TO THE EDITOR:
Heparin-induced thrombocytopenia (HIT) is a prothrombotic disorder caused by platelet-activating antibodies that recognize PF4/heparin complexes.1-3 Although PF4-dependent enzyme-immunoassays (EIAs) have high diagnostic sensitivity for HIT (∼97% to 99%),4,5 they frequently detect nonpathogenic antibodies in heparin-exposed patients (ie, low specificity), and test results are usually not available for many hours or even several days. Accordingly, it has been recommended (Choosing Wisely6) that diagnostic testing for HIT should not be performed in low-clinical-probability settings (eg, 4Ts score of ≤3 points; HIT risk ≤2%).7,8
However, if a test for HIT antibodies was both highly sensitive and specific, this could make the test valuable even in certain low-probability situations, especially if the result was quickly available. We used a recently US Food and Drug Administration–cleared, rapid, automated, immunoglobulin G (IgG)–specific chemiluminescence-based immunoassay (CLIA) to evaluate stored blood samples from a previously reported prospective clinical study8 that evaluated the 4Ts clinical scoring system and an additional 135 HIT-positive samples collected from a local hospital. We compared the CLIA’s operating characteristics for HIT antibody detection against a widely used IgG-specific commercial EIA with addition of the “high heparin” inhibition step9 (thus maximizing EIA specificity).
Stored sera (n = 509) and citrated plasma (n = 429) were available from a prospective evaluation of the 4Ts scoring system.8 All patients had a 4Ts score, and results from the serotonin-release assay (SRA)10,11 and a polyspecific EIA that detects anti-PF4/polyvinylsulfonate antibodies of IgG/IgA/IgM classes (LIFECODES PF4 Enhanced assay; Immucor GTI Diagnostics, Waukesha, WI).12 For this new study, all serum/plasma samples were also tested using an IgG-specific CLIA (HemosIL AcuStar HIT-IgG(PF4-H), Instrumentation Laboratory, Bedford, MA)13; per the manufacturer, a result ≥1.00 U/mL was considered positive. In addition, we tested sera in a commercial IgG-specific EIA (LIFECODES PF4 IgG assay; Immucor GTI Diagnostics)14 using a high-heparin step per the manufacturer’s recommendations (reactivity of anti-PF4/heparin antibodies is inhibited at suprapharmacologic heparin concentrations).9 All testing was performed by laboratory personnel blinded to sample classification.
“HIT-positive” patients were defined as patients whose blood tested positive in both the SRA and polyspecific EIA and whose clinical course was considered consistent with HIT, either by investigators’ 4Ts scoring (≥4 points) or (in case of low 4Ts scoring) upon case review. (As previously reported,8 4Ts discrepant cases could be explained by incorrect scoring or missing information.) All other patients were classified as “HIT negative.” Each patient’s HIT status was determined before CLIA testing.
For each test, we calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) (with exact Clopper-Pearson 95% confidence intervals [CIs]), and likelihood ratios (LR+ and LR−; 95% CIs were calculated per Altman et al15). We also included 135 consecutive SRA-positive HIT patients from a single hospital (consecutive case series) to aid in calculating test sensitivity. Comparisons between assays were performed using the χ2 test. Pretest probabilities for the prospective cohort study were determined by the frequency of SRA-positive status within each 4Ts score category (as assessed in real time by the participating clinicians); post-test probabilities were calculated according to Bayes theorem by multiplying the pretest odds by the LR+, at the manufacturer’s cutoff, and at different stratum-specific likelihood ratios. The Hamilton Integrated Research Ethics Board approved this study (#1288-T).
The 168 HIT-positive patients (33 in the prospective cohort study; 135 in the consecutive case series) comprised 79 males and 89 females, with a median age of 70 years (interquartile range [IQR], 62, 78; range, 29-94). Approximately three-quarters (74.4%) of the patients were surgical. HIT-associated thrombosis occurred in 101 (60.1%) patients. The median platelet count nadir was 59 × 109/L (IQR, 33, 82; range, 2-279), and the median percent platelet count fall was 70.1% (IQR, 55.0, 84.1; range, 26.7-97.0).
