALTH ON VITAL RATES
For most species, few empirical data are available to quantify the relation between an individual's health and its vital rates. New et al. (2014) and Costa, Schwarz, et al. (2016) used empirical data on the relation between a female elephant seal's energy stores at the start of lactation and the weaning mass of her pup, which affects the pup's survival probability (McMahon, Burton, & Bester, 2000, 2003), as the basis for the relation between health and reproductive success. Schick, Kraus, et al. (2013) and Rolland et al. (2016) used state‐space models linking the health of individual North Atlantic right whales (Eubalaena glacialis), obtained from the integration of multiple photographic assessments, to their survival and fertility. Schwacke et al. (2017) used a respiratory metric of health to quantify the effect of the Deepwater Horizon oil spill on vital rates of bottlenose dolphins in the Gulf of Mexico. In the absence of a direct estimate of calf survival, Christiansen and Lusseau (2015) used the fetal length of minke whales (Balaenoptera acutorostrata) as a proxy, and investigated how fetal length was associated with female body condition (Christiansen, Víkingsson, Rasmussen, & Lusseau, 2014). All other PCoD studies of marine mammals have assumed a simple relationship between various health metrics and vital rates (McHuron, Costa, et al., 2017; Nabe‐Nielsen et al., 2014, 2018; Pirotta, Mangel, et al., 2018; Villegas‐Amtmann et al., 2015, 2017).
6. A DIRECT LINK BETWEEN EXPOSURE AND VITAL RATES
Few monitoring programs collect information on the changes in individual health and vital rates that may result from behavioral responses to disturbance. In situations where a management decision is needed and this information is not available, a pragmatic alternative is to use a single function to link behavioral responses directly to vital rates. This has been referred to as an interim PCoD approach (King et al., 2015) because this function should be replaced with one based on empirically derived values as soon as they become available. In some of these cases, structured elicitation of information from multiple experts (known as expert elicitation) can provide both estimates of the appropriate parameters and a measure of the associated uncertainty (King et al., 2015; Martin et al., 2012; Oedekoven, Fleishman, Hamilton, Clark, & Schick, 2015). In alternative, the measured effect of changes in prey availability can be used as a proxy for the relation between energy intake and vital rates (Williams, Thomas, Ashe, Clark, & Hammond, 2016). The latter requires the assumption that a reduction in foraging time resulting from disturbance is equivalent to a reduction in the availability of prey.
7. MODELING THE EFFECT OF VITAL RATES ON POPULATION DYNAMICS
The final step in the PCoD conceptual model is the propagation of changes in individuals' vital rates to the population. It is beyond the scope of this study to review methods for modeling the dynamics of wildlife populations. Here, we describe the types of population models that have been used, or could be used, to estimate the population‐level effects of disturbance. They lie along a continuum from treating all animals in a population as identical to considering all animals as unique individuals that are followed from birth to death (i.e., individual‐ or agent‐based models [IBMs]).
Because traditional matrix models (Caswell, 2001) are formulated in discrete time, they generally have a birth‐pulse structure. In this structure, all births and deaths are assumed to occur at the same moment in time, which usually corresponds to the transition from one age class to the next, and all individuals within a class are treated as identical. However, classes may be subdivided to reflect the different vital rates of disturbed and undisturbed animals (King et al., 2015). Most PCoD applications have used a simple Leslie matrix to predict the trajectory of a population under different scenarios of anthropogenic disturbance (King et al., 2015; New et al., 2014; Schwacke et al., 2017). Matrix models historically assumed that vital rates are simply a function of an individual's age or stage, but integral projection models (IPMs) account for the additional effects of continuously varying traits (such as physical size) on vital rates (Ellner & Rees, 2006). In principle, a continuous measure of health or the amount of disturbance experienced by different individuals could be modeled as a fitness‐related trait (Coulson, 2012). However, because IPMs do not assign traits to specific individuals, individuals are still not consistently followed over time, and models are formulated in discrete time.
In reality, survival and reproduction are affected by an individual's physiological status and behavior in a complex manner (Houston & McNamara, 1999), and changes induced by disturbance can affect vital rates from the moment at which disturbance occurs. Continuous‐time life‐history models (De Roos, 2008) could therefore be more appropriate for estimating the population‐level effects of disturbance. Although continuous‐time life‐history models also assume individuals are identical, they can readily be structured into multiple classes (De Roos, Galic, & Heesterbeek, 2009).
IBMs follow simulated individuals throughout their life, allowing for explicit modeling of individual heterogeneity and environmental stochasticity in quasi‐continuous time (Grimm & Railsback, 2013). Some PCoD applications developed IBMs that simulate individuals moving, accessing prey, and accumulating energy stores to sustain survival and reproduction (New, Harwood, et al., 2013; Pirotta, Harwood, et al., 2015; Pirotta et al., 2014; Villegas‐Amtmann et al., 2015). However, only three studies (Nabe‐Nielsen et al., 2014, 2018; Villegas‐Amtmann et al., 2017) have used IBMs to predict the dynamics of a population over time. Although IBMs require considerable data, they are extremely flexible. In addition, simple models with sufficient realism can often be constructed on the basis of a relatively small amount of empirical information. Unknown parameters may, as an interim measure, be extrapolated from a species with a comparable life history (Sibly et al., 2013), as long as their influence on the model's outcome is explicitly quantified and acknowledged, for example, using sensitivity analysis. Model parameters then can be optimized with standard calibration techniques (Grimm & Railsback, 2013), or fitted to data with Bayesian inference (Kattwinkel & Reichert, 2017). IBMs can also be implemented via stochastic dynamic programming (Mangel & Clark, 1988) and used to estimate optimal behavior given estimates of state variables over time. The ability of this approach to forecast population‐level effects of disturbance was demonstrated for pinnipeds by McHuron, Costa, et al. (2017) and for Eastern North Pacific blue whales (Balaenoptera musculus) by Pirotta, Mangel, et al. (2018), whereas Klaassen, Bauer, Madsen, and Tombre (2006) used stochastic dynamic programming to quantify the effects of disturbance on the survival of Svalbard pink‐footed geese (Anser brachyrhynchus).
8. CHOOSING A MODEL STRUCTURE
In most situations, selection of a model structure to forecast the population‐level effects of disturbance is likely to be driven by data availability (Figure 3). No PCoD model to date has been fully parameterized with empirical data. Even models that encompass the chain from exposure to population dynamics (King et al., 2015; Nabe‐Nielsen et al., 2014, 2018; New et al., 2014; Villegas‐Amtmann et al., 2017; Williams et al., 2016) have used data extrapolated from other species, expert judgments, proxy relations, or informed assumptions.
Figure 3.