Table 1 shows the CLIA’s operating characteristics (per 509 sera, 4Ts trial). Sensitivity, NPV, and LR− were similarly high for all immunoassays evaluated, reflecting high assay sensitivity. Specificity was highest for the CLIA, even compared with the IgG-specific EIA with the high-heparin step (98.5% vs 94.1%; P < .0001). Similarly, PPV and LR+ were higher for the CLIA than for EIAs.
Table 1.
Operating characteristics of the CLIA vs 2 other commercial PF4-dependent EIAs (one performed with the high-heparin step)
| CLIA (95% CI) | EIA-IgG with high-heparin step (95% CI) P value (vs CLIA) | EIA-IgG without high-heparin step (95% CI) P value (vs CLIA) | EIA-IgGAM without high-heparin step (95% CI) P value (vs CLIA) | |
|---|---|---|---|---|
| Se | 32/33 = 97.0% (84.2%, 99.9%) | 32/33 = 97.0% (84.2%, 99.9%) P = 1.0 | 33/33 = 100% (89.4%, 100%) P = .3173 | 33/33 = 100% (89.4%, 100%) P = .3173 |
| Se* | 166/168 = 98.8% (95.7%, 99.8%) | 162/168 = 96.4% (92.4%, 98.7%) P = 0.1573 | 167/168 = 99.4% (96.7%, 100.0%) P = .5637 | 168/168 = 100% (97.83%, 100.1%) P = .1573 |
| Sp | 469/476 = 98.5% (97.0%, 99.4%) | 448/476 = 94.1% (91.6%, 96.1%) P < .0001 | 435/476 = 91.4% (88.5%, 93.7%) P < .0001 | 393/476 = 82.6% (78.8%, 85.9%) P < .0001 |
| PPV | 32/39 = 82.1% (66.5%, 92.5) | 32/60 = 53.3% (40.0%, 66.3%) P < .0001 | 33/74 = 44.6% (33.0%, 56.6%) P < .0001 | 33/116 = 28.5% (20.5%, 37.6%) P < .0001 |
| NPV | 469/470 = 99.8% (98.8%, 99.99%) | 448/449 = 99.8% (98.8%, 99.99%) P = .9742 | 435/435 = 100% (99.2%, 100%) P = .3168 | 393/393 = 100% (99.1%, 100%) P = .3167 |
| LR+ | 65.9 (31.5, 137.9) | 16.5 (11.5, 23.7) P = .0002 | 11.6 (8.6, 15.6) | 5.7 (4.7, 7.0) |
| LR− | 0.031 (0.004, 0.212) | 0.032 (0.005, 0.222) P = .9742 | 0.0 (undefined) | 0.0 (undefined) |
| AUC | 0.997 (0.994, 1.000) | 0.994 (0.985, 1.000) P = .4016 | 0.997 (0.994, 1.000) P = .7225 | 0.992 (0.986, 0.999) P = .1053 |
The table presents the results of 3 different PF4-dependent immunoassays (including 1 assay, the EIA-IgG, performed with and without the high-heparin step) tested on 509 patients (33 SRA-positive) from a prospective study of the 4Ts scoring system.8 All samples tested were sera, with the exception of 2 plasma samples tested in the EIA-IgGAM (without high heparin). CLIA results using plasma are as follows: for 429 of the 509 patients, citrated plasma was available for testing in the CLIA, with the following operating characteristics found: (1) sensitivity = 30/31 (96.8%; 95% CI, 83.3%, 99.9%); (2) sensitivity (including 135 consecutive HIT-positive patients from one hospital) = 162/166 (97.6%; 95% CI, 93.9%, 99.3%); (3) specificity = 391/398 (98.2%; 95% CI, 96.4%, 99.3%); (4) PPV = 30/37 (81.1%; 95% CI, 64.8%, 92.0%); (5) NPV = 391/392 (99.7%; 95% CI, 98.6%, 100%); (6) LR+ = 55.0 (95% CI, 26.3, 115.0); and (7) LR− = 0.033 (95% CI, 0.005, 0.226). All comparisons were not significantly different than the results seen with serum (P ≥ .3120 for all 6 comparisons).