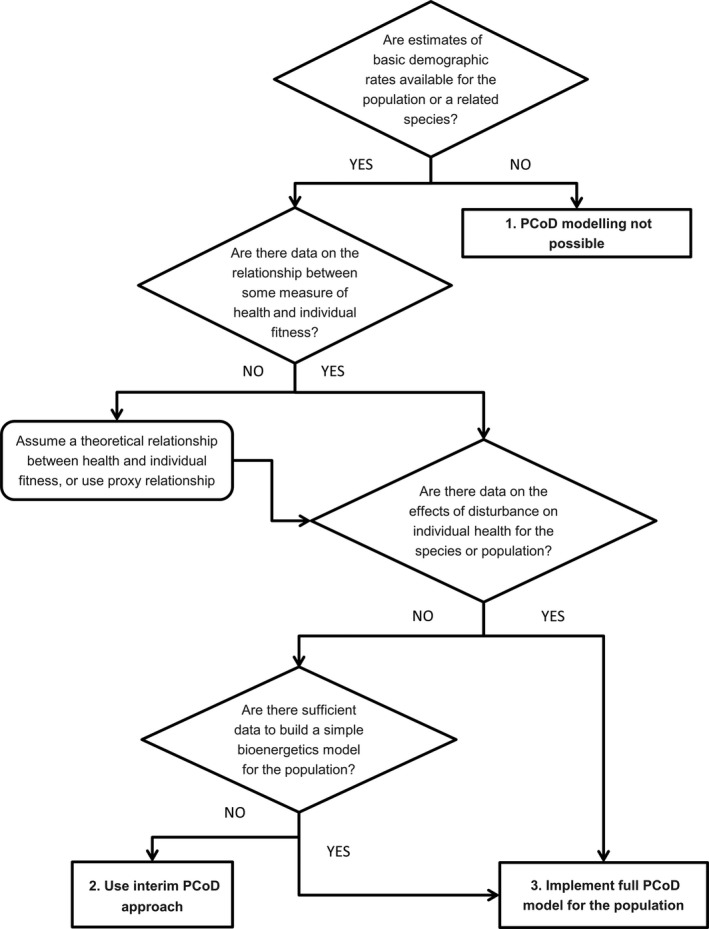
Decision tree to guide selection of the most suitable Population Consequences of Disturbance (PCoD) model for a given population, given data availability. Decision points are represented by diamonds, and possible outcomes by rectangles
The first step in choosing a model is to evaluate the availability of estimates of basic demographic parameters, such as vital rates, population growth rate, or age at first breeding or maturity. Precise estimates of these parameters are not essential, and missing values can be inferred if reliable estimates of other demographic attributes are available. If no demographic information is available for the target population or a related species, PCoD modeling is not possible (outcome 1 in Figure 3), but insights into a population's propensity for PCoD can be obtained from first principles using prospective approaches (Nattrass & Lusseau, 2016). Although some demographic parameters are emergent properties of bioenergetic models (see below), estimates of these parameters are needed to calibrate the models and validate their predictions.
The second step is to assess evidence of empirical relations between measures of individual health and fitness. For example, New et al. (2014) estimated a relation between pup survival and total body lipid of adult female elephant seals. If no empirical information is available, a simple, theoretical relation between an individual's health and its subsequent survival, fecundity, or growth can be assumed (New, Moretti, Hooker, Costa, & Simmons, 2013). Typically, such relations are hyperbolic (Nabe‐Nielsen et al., 2014) or sigmoidal (McHuron, Costa, et al., 2017).
The third step is to investigate whether there are empirical data on the relation between behavioral change and individual health. If such information is unavailable, bioenergetic models can be used to examine the potential effects of lost foraging opportunities on an individual's health, as measured by its energy stores (McHuron, Costa, et al., 2017; Nabe‐Nielsen et al., 2014, 2018; Pirotta, Mangel, et al., 2018; Villegas‐Amtmann et al., 2015, 2017). This allows construction of a full PCoD model for the population (outcome 3 in Figure 3), as in New et al. (2014). The basic data required to construct such bioenergetic models are duration of gestation and lactation, a growth curve to predict mass at different ages, and an estimate of field metabolic rate (Costa & Maresh, 2017; Villegas‐Amtmann et al., 2015). Ideally, these data should be collected from the target species or population, but they can be derived theoretically or from related species. The resulting models can be calibrated using information on the demography of the population, such as the ratio of calves to mature females. Expert elicitation can be used to fill some knowledge gaps, for example, to estimate the level of energy stores below which starvation mortality may occur, or to establish whether the rate of milk transfer is determined by the offspring or the mother.
If there is insufficient information to develop a bioenergetic model, expert elicitation can be used to estimate a direct relation between behavioral change and vital rates. This is represented by outcome 2 of the decision tree (Figure 3): the use of an interim PCoD approach (King et al., 2015).
9. INCORPORATING UNCERTAINTY
Whichever model is chosen, it is necessary to quantify uncertainty at all stages of modeling (Harwood & Stokes, 2003; Milner‐Gulland & Shea, 2017). Uncertainty arises through the precise choice of model parameterization, the specification of input parameter values, environmental stochasticity, and variation among individuals. Incorporation and propagation of these uncertainties vary among the modeling approaches described above. For example, in IBMs, it is possible to simulate from distributions on input parameters, and to include environmental stochasticity and variation among individuals in the simulation (Grimm & Railsback, 2013). To incorporate model uncertainty, simulations can sample from alternative parameterizations, although this rarely is done in practice. By contrast, life‐history models are intrinsically deterministic, although it is possible to repeat the modeling with different inputs. In all cases where uncertainty is not explicitly incorporated into the modeling, we suggest that a sensitivity analysis be performed post hoc to determine the robustness of conclusions to plausible violation of model assumptions and variation in the inputs. Uncertainty in the estimated population consequence ultimately can be reported as a distribution of potential outcomes. This will allow the precautionary principle to be applied if the results are used to make management decisions.
10. APPLICATIONS OF PCoD MODELS
Since its formulation (National Research Council, 2005), the PCoD conceptual model has served as a common framework for examining the potential effects of nonlethal human disturbance on marine mammal populations, accounting for the uncertainties associated with each step in the process. Use of this model has changed the focus of the scientific discussion from establishing subjective thresholds of acceptable behavioral change to quantifying long‐term, population‐level effects (National Academies, 2017).
Real‐world applications of the PCoD framework in the last decade used a range of modeling approaches to translate the conceptual model into a mathematical structure, and highlighted challenges and data gaps (Figure 2). The effects of disturbance on population size predicted in these studies were generally too small for short‐term detection with conventional methods of abundance estimation (Taylor, Martinez, Gerrodette, Barlow, & Hrovat, 2007). Yet these effects could have substantial medium‐term effects on population status.
To remain tractable, most PCoD models to date considered one disturbance source or scenario in isolation. However, multiple sources of disturbance are likely to occur in an area at any given time, together with other, concurrent environmental and ecological processes. Attributing causation to a single stressor and developing mitigation measures therefore is challenging in practice. Accordingly, the PCoD framework recently was expanded to incorporate the cumulative effects of multiple stressors and ecological drivers (National Academies, 2017).