AUC, area under the curve; EIA-IgG, enzyme immunoassay (IgG specific); EIA-IgGAM, enzyme immunoassay (polyspecific); Se, sensitivity; Sp, specificity.
Sensitivity calculations performed with the addition of 135 consecutive HIT-positive patients from one hospital (total HIT-positive patients, n = 168), identified from February 1999 until January 2018, inclusive.
To obtain a more precise estimate of test sensitivity, we evaluated the CLIA in 135 consecutive HIT-positive patients. Combined with the 33 HIT-positive patients (4Ts trial), the sensitivity of the CLIA using serum was 166 out of 168 (98.8%; 95% CI, 95.8, 99.9%). The IgG-specific CLIA thus has a notably high combination of sensitivity and specificity (98.8% and 98.5%, respectively) relative to other HIT immunoassays.5,16
Results were similar when 429 available plasmas (HIT positive, n = 31) from the 4Ts trial were tested with the CLIA. When results of the 135 HIT-positive consecutive case-series patient plasmas were combined with 4Ts study patient data, the CLIA’s sensitivity for detecting HIT-positive status was 162 out of 166 (97.6%; 95% CI, 93.9%, 99.3%) (see Table 1 legend). Of the 4 false-negative samples, 2 yielded borderline-negative results when using plasma (0.89 and 0.97 U/mL) while the corresponding sera yielded borderline-positive results (1.07 and 1.06, respectively); the other 2 samples tested negative using both plasma and serum. These 4 samples were retested in the SRA and EIA-IgGAM, which confirmed their HIT-positive status. These samples remained negative upon retesting in the CLIA. Overall, we found a strong correlation between the CLIA quantitative results for HIT-positive serum–plasma pairs (r2 = 0.848; slope = 1.2113; y-intercept = 0.1404; P < .0001).
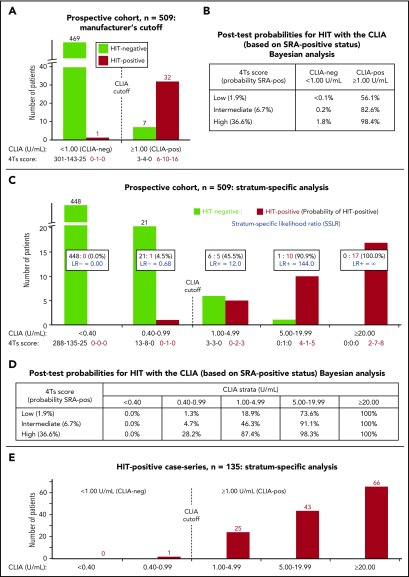
Figure 1A shows the results of the CLIA for 509 sera (4Ts trial). The overall agreement with the SRA was high (501/509 = 98.4%). Figure 1B shows the corresponding post-test probabilities of HIT for a positive test result (using 1.00 U/mL cutoff), which range from 56.1% (low 4Ts score) to 82.6% (intermediate score) to 98.4% (high score). In comparison with these Bayesian estimates, the actual results were similar, as follows: for the 310 patients with low 4Ts scores, 9 tested CLIA positive, of whom 6 out of 9 (66.7%) tested SRA positive (ie, ∼52 patients tested to identify 1 HIT patient). Thus, at a cost of 3 false-positive patients (for whom HIT was ruled out by further testing with the SRA), 6 patients with low 4Ts scores were identified as having HIT (positive SRA) by a positive CLIA. For the 158 patients with intermediate 4Ts scores, 14 tested CLIA positive, of whom 10 (71.4%) tested SRA positive (∼16 patients tested to identify 1 HIT patient). For the 41 patients with high 4Ts scores, 16 tested CLIA positive, of whom all 16 (100%) tested SRA positive (∼3 patients tested to identify 1 HIT patient). Only 1 of the 470 CLIA-negative patients tested SRA positive; this patient had been classified as 4Ts intermediate.