Although models of the population consequences of anthropogenic disturbance have been developed to assess the effects of expanding human activities in the ocean on marine mammal populations, the PCoD framework is relevant for other marine and terrestrial taxa. The literature on the effects of human disturbance on wildlife behavior is extensive (e.g., Blumstein, Fernández‐Juricic, Zollner, & Garity, 2005; Stankowich, 2008). Moreover, many studies have linked changes in behavior deriving from interactions with humans to the survival and reproductive success of individuals (e.g., Broekhuis, 2018; Dussault, Pinard, Ouellet, Courtois, & Fortin, 2012; Ellenberg, Mattern, Seddon, & Jorquera, 2006; Giese, 1996; Gosselin, Zedrosser, Swenson, & Pelletier, 2014; Kerley et al., 2002; Kight & Swaddle, 2007; McClung et al., 2004; Rodriguez‐Prieto & Fernandez‐Juricic, 2005), and some have quantified the long‐term effects on population dynamics (e.g., Coetzee & Chown, 2016; Green et al., 2018; Iverson, Converse, Smith, & Valiulis, 2006; Wood et al., 2015). These studies could be incorporated into the unifying framework we describe here to model the effects of many forms of nonlethal anthropogenic disturbance.
There is interest in integrating proximate (mechanisms and functions) and ultimate (adaptation and fitness value) aspects of behavior into conservation (Cooke et al., 2014; Sutherland, 1998). Knowledge of the physiological mechanisms of an animal's interaction with its environment is also relevant to conservation (Wikelski & Cooke, 2006). The PCoD approach provides a means for investigating the physiological and the behavioral drivers of an individual's response to human disturbance, and therefore a population's viability (Cooke et al., 2014). Linking behavioral and physiological changes to demography also facilitates prediction of the effects of climate change on wildlife populations (e.g., Desprez, Jenouvrier, Barbraud, Delord, & Weimerskirch, 2018; Pagano et al., 2018; Weimerskirch, 2018). More generally, the nonlethal effects of human disturbance and environmental change and their repercussions at a population level can be viewed as examples of the fundamental processes regulating trait‐mediated, indirect ecological interactions (Ripple & Beschta, 2012; Werner & Peacor, 2003). Disentangling consumptive and nonconsumptive effects requires an understanding of the interactions among the individual, population, and community levels (Schmitz et al., 2004), and integrative approaches are necessary to link individual behavior, energy balance, and life history and to investigate demographic effects (Middleton et al., 2013). The PCoD approach therefore could offer a formal framework for the investigation of the proximate mechanisms of these and other ecological phenomena that operate via changes at the individual level. Experience already gained from application of PCoD models to marine mammal populations provides practical guidance for model development and data collection (Fleishman et al., 2016).
CONFLICT OF INTEREST
None declared.
AUTHORS' CONTRIBUTIONS
All authors contributed to discussions that supported the development of the framework and led to the present review of its applications. EP and JH led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
DATA ACCESSIBILITY
No data are associated with this manuscript.
ACKNOWLEDGMENTS
This review was supported by Office of Naval Research grant N00014‐16‐1‐2858: “PCoD+: Developing widely‐applicable models of the population consequences of disturbance.” The work benefited from discussions with participants in a working group supported by Office of Naval Research (ONR) grants N00014‐09‐1‐0896 to the University of California, Santa Barbara and N00014‐12‐1‐0274 to the University of California, Davis. It also benefited from discussions and analyses funded by the E&P Sound and Marine Life Joint Industry Project of the International Association of Oil and Gas Producers. PLT and DL acknowledge support from the MASTS pooling initiative (Marine Alliance for Science and Technology for Scotland; supported by the Scottish Funding Council, grant reference HR09011, and contributing institutions) and PLT acknowledges support from ONR grant N00014‐15‐1‐2553. We thank two anonymous reviewers for their comments on the manuscript.
Pirotta E, Booth CG, Costa DP, et al. Understanding the population consequences of disturbance. Ecol Evol. 2018;8:9934–9946. 10.1002/ece3.4458
Funding information
This review was supported by Office of Naval Research grant N00014‐16‐1‐2858: “PCoD+: Developing widely‐applicable models of the population consequences of disturbance.” The work benefited from discussions with participants in a working group supported by Office of Naval Research (ONR) grants N00014‐09‐1‐0896 to the University of California, Santa Barbara and N00014‐12‐1‐0274 to the University of California, Davis. It also benefited from discussions and analyses funded by the E&P Sound and Marine Life Joint Industry Project of the International Association of Oil and Gas Producers. PLT and DL acknowledge support from the MASTS pooling initiative (Marine Alliance for Science and Technology for Scotland; supported by the Scottish Funding Council, grant reference HR09011, and contributing institutions) and PLT acknowledges support from ONR grant N00014‐15‐1‐2553.
REFERENCES
- Andersen, S. M. , Teilmann, J. , Dietz, R. , Schmidt, N. M. , & Miller, L. A. (2014). Disturbance‐induced responses of VHF and satellite tagged harbour seals. Aquatic Conservation: Marine and Freshwater Ecosystems, 24, 712–723. 10.1002/aqc.2393 [DOI] [Google Scholar]
- Beale, C. M. , & Monaghan, P. (2004). Human disturbance: People as predation‐free predators? Journal of Applied Ecology, 41, 335–343. 10.1111/j.0021-8901.2004.00900.x [DOI] [Google Scholar]
- Beltran, R. S. , Testa, J. W. , & Burns, J. M. (2017). An agent‐based bioenergetics model for predicting impacts of environmental change on a top marine predator, the Weddell seal. Ecological Modelling, 351, 36–50. 10.1016/j.ecolmodel.2017.02.002 [DOI] [Google Scholar]
- Blumstein, D. T. , Fernández‐Juricic, E. , Zollner, P. A. , & Garity, S. C. (2005). Inter‐specific variation in avian responses to human disturbance. Journal of Applied Ecology, 42, 943–953. 10.1111/j.1365-2664.2005.01071.x [DOI] [Google Scholar]