Figure 1.
CLIA performance for diagnosis of HIT, including Bayesian analysis. (A) Results of the CLIA for the prospective study (n = 509 sera); analysis per manufacturer’s recommended cutoff (1.00 U/mL). HIT-positive vs HIT-negative status in relation to CLIA results, CLIA-pos (positive) vs CLIA-neg (negative). The corresponding 4Ts scores, shown as xx-xx-xx for each of the data groupings, correspond to low-intermediate-high (per real-time scoring by the investigators). (B) Post-test probabilities of HIT based upon combining pretest probability of HIT (per investigators’ 4Ts score) and CLIA test result at manufacturer’s recommended cutoff (Bayesian analysis). For each 4Ts classification (low, intermediate, and high), the probability of an SRA-positive (SRA-pos) test result is indicated. (C) Probability of HIT-positive vs HIT-negative result per strength of CLIA result (stratum-specific analysis). LR− indicates likelihood ratio for HIT-negative status, whereas LR+ indicates likelihood ratio for HIT-positive status. As above, the corresponding 4Ts scores, shown as xx-xx-xx for each of the data groupings, correspond to low-intermediate-high (per real-time scoring by the investigators). (D) Post-test probabilities of HIT based upon combining pretest probability of HIT (per investigators’ 4Ts score) and stratum-specific CLIA test result (Bayesian analysis). The data shown are for the prospective study (n = 509 sera). (E) Distribution of CLIA test results for single-hospital consecutive patients with HIT. The figure shows the results using serum (n = 135). When plasma was used, the corresponding data distribution was similar, as follows: <0.40, n = 0; 0.40-0.99, n = 3; 1.00-4.99, n = 23; 5.00-19.99, n = 50; and ≥ 20.0, n = 59.
It is known that a high quantitative optical density value of a positive EIA test is associated with a high likelihood of HIT.4,17 Figure 1C shows a similar relationship for the CLIA: for a result of ≥5.00 U/mL, the probability of HIT was 96.4% (27/28), whereas for a weak-positive result (1.00-4.99 U/mL), the overall probability of HIT was 45.5% (5/11). Figure 1D summarizes the approximate post-test probabilities of HIT for various strata of positive and negative results (Bayesian analysis). Finally, the distribution of positive results in the 4Ts trial (Figure 1C) was similar to that of the consecutive HIT-positive patients (Figure 1E).
In conclusion, our evaluation of the CLIA shows a high sensitivity/specificity tradeoff for a PF4-dependent immunoassay. Particularly given its rapid, on-demand test capabilities (results available within 30 minutes13,16 following preparation of test serum or plasma), the clinical usefulness of diagnostic testing for HIT even in some low-probability situations requires further consideration.
Acknowledgments
This study was funded by Instrumentation Laboratory (Bedford, MA), the manufacturer of the IgG-specific CLIA [HemosIL AcuStar HIT-IgG(PF4-H)]. The sponsor had no role in the design of the study, performance of the assays, or analysis of the results. The sponsor provided comments to the manuscript, but all final decisions regarding manuscript content were made by the authors.
Authorship
Contribution: T.E.W. designed the study, determined 4Ts scores for the consecutive patient series, and wrote the first draft of the manuscript; J.-A.I.S. performed all of the assays; T.E.W. and J-A.I.S. performed data analyses; L.-A.L. was responsible for the 4Ts trial and oversaw the Bayesian analyses in the current study; D.M.A. and I.N. oversaw the laboratory investigations and edited the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: T.E.W. has received royalties from Informa (Taylor & Francis) and lecture honoraria from Instrumentation Laboratory; has provided consulting services to and/or has received research funding from Aspen Global, Instrumentation Laboratory, Medtronic Diabetes, Octapharma, and W.L. Gore; and has provided expert witness testimony relating to HIT and non-HIT thrombocytopenic and coagulopathic disorders. L.-A.L. has received consultancy fees and research funding from Bayer. The remaining authors declare no competing financial interests.