- Braithwaite, J. E. , Meeuwig, J. J. , & Hipsey, M. R. (2015). Optimal migration energetics of humpback whales and the implications of disturbance. Conservation Physiology, 3, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, M. , Diederichs, A. , Betke, K. , & Nehls, G. (2011). Responses of harbour porpoises to pile driving at the Horns Rev II offshore wind farm in the Danish North Sea. Marine Ecology Progress Series, 421, 205–216. 10.3354/meps08888 [DOI] [Google Scholar]
- Broekhuis, F. (2018). Natural and anthropogenic drivers of cub recruitment in a large carnivore. Ecology and Evolution, 8, 6748–6755. 10.1002/ece3.4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calambokidis, J. , Barlow, J. , Ford, J. K. B. , Chandler, T. E. , & Douglas, A. B. (2009). Insights into the population structure of blue whales in the Eastern North Pacific from recent sightings and photographic identification. Marine Mammal Science, 25, 816–832. 10.1111/j.1748-7692.2009.00298.x [DOI] [Google Scholar]
- Caswell, H. (2001). Matrix population models. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Christiansen, F. , Bertulli, C. G. , Rasmussen, M. H. , & Lusseau, D. (2015). Estimating cumulative exposure of wildlife to non‐lethal disturbance using spatially explicit capture–recapture models. The Journal of Wildlife Management, 79, 311–324. 10.1002/jwmg.836 [DOI] [Google Scholar]
- Christiansen, F. , Dujon, A. M. , Sprogis, K. R. , Arnould, J. P. Y. , & Bejder, L. (2016). Non‐invasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere, 7, e01468 10.1002/ecs2.1468 [DOI] [Google Scholar]
- Christiansen, F. , & Lusseau, D. (2015). Linking behaviour to vital rates to measure the effects of non‐lethal disturbance on wildlife. Conservation Letters, 8, 424–431. 10.1111/conl.12166 [DOI] [Google Scholar]
- Christiansen, F. , Rasmussen, M. , & Lusseau, D. (2013). Whale watching disrupts feeding activities of minke whales on a feeding ground. Marine Ecology Progress Series, 478, 239–251. 10.3354/meps10163 [DOI] [Google Scholar]
- Christiansen, F. , Rasmussen, M. H. , & Lusseau, D. (2014). Inferring energy expenditure from respiration rates in minke whales to measure the effects of whale watching boat interactions. Journal of Experimental Marine Biology and Ecology, 459, 96–104. 10.1016/j.jembe.2014.05.014 [DOI] [Google Scholar]
- Christiansen, F. , Víkingsson, G. A. , Rasmussen, M. H. , & Lusseau, D. (2014). Female body condition affects foetal growth in a capital breeding mysticete. Functional Ecology, 28, 579–588. 10.1111/1365-2435.12200 [DOI] [Google Scholar]
- Coetzee, B. W. T. , & Chown, S. L. (2016). A meta‐analysis of human disturbance impacts on Antarctic wildlife. Biological Reviews, 91, 578–596. 10.1111/brv.12184 [DOI] [PubMed] [Google Scholar]
- Cooke, S. J. , Blumstein, D. T. , Buchholz, R. , Caro, T. , Fernández‐Juricic, E. , Franklin, C. E. , … Wikelski, M. (2014). Physiology, behavior, and conservation. Physiological and Biochemical Zoology, 87, 1–14. 10.1086/671165 [DOI] [PubMed] [Google Scholar]
- Costa, D. P. , Crocker, D. E. , Gedamke, J. , Webb, P. M. , Houser, D. S. , Blackwell, S. B. , … Le Boeuf, B. J. (2003). The effect of a low‐frequency sound source (acoustic thermometry of the ocean climate) on the diving behavior of juvenile northern elephant seals, Mirounga angustirostris . Journal of the Acoustical Society of America, 113, 1155–1165. 10.1121/1.1538248 [DOI] [PubMed] [Google Scholar]
- Costa, D. P. , Hückstädt, L. A. , Schwarz, L. K. , Friedlaender, A. S. , Mate, B. R. , Zerbini, A. N. , … Gales, N. J. (2016). Assessing the exposure of animals to acoustic disturbance: Towards an understanding of the population consequences of disturbance. Proceedings of Meetings on Acoustics, 27, 010027 10.1121/2.0000298 [DOI] [Google Scholar]
- Costa, D. P. , & Maresh, J. L. (2017). Energetics In Würsig B., Thewissen J. G. M., & Kovacs K. (Eds.), Encyclopedia of marine mammals (pp. 329–335). San Diego, CA: Academic Press. [Google Scholar]
- Costa, D. P. , Schwarz, L. , Robinson, P. , Schick, R. S. , Morris, P. A. , Condit, R. , … Kilpatrick, A. M. (2016). A bioenergetics approach to understanding the population consequences of disturbance: Elephant seals as a model system In Popper A. N. & Hawkins A. (Eds.), Effects of noise on aquatic life II (pp. 161–169). New York, NY: Springer Science+Business Media; 10.1007/978-1-4939-2981-8 [DOI] [PubMed] [Google Scholar]
- Coulson, T. (2012). Integral projections models, their construction and use in posing hypotheses in ecology. Oikos, 121, 1337–1350. 10.1111/j.1600-0706.2012.00035.x [DOI] [Google Scholar]
- Cowling, M. , Kirkwood, R. , Boren, L. , Sutherland, D. , & Scarpaci, C. (2015). The effects of vessel approaches on the New Zealand fur seal (Arctocephalus forsteri) in the bay of plenty, New Zealand. Marine Mammal Science, 31, 501–519. 10.1111/mms.12171 [DOI] [Google Scholar]
- De Roos, A. M. (2008). Demographic analysis of continuous‐time life‐history models. Ecology Letters, 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roos, A. M. , Galic, N. , & Heesterbeek, H. (2009). How resource competition shapes individual life history for nonplastic growth: Ungulates in seasonal food environments. Ecology, 90, 945–960. 10.1890/07-1153.1 [DOI] [PubMed] [Google Scholar]
- Desprez, M. , Jenouvrier, S. , Barbraud, C. , Delord, K. , & Weimerskirch, H. (2018). Linking oceanographic conditions, migratory schedules and foraging behaviour during the non‐breeding season to reproductive performance in a long‐lived seabird. Functional Ecology, 32, 2040–2053. 10.1111/1365-2435.13117 [DOI] [Google Scholar]
- Dunlop, R. A. , Noad, M. J. , Cato, D. H. , Kniest, E. , Miller, P. J. O. , Smith, J. N. , & Stokes, M. D. (2013). Multivariate analysis of behavioural response experiments in humpback whales (Megaptera novaeangliae). Journal of Experimental Biology, 216, 759–770. 10.1242/jeb.071498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussault, C. , Pinard, V. , Ouellet, J.‐P. , Courtois, R. , & Fortin, D. (2012). Avoidance of roads and selection for recent cutovers by threatened caribou: Fitness‐rewarding or maladaptive behaviour? Proceedings of the Royal Society of London. Series B: Biological Sciences, 279, 4481–4488. 10.1098/rspb.2012.1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberg, U. , Mattern, T. , Seddon, P. J. , & Jorquera, G. L. (2006). Physiological and reproductive consequences of human disturbance in Humboldt penguins: The need for species‐specific visitor management. Biological Conservation, 133, 95–106. 10.1016/j.biocon.2006.05.019 [DOI] [Google Scholar]
- Ellison, W. T. , Racca, R. , Clark, C. W. , Streever, B. , Frankel, A. S. , Fleishman, E. , … Thomas, L. (2016). Modeling the aggregated exposure and responses of bowhead whales Balaena mysticetus to multiple sources of anthropogenic underwater sound. Endangered Species Research, 30, 95–108. 10.3354/esr00727 [DOI] [Google Scholar]
- Ellner, S. P. , & Rees, M. (2006). Integral projection models for species with complex demography. The American Naturalist, 167, 410–428. 10.1086/499438 [DOI] [PubMed] [Google Scholar]
- Falcone, E. A. , Schorr, G. S. , Watwood, S. L. , Deruiter, S. L. , Zerbini, A. N. , Andrews, R. D. , … Moretti, D. J. (2017). Diving behaviour of Cuvier's beaked whales exposed to two types of military sonar. Royal Society Open Science, 4, 170629 10.1098/rsos.170629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, N. A. , Noren, D. P. , Fougères, E. M. , Machernis, A. , & Baker, K. (2018). Resilience of the endangered sperm whale Physeter macrocephalus to foraging disturbance in the Gulf of Mexico, USA: A bioenergetic approach. Marine Ecology Progress Series, 589, 241–261. 10.3354/meps12457 [DOI] [Google Scholar]
- Fleishman, E. , Costa, D. P. , Harwood, J. , Kraus, S. , Moretti, D. , New, L. F. , … Wells, R. S. (2016). Monitoring population‐level responses of marine mammals to human activities. Marine Mammal Science, 32, 1004–1021. 10.1111/mms.12310 [DOI] [Google Scholar]
- Frid, A. , & Dill, L. (2002). Human‐caused disturbance stimuli as a form of predation risk. Conservation Ecology, 6, 11 10.5751/ES-00404-060111 [DOI] [Google Scholar]
- Giese, M. (1996). Effects of human activity on adelie penguin Pygoscelis adeliae breeding success. Biological Conservation, 75, 157–164. 10.1016/0006-3207(95)00060-7 [DOI] [Google Scholar]
- Gill, J. A. , Norris, K. , & Sutherland, W. J. (2001). Why behavioural responses may not reflect the population consequences of human disturbance. Biological Conservation, 97, 265–268. 10.1016/S0006-3207(00)00002-1 [DOI] [Google Scholar]
- Gosselin, J. , Zedrosser, A. , Swenson, J. E. , & Pelletier, F. (2014). The relative importance of direct and indirect effects of hunting mortality on the population dynamics of brown bears. Proceedings of the Royal Society of London. Series B: Biological Sciences, 282, 20141840 10.1098/rspb.2014.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D. S. , Johnson‐Ulrich, L. , Couraud, H. E. , & Holekamp, K. E. (2018). Anthropogenic disturbance induces opposing population trends in spotted hyenas and African lions. Biodiversity and Conservation, 27, 871–889. 10.1007/s10531-017-1469-7 [DOI] [Google Scholar]
- Grimm, V. , & Railsback, S. F. (2013). Individual‐based modeling and ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- Hammond, P. S. , Berggren, P. , Benke, H. , Borchers, D. L. , Collet, A. , Heide‐Jørgensen, M. P. , … Øien, N. (2002). Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. Journal of Applied Ecology, 39, 361–376. 10.1046/j.1365-2664.2002.00713.x [DOI] [Google Scholar]
- Harris, C. M. , Thomas, L. , Falcone, E. A. , Hildebrand, J. , Houser, D. , Kvadsheim, P. H. , … Janik, V. M. (2018). Marine mammals and sonar: Dose‐response studies, the risk‐disturbance hypothesis and the role of exposure context. Journal of Applied Ecology, 55, 396–404. 10.1111/1365-2664.12955 [DOI] [Google Scholar]
- Harwood, J. , & Stokes, K. (2003). Coping with uncertainty in ecological advice: Lessons from fisheries. Trends in Ecology & Evolution, 18, 617–622. 10.1016/j.tree.2003.08.001 [DOI] [Google Scholar]
- Hogg, C. J. , Rogers, T. L. , Shorter, A. , Barton, K. , Miller, P. J. O. , & Nowacek, D. (2009). Determination of steroid hormones in whale blow: It is possible. Marine Mammal Science, 25, 605–618. 10.1111/j.1748-7692.2008.00277.x [DOI] [Google Scholar]
- Hooker, S. K. , Fahlman, A. , Moore, M. J. , Aguilar de Soto, N. , Bernaldo de Quiros, Y. , Brubakk, A. O. , … Tyack, P. L. (2012). Deadly diving? Physiological and behavioural management of decompression stress in diving mammals. Proceedings of the Royal Society B: Biological Sciences, 279, 1041–1050. 10.1098/rspb.2011.2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston, A. I. , & McNamara, J. M. (1999). Models of adaptive behavior: An approach based on state. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Iverson, J. B. , Converse, S. J. , Smith, G. R. , & Valiulis, J. M. (2006). Long‐term trends in the demography of the Allen Cays Rock Iguana (Cyclura cychlura inornata): Human disturbance and density‐dependent effects. Biological Conservation, 132, 300–310. 10.1016/j.biocon.2006.04.022 [DOI] [Google Scholar]
- Jones, E. L. , Hastie, G. D. , Smout, S. , Onoufriou, J. , Merchant, N. D. , Brookes, K. L. , & Thompson, D. (2017). Seals and shipping: Quantifying population risk and individual exposure to vessel noise. Journal of Applied Ecology, 54, 1930–1940. 10.1111/1365-2664.12911 [DOI] [Google Scholar]
- Karpovich, S. A. , Skinner, J. P. , Mondragon, J. E. , & Blundell, G. M. (2015). Combined physiological and behavioral observations to assess the influence of vessel encounters on harbor seals in glacial fjords of southeast Alaska. Journal of Experimental Marine Biology and Ecology, 473, 110–120. 10.1016/j.jembe.2015.07.016 [DOI] [Google Scholar]
- Kattwinkel, M. , & Reichert, P. (2017). Bayesian parameter inference for individual‐based models using a Particle Markov Chain Monte Carlo method. Environmental Modelling and Software, 87, 110–119. 10.1016/j.envsoft.2016.11.001 [DOI] [Google Scholar]
- Kerley, L. L. , Goodrich, J. M. , Miquelle, D. G. , Smirnov, E. N. , Quigley, H. B. , & Hornocker, M. G. (2002). Effects of roads and human disturbance on Amur tigers. Conservation Biology, 16, 97–108. 10.1046/j.1523-1739.2002.99290.x [DOI] [PubMed] [Google Scholar]
- Kight, C. R. , & Swaddle, J. P. (2007). Associations of anthropogenic activity and disturbance with fitness metrics of eastern bluebirds (Sialia sialis). Biological Conservation, 138, 189–197. 10.1016/j.biocon.2007.04.014 [DOI] [Google Scholar]
- King, S. L. , Schick, R. S. , Donovan, C. , Booth, C. G. , Burgman, M. , Thomas, L. , & Harwood, J. (2015). An interim framework for assessing the population consequences of disturbance. Methods in Ecology and Evolution, 6, 1150–1158. 10.1111/2041-210X.12411 [DOI] [Google Scholar]
- Klaassen, M. , Bauer, S. , Madsen, J. , & Tombre, I. (2006). Modelling behavioural and fitness consequences of disturbance for geese along their spring flyway. Journal of Applied Ecology, 43, 92–100. [Google Scholar]