Correspondence: Theodore E. Warkentin, Department of Pathology and Molecular Medicine, and Department of Medicine, Michael G. DeGroote School of Medicine, McMaster University, Hamilton General Site, 237 Barton St East, Hamilton, ON L8L 2X2, Canada; e-mail: twarken@mcmaster.ca.
REFERENCES
- 1.Cuker A. Clinical and laboratory diagnosis of heparin-induced thrombocytopenia: an integrated approach. Semin Thromb Hemost. 2014;40(1):106-114. [DOI] [PubMed] [Google Scholar]
- 2.Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261. [DOI] [PubMed] [Google Scholar]
- 3.Arepally GM. Heparin-induced thrombocytopenia. Blood. 2017;129(21):2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greinacher A, Juhl D, Strobel U, et al. Heparin-induced thrombocytopenia: a prospective study on the incidence, platelet-activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. J Thromb Haemost. 2007;5(8):1666-1673. [DOI] [PubMed] [Google Scholar]
- 5.Husseinzadeh HD, Gimotty PA, Pishko AM, Buckley M, Warkentin TE, Cuker A. Diagnostic accuracy of IgG-specific versus polyspecific enzyme-linked immunoassays in heparin-induced thrombocytopenia: a systematic review and meta-analysis. J Thromb Haemost. 2017;15(6):1203-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hicks LK, Bering H, Carson KR, et al. Five hematologic tests and treatments to question. Blood. 2014;124(24):3524-3528. [DOI] [PubMed] [Google Scholar]
- 7.Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linkins LA, Bates SM, Lee AYY, Heddle NM, Wang G, Warkentin TE. Combination of 4Ts score and PF4/H-PaGIA for diagnosis and management of heparin-induced thrombocytopenia: prospective cohort study. Blood. 2015;126(5):597-603. [DOI] [PubMed] [Google Scholar]
- 9.Whitlatch NL, Kong DF, Metjian AD, Arepally GM, Ortel TL. Validation of the high-dose heparin confirmatory step for the diagnosis of heparin-induced thrombocytopenia. Blood. 2010;116(10):1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(1):27-30. [PubMed] [Google Scholar]
- 11.Warkentin TE, Arnold DM, Kelton JG, Sheppard JI, Smith JW, Nazy I. Platelet-activating antibodies are detectable at the earliest onset of heparin-induced thrombocytopenia, with implications for the operating characteristics of the serotonin-release assay. Chest. 2018;153(6):1396-1404. [DOI] [PubMed] [Google Scholar]
- 12.Visentin GP, Moghaddam M, Beery SE, McFarland JG, Aster RH. Heparin is not required for detection of antibodies associated with heparin-induced thrombocytopenia/thrombosis. J Lab Clin Med. 2001;138(1):22-31. [DOI] [PubMed] [Google Scholar]
- 13.Legnani C, Cini M, Pili C, Boggian O, Frascaro M, Palareti G. Evaluation of a new automated panel of assays for the detection of anti-PF4/heparin antibodies in patients suspected of having heparin-induced thrombocytopenia. Thromb Haemost. 2010;104(2):402-409. [DOI] [PubMed] [Google Scholar]
- 14.Warkentin TE, Sheppard JI, Moore JC, Kelton JG. The use of well-characterized sera for the assessment of new diagnostic enzyme-immunoassays for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2010;8(1):216-218. [DOI] [PubMed] [Google Scholar]
- 15.Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed London, England: BMJ Books; 2000:109. [Google Scholar]
- 16.Sun L, Gimotty PA, Lakshmanan S, Cuker A. Diagnostic accuracy of rapid immunoassays for heparin-induced thrombocytopenia. A systematic review and meta-analysis. Thromb Haemost. 2016;115(5):1044-1055. [DOI] [PubMed] [Google Scholar]
- 17.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6(8):1304-1312. [DOI] [PubMed] [Google Scholar]