- Kvadsheim, P. H. , Sevaldsen, E. M. , Folkow, L. P. , & Blix, A. S. (2010). Behavioural and physiological responses of hooded seals (Cystophora cristata) to 1 to 7 kHz sonar signals. Aquatic Mammals, 36, 239–247. 10.1578/AM.36.3.2010.239 [DOI] [Google Scholar]
- Lima, S. (1998). Nonlethal effects in the ecology of predator–prey interactions. BioScience, 48, 25–34. 10.2307/1313225 [DOI] [Google Scholar]
- Lusseau, D. (2003). Effects of tour boats on the behavior of bottlenose dolphins: Using Markov chains to model anthropogenic impacts. Conservation Biology, 17, 1785–1793. 10.1111/j.1523-1739.2003.00054.x [DOI] [Google Scholar]
- Madsen, P. T. , Johnson, M. , Miller, P. J. O. , Aguilar Soto, N. , Lynch, J. , & Tyack, P. (2006). Quantitative measures of air‐gun pulses recorded on sperm whales (Physeter macrocephalus) using acoustic tags during controlled exposure experiments. The Journal of the Acoustical Society of America, 120, 2366–2379. 10.1121/1.2229287 [DOI] [PubMed] [Google Scholar]
- Mangel, M. , & Clark, C. W. (1988). Dynamic modeling in behavioral ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- Martin, T. G. , Burgman, M. A. , Fidler, F. , Kuhnert, P. M. , Low‐Choy, S. , McBride, M. , & Mengersen, K. (2012). Eliciting expert knowledge in conservation science. Conservation Biology, 26, 29–38. 10.1111/j.1523-1739.2011.01806.x [DOI] [PubMed] [Google Scholar]
- McClung, M. R. , Seddon, P. J. , Massaro, M. , & Setiawan, A. N. (2004). Nature‐based tourism impacts on yellow‐eyed penguins Megadyptes antipodes: Does unregulated visitor access affect fledging weight and juvenile survival? Biological Conservation, 119, 279–285. 10.1016/j.biocon.2003.11.012 [DOI] [Google Scholar]
- McDonald, B. I. , Crocker, D. E. , Burns, J. M. , & Costa, D. P. (2008). Body condition as an index of winter foraging success in crabeater seals (Lobodon carcinophaga). Deep‐Sea Research Part II: Topical Studies in Oceanography, 55, 515–522. 10.1016/j.dsr2.2007.11.002 [DOI] [Google Scholar]
- McHuron, E. , Costa, D. , Schwarz, L. , & Mangel, M. (2017). A behavioral framework for assessing the population consequences of anthropogenic disturbance on pinnipeds. Methods in Ecology and Evolution, 8, 552–560. 10.1111/2041-210X.12701 [DOI] [Google Scholar]
- McHuron, E. A. , Mangel, M. , Schwarz, L. K. , & Costa, D. P. (2017). Energy and prey requirements of California sea lions under variable environmental conditions. Marine Ecology Progress Series, 567, 235–247. 10.3354/meps12041 [DOI] [Google Scholar]
- McMahon, C. R. , Burton, H. R. , & Bester, M. N. (2000). Weaning mass and the future survival of juvenile southern elephant seals, Mirounga leonina, at Macquarie Island. Antarctic Science, 12, 149–153. [Google Scholar]
- McMahon, C. R. , Burton, H. R. , & Bester, M. N. (2003). A demographic comparison of two southern elephant seal populations. Journal of Animal Ecology, 72, 61–74. 10.1046/j.1365-2656.2003.00685.x [DOI] [Google Scholar]
- McMahon, C. R. , Harcourt, R. G. , Burton, H. R. , Daniel, O. , & Hindell, M. A. (2017). Seal mothers expend more on offspring under favourable conditions and less when resources are limited. Journal of Animal Ecology, 86, 359–370. 10.1111/1365-2656.12611 [DOI] [PubMed] [Google Scholar]
- Merchant, N. D. , Faulkner, R. C. , & Martinez, R. (2018). Marine noise budgets in practice. Conservation Letters, 11, e12420 [Google Scholar]
- Middleton, A. D. , Kauffman, M. J. , Mcwhirter, D. E. , Jimenez, M. D. , Cook, R. C. , Cook, J. G. , … White, P. J. (2013). Linking anti‐predator behaviour to prey demography reveals limited risk effects of an actively hunting large carnivore. Ecology Letters, 16, 1023–1030. 10.1111/ele.12133 [DOI] [PubMed] [Google Scholar]
- Miksis, J. , Grund, M. , Nowacek, D. , Solow, A. , Connor, R. , & Tyack, P. (2001). Cardiac responses to acoustic playback experiments in the captive bottlenose dolphin (Tursiops truncatus). Journal of Comparative Phsychology, 115, 227–232. 10.1037/0735-7036.115.3.227 [DOI] [PubMed] [Google Scholar]
- Miller, C. , Best, P. , Perryman, W. , Baumgartner, M. , & Moore, M. (2012). Body shape changes associated with reproductive status, nutritive condition and growth in right whales Eubalaena glacialis and E. australis . Marine Ecology Progress Series, 459, 135–156. 10.3354/meps09675 [DOI] [Google Scholar]
- Miller, P. J. O. , Johnson, M. P. , Madsen, P. T. , Biassoni, N. , Quero, M. , & Tyack, P. L. (2009). Using at‐sea experiments to study the effects of airguns on the foraging behavior of sperm whales in the Gulf of Mexico. Deep‐Sea Research Part I: Oceanographic Research Papers, 56, 1168–1181. 10.1016/j.dsr.2009.02.008 [DOI] [Google Scholar]
- Milner‐Gulland, E. J. , & Shea, K. (2017). Embracing uncertainty in applied ecology. Journal of Applied Ecology, 54, 2063–2068. 10.1111/1365-2664.12887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti, D. , Thomas, L. , Marques, T. , Harwood, J. , Dilley, A. , Neales, B. , … Morrissey, R. (2014). A risk function for behavioral disruption of Blainville's beaked whales (Mesoplodon densirostris) from mid‐frequency active sonar. PLoS ONE, 9, e85064 10.1371/journal.pone.0085064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabe‐Nielsen, J. , Sibly, R. M. , Tougaard, J. , Teilmann, J. , & Sveegaard, S. (2014). Effects of noise and by‐catch on a Danish harbour porpoise population. Ecological Modelling, 272, 242–251. 10.1016/j.ecolmodel.2013.09.025 [DOI] [Google Scholar]
- Nabe‐Nielsen, J. , van Beest, F. M. , Grimm, V. , Sibly, R. , Teilmann, J. , & Thompson, P. M. (2018). Predicting the impacts of anthropogenic disturbances on marine populations. Conservation Letters. [Google Scholar]
- National Academies (2017). Approaches to understanding the cumulative effects of stressors on marine mammals. Washington, DC: The National Academies Press. [Google Scholar]
- National Research Council (2005). Marine mammal populations and ocean noise: Determining when noise causes biologically significant effects. Washington, DC: The National Academies Press. [Google Scholar]
- Nattrass, S. , & Lusseau, D. (2016). Using resilience to predict the effects of disturbance. Scientific Reports, 6, 25539 10.1038/srep25539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- New, L. F. , Clark, J. S. , Costa, D. P. , Fleishman, E. , Hindell, M. A. , Klanjšček, T. , … Harwood, J. (2014). Using short‐term measures of behaviour to estimate long‐term fitness of southern elephant seals. Marine Ecology Progress Series, 496, 99–108. 10.3354/meps10547 [DOI] [Google Scholar]
- New, L. F. , Hall, A. J. , Harcourt, R. , Kaufman, G. , Parsons, E. C. M. , Pearson, H. C. , … Schick, R. S. (2015). The modelling and assessment of whale‐watching impacts. Ocean and Coastal Management, 115, 10–16. 10.1016/j.ocecoaman.2015.04.006 [DOI] [Google Scholar]
- New, L. F. , Harwood, J. , Thomas, L. , Donovan, C. , Clark, J. S. , Hastie, G. , … Lusseau, D. (2013). Modeling the biological significance of behavioral change in coastal bottlenose dolphins in response to disturbance. Functional Ecology, 27, 314–322. 10.1111/1365-2435.12052 [DOI] [Google Scholar]
- New, L. F. , Moretti, D. J. , Hooker, S. K. , Costa, D. P. , & Simmons, S. E. (2013). Using energetic models to investigate the survival and reproduction of beaked whales (family Ziphiidae). PLoS ONE, 8, e68725 10.1371/journal.pone.0068725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, D. P. (2011). Estimated field metabolic rates and prey requirements of resident killer whales. Marine Mammal Science, 27, 60–77. 10.1111/j.1748-7692.2010.00386.x [DOI] [Google Scholar]
- Nowacek, D. P. , Christiansen, F. , Bejder, L. , Goldbogen, J. A. , & Friedlaender, A. S. (2016). Studying cetacean behaviour: New technological approaches and conservation applications. Animal Behaviour, 120, 235–244. 10.1016/j.anbehav.2016.07.019 [DOI] [Google Scholar]
- Oedekoven, C. , Fleishman, E. , Hamilton, P. , Clark, J. , & Schick, R. (2015). Expert elicitation of seasonal abundance of North Atlantic right whales Eubalaena glacialis in the mid‐Atlantic. Endangered Species Research, 29, 51–58. 10.3354/esr00699 [DOI] [Google Scholar]
- Osterrieder, S. K. , Salgado Kent, C. , & Robinson, R. W. (2017). Responses of Australian sea lions, Neophoca cinerea, to anthropogenic activities in the Perth metropolitan area, Western Australia. Aquatic Conservation: Marine and Freshwater Ecosystems, 27, 414–435. 10.1002/aqc.2668 [DOI] [Google Scholar]
- Pagano, A. M. , Durner, G. M. , Rode, K. D. , Atwood, T. C. , Atkinson, S. N. , Peacock, E. , … Williams, T. M. (2018). High‐energy, high‐fat lifestyle challenges an Arctic apex predator, the polar bear. Science, 359, 568–572. 10.1126/science.aan8677 [DOI] [PubMed] [Google Scholar]
- Peckarsky, B. L. , Abrams, P. A. , Bolnick, D. I. , Dill, L. M. , Grabowski, J. H. , Luttbeg, B. , … Trussell, G. C. (2008). Revisiting the classics: Considering nonconsumptive effects in textbook examples of predator–prey interactions. Ecology, 89, 2416–2425. 10.1890/07-1131.1 [DOI] [PubMed] [Google Scholar]
- Pettis, H. M. , Rolland, R. M. , Hamilton, P. K. , Knowlton, A. R. , Burgess, E. A. , & Kraus, S. D. (2017). Body condition changes arising from natural factors and fishing gear entanglements in North Atlantic right whales Eubalaena glacialis . Endangered Species Research, 32, 237–249. 10.3354/esr00800 [DOI] [Google Scholar]
- Pirotta, E. , Harwood, J. , Thompson, P. M. , New, L. , Cheney, B. , Arso, M. , … Lusseau, D. (2015). Predicting the effects of human developments on individual dolphins to understand potential long‐term population consequences. Proceedings of the Royal Society B: Biological Sciences, 282, 20152109 10.1098/rspb.2015.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotta, E. , Mangel, M. , Costa, D. P. , Mate, B. , Goldbogen, J. , Palacios, D. M. , … New, L. (2018). A dynamic state model of migratory behavior and physiology to assess the consequences of environmental variation and anthropogenic disturbance on marine vertebrates. The American Naturalist, 191, E40–E56. 10.1086/695135 [DOI] [PubMed] [Google Scholar]
- Pirotta, E. , Merchant, N. D. , Thompson, P. M. , Barton, T. R. , & Lusseau, D. (2015). Quantifying the effect of boat disturbance on bottlenose dolphin foraging activity. Biological Conservation, 181, 82–89. 10.1016/j.biocon.2014.11.003 [DOI] [Google Scholar]
- Pirotta, E. , New, L. , Harwood, J. , & Lusseau, D. (2014). Activities, motivations and disturbance: An agent‐based model of bottlenose dolphin behavioral dynamics and interactions with tourism in Doubtful Sound, New Zealand. Ecological Modelling, 282, 44–58. 10.1016/j.ecolmodel.2014.03.009 [DOI] [Google Scholar]
- Pirotta, E. , New, L. , & Marcoux, M. (2018). Modelling beluga habitat use and baseline exposure to shipping traffic to design effective protection against prospective industrialization in the Canadian Arctic. Aquatic Conservation: Marine and Freshwater Ecosystems, 28, 713–722. 10.1002/aqc.2892 [DOI] [Google Scholar]
- Pirotta, E. , Thompson, P. M. , Cheney, B. , Donovan, C. R. , & Lusseau, D. (2015). Estimating spatial, temporal and individual variability in dolphin cumulative exposure to boat traffic using spatially explicit capture–recapture methods. Animal Conservation, 18, 20–31. 10.1111/acv.12132 [DOI] [Google Scholar]
- Ripple, W. J. , & Beschta, R. L. (2012). Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biological Conservation, 145, 205–213. 10.1016/j.biocon.2011.11.005 [DOI] [Google Scholar]
- Rodriguez‐Prieto, I. , & Fernandez‐Juricic, E. (2005). Effects of direct human disturbance on the endemic Iberian frog at individual and population levels. Biological Conservation, 123, 1–9. 10.1016/j.biocon.2004.10.003 [DOI] [Google Scholar]
- Rolland, R. M. , Parks, S. E. , Hunt, K. E. , Castellote, M. , Corkeron, P. J. , Nowacek, D. P. , … Kraus, S. D. (2012). Evidence that ship noise increases stress in right whales. Proceedings of the Royal Society B: Biological Sciences, 279, 2363–2368. 10.1098/rspb.2011.2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland, R. M. , Schick, R. S. , Pettis, H. M. , Knowlton, A. R. , Hamilton, P. K. , Clark, J. S. , & Kraus, S. D. (2016). Health of North Atlantic right whales, Eubalaena glacialis, over three decades: From individual health to demographic and population health trends. Marine Ecology Progress Series, 542, 265–282. 10.3354/meps11547 [DOI] [Google Scholar]
- Russell, D. J. F. , Hastie, G. D. , Thompson, D. , Janik, V. M. , Hammond, P. S. , Scott‐Hayward, L. A. S. , … Votier, S. (2016). Avoidance of wind farms by harbour seals is limited to pile driving activities. Journal of Applied Ecology, 53, 1642–1652. 10.1111/1365-2664.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick, R. S. , Kraus, S. D. , Rolland, R. M. , Knowlton, A. R. , Hamilton, P. K. , Pettis, H. M. , … Clark, J. S. (2013). Using hierarchical Bayes to understand movement, health, and survival in the endangered north Atlantic right whale. PLoS ONE, 8, e64166 10.1371/journal.pone.0064166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick, R. S. , New, L. F. , Thomas, L. , Costa, D. P. , Hindell, M. A. , McMahon, C. R. , … Clark, J. S. (2013). Estimating resource acquisition and at‐sea body condition of a marine predator. Journal of Animal Ecology, 82, 1300–1315. 10.1111/1365-2656.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, O. J. , Krivan, V. , & Ovadia, O. (2004). Trophic cascades: The primacy of trait‐mediated indirect interactions. Ecology Letters, 7, 153–163. 10.1111/j.1461-0248.2003.00560.x [DOI] [Google Scholar]
- Schwacke, L. H. , Thomas, L. , Wells, R. S. , McFee, W. E. , Hohn, A. A. , Mullin, K. D. , … Schwacke, J. H. (2017). Quantifying injury to common bottlenose dolphins from the Deepwater Horizon oil spill using an age‐, sex‐ and class‐structured population model. Endangered Species Research, 33, 265–279. 10.3354/esr00777 [DOI] [Google Scholar]
- Shero, M. R. , Krotz, R. T. , Costa, D. P. , Avery, J. P. , & Burns, J. M. (2015). How do overwinter changes in body condition and hormone profiles influence Weddell seal reproductive success? Functional Ecology, 29, 1278–1291. 10.1111/1365-2435.12434 [DOI] [Google Scholar]
- Sibly, R. M. , Grimm, V. , Martin, B. T. , Johnston, A. S. A. , Kulakowska, K. , Topping, C. J. , … Deangelis, D. L. (2013). Representing the acquisition and use of energy by individuals in agent‐based models of animal populations. Methods in Ecology and Evolution, 4, 151–161. 10.1111/2041-210x.12002 [DOI] [Google Scholar]
- Stankowich, T. (2008). Ungulate flight responses to human disturbance: A review and meta‐analysis. Biological Conservation, 141, 2159–2173. 10.1016/j.biocon.2008.06.026 [DOI] [Google Scholar]
- Sutherland, W. J. (1998). The importance of behavioural studies in conservation biology. Animal Behaviour, 56, 801–809. 10.1006/anbe.1998.0896 [DOI] [PubMed] [Google Scholar]
- Taylor, B. L. , Martinez, M. , Gerrodette, T. , Barlow, J. , & Hrovat, Y. N. (2007). Lessons from monitoring trends in abundance of marine mammals. Marine Mammal Science, 23, 157–175. 10.1111/j.1748-7692.2006.00092.x [DOI] [Google Scholar]
- Thomas, J. A. , Kastelein, R. A. , & Awbrey, F. T. (1990). Behavior and blood catecholamines of captive belugas during playbacks of noise from an oil drilling platform. Zoo Biology, 9, 393–402. 10.1002/(ISSN)1098-2361 [DOI] [Google Scholar]
- Tyack, P. L. , Zimmer, W. M. X. , Moretti, D. , Southall, B. L. , Claridge, D. E. , Durban, J. W. , … Boyd, I. L. (2011). Beaked whales respond to simulated and actual navy sonar. PLoS ONE, 6, e17009 10.1371/journal.pone.0017009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas‐Amtmann, S. , Schwarz, L. K. , Gailey, G. , Sychenko, O. , & Costa, D. P. (2017). East or west: The energetic cost of being a gray whale and the consequence of losing energy to disturbance. Endangered Species Research, 34, 167–183. 10.3354/esr00843 [DOI] [Google Scholar]
- Villegas‐Amtmann, S. , Schwarz, L. K. , Sumich, J. L. , & Costa, D. P. (2015). A bioenergetics model to evaluate demographic consequences of disturbance in marine mammals applied to gray whales. Ecosphere, 6, 1–19. [Google Scholar]
- Weimerskirch, H. (2018). Linking demographic processes and foraging ecology in wandering albatross—Conservation implications. Journal of Animal Ecology, 87, 945–955. 10.1111/1365-2656.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, R. S. , Rhinehart, H. L. , Hansen, L. J. , Sweeney, J. C. , Townsend, F. I. , Stone, R. , … Rowles, T. K. (2004). Bottlenose dolphins as marine ecosystem sentinels: Developing a health monitoring system. EcoHealth, 1, 246–254. [Google Scholar]
- Wensveen, P. J. , Kvadsheim, P. H. , Lam, F. A. , von Benda‐Beckmann, A. M. , Sivle, L. D. , Visser, F. , … Miller, P. J. O. (2017). Lack of behavioural responses of humpback whales (Megaptera novaeangliae) indicate limited effectiveness of sonar mitigation. Journal of Experimental Biology, 220, 4150–4161. 10.1242/jeb.161232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, E. E. , & Peacor, S. D. (2003). A review of trait‐mediated indirect interactions in ecological communities. Ecology, 84, 1083–1100. 10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2 [DOI] [Google Scholar]
- Wheatley, K. E. , Bradshaw, C. J. A. , Davis, L. S. , Harcourt, R. G. , & Hindell, M. A. (2006). Influence of maternal mass and condition on energy transfer in Weddell seals. Journal of Animal Ecology, 75, 724–733. 10.1111/j.1365-2656.2006.01093.x [DOI] [PubMed] [Google Scholar]
- Wikelski, M. , & Cooke, S. J. (2006). Conservation physiology. Trends in Ecology & Evolution, 21, 38–46. 10.1016/j.tree.2005.10.018 [DOI] [PubMed] [Google Scholar]
- Williams, T. M. , Blackwell, S. B. , Richter, B. , Sinding, M.‐H. S. , & Heide‐Jørgensen, M. P. (2017). Paradoxical escape responses by narwhals (Monodon monoceros). Science, 23, 1328–1331. 10.1126/science.aao2740 [DOI] [PubMed] [Google Scholar]
- Williams, T. M. , Kendall, T. L. , Richter, B. P. , Ribeiro‐French, C. R. , John, J. S. , Odell, K. L. , … Stamper, M. A. (2017). Swimming and diving energetics in dolphins: A stroke‐by‐stroke analysis for predicting the cost of flight responses in wild odontocetes. Journal of Experimental Biology, 220, 1135–1145. 10.1242/jeb.154245 [DOI] [PubMed] [Google Scholar]
- Williams, R. , Lusseau, D. , & Hammond, P. S. (2006). Estimating relative energetic costs of human disturbance to killer whales (Orcinus orca). Biological Conservation, 133, 301–311. 10.1016/j.biocon.2006.06.010 [DOI] [Google Scholar]
- Williams, R. , Thomas, L. , Ashe, E. , Clark, C. W. , & Hammond, P. S. (2016). Gauging allowable harm limits to cumulative, sub‐lethal effects of human activities on wildlife: A case‐study approach using two whale populations. Marine Policy, 70, 58–64. 10.1016/j.marpol.2016.04.023 [DOI] [Google Scholar]
- Williams, R. , Trites, A. W. , & Bain, D. E. (2002). Behavioural responses of killer whales (Orcinus orca) to whale‐watching boats: Opportunistic observations and experimental approaches. Journal of Zoology, 256, 255–270. [Google Scholar]
- Wilson, A. D. M. , Wikelski, M. , Wilson, R. P. , & Cooke, S. J. (2015). Utility of biological sensor tags in animal conservation. Conservation Biology, 29, 1065–1075. 10.1111/cobi.12486 [DOI] [PubMed] [Google Scholar]
- Wood, K. A. , Stillman, R. A. , & Goss‐Custard, J. D. (2015). Co‐creation of individual‐based models by practitioners and modellers to inform environmental decision‐making. Journal of Applied Ecology, 52, 810–815. 10.1111/1365-2664.12419 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are associated with this manuscript.


