Short abstract
Chikungunya virus (CHIKV) is a mosquito-borne virus that has recently emerged in the Western Hemisphere. Approved antiviral therapies or vaccines for the treatment or prevention of CHIKV infections are not available. This study aims to evaluate the antiviral activity of commercially available broad-spectrum antivirals against CHIKV. Due to host cell-specific variability in uptake and intracellular processing of drug, we evaluated the antiviral effects of each agent in three cell lines. Antiviral activities of ribavirin (RBV), interferon-alfa (IFN-α) and favipiravir (FAV) were assessed in CHIKV-infected Vero, HUH-7, and A549 cells. CHIKV-infected cells were treated with increasing concentrations of each agent for three days and viral burden was quantified by plaque assay on Vero cells. Cytotoxic effects of RBV, FAV and IFN-α were also evaluated. Antiviral activity differed depending on the cell line used for evaluation. RBV had the greatest antiviral effect in HUH-7 cells (EC50 = 2.575 µg/mL); IFN-α was most effective in A549 cells (EC50 = 4.235 IU/mL); and FAV in HUH-7 cells (EC50 = 20.00 μg/mL). The results of our study show FAV and IFN-α are the most promising candidates, as their use led to substantial reductions in viral burden at clinically achievable concentrations in two human-derived cell lines. FAV is an especially attractive candidate for further investigation due to its oral bioavailability. These findings also highlight the importance of cell line selection for preclinical drug trials.
Keywords: Chikungunya virus, antiviral agents, drug repurposing, ribavirin, favipiravir, interferon-alpha
Introduction
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus that is mainly transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Although endemic to Africa, India, and Southeast Asia, widespread distribution of the mosquito vectors and the ability to be transmitted by travelers have facilitated CHIKV outbreaks in previously unaffected regions.1,2 Over the past few years, CHIKV has spread rapidly throughout the Western hemisphere with >2 million cases being reported in the Americas, including the United States and its territories.3 Viral infection contributes to significant morbidity as approximately 75–97% of infected patients are symptomatic, exhibiting fever, debilitating joint pain, swelling of the joints, rash, headache, and muscle pain. Symptoms usually resolve 7–10 days post-infection; however, in 10–20% of cases, joint symptoms are recurrent and may persist for months to years,2,4,5 severely deteriorating the quality of life in these patients.6 For these reasons, CHIKV has increasingly become a significant public health concern.
To date, there are no approved antiviral therapies or vaccines against CHIKV. Current therapeutic protocols involve the use of supportive treatments such as analgesics and antipyretics (i.e. acetaminophen), as well as rest and maintaining adequate fluid intake to prevent dehydration.7,8 Due to the lack of licensed antivirals, it is imperative to identify new treatment strategies for CHIKV. One promising approach is to evaluate the antiviral activity of currently approved anti-infectives against CHIKV in an effort to repurpose these agents. A drug repurposing approach can greatly reduce the time to availability of treatment to affected patients because the safety, efficacy, and pharmacokinetic (PK) profiles for these approved drugs are already well defined.
For these studies, we selected three licensed agents, ribavirin (RBV), favipiravir (FAV), and interferon-alfa (IFN-α), that have broad-spectrum activity against a variety of RNA viruses. RBV is an orally available antiviral that is approved for the treatment of chronic hepatitis C infection. Upon uptake, RBV is phosphorylated by host cell kinases into mono- (RMP), di- (RDP) and triphosphate (RTP) metabolites.9 RMP inhibits the host enzyme inosine monophosphate dehydrogenase (IMPDH), resulting in depletion of intracellular guanosine triphosphate (GTP) pools;10,11 RTP accumulates in the host cell where it can act as a GTP analogue that is incorporated into the viral genome, resulting in lethal mutagenesis.11–14 FAV is a nucleoside polymerase inhibitor approved in Japan and undergoing phase III clinical trials in the US for the treatment of influenza virus;15 in addition, FAV has been evaluated for its activity against Ebola virus during a 2014 outbreak in Guinea.16 Like RBV, FAV is also converted to an active triphosphate form (FAV-RTP) by host cell kinases through monophosphate and diphosphate metabolites.15,17 Finally, IFN-α is an immunomodulator that stimulates a host antiviral response, making host cells refractory to viral infection.18 It is approved for the treatment of chronic hepatitis C virus as monotherapy or in combination with RBV.19
Since a cellular target for human infection has yet to be defined, we employed three cell lines derived from different tissues and species to evaluate the antiviral activity of RBV, FAV and IFN-α against CHIKV. We hypothesized that antiviral effect will be influenced by cell line, especially for drugs (i.e. RBV and FAV) that require activation by host cell enzymes. Vero (African Green Monkey Kidney) cells were selected because they are often considered the standard cell line used in CHIKV assays due to the fact that they are highly permissive to infection and show a robust cytopathic effect. A549 (Human Alveolar Basal Epithelial cells) and HUH-7 (Human Hepatocellular Carcinoma) cells were selected, as both lines are derived from human tissue and support robust CHIKV replication kinetics, which are ideal for antiviral evaluations. Overall, this preclinical experimental approach was used to evaluate the therapeutic potential of repurposed antiviral agents as a treatment strategy for CHIKV-infected patients. Additionally, this work highlights the importance of careful host cell selection for antiviral evaluations.
Material and methods
Cell lines
Vero cells (ATCC #CCL-81) were maintained in Eagle’s minimum essential medium (Corning Cellgro; Manassas) with 5% fetal bovine serum (Sigma Aldrich; St. Louis, Missouri) and 1% penicillin–streptomycin solution (HyClone; Logan, Utah). HUH-7 and A549 cells (ATCC #CCL-185) cells were maintained in DMEM with 5% FBS (Sigma Aldrich; St. Louis, Missouri) and 1% penicillin–streptomycin solution (HyClone; Logan, Utah). Cells were incubated at 37°C, 5% CO2 and split twice weekly to maintain subconfluency.
Virus
The vaccine strain of CHIKV (181/clone 25) obtained from Biodefense and Emerging Infections Research Resources Repository (BEI Resources, Manassas, Virginia). Viral stocks were prepared as previously described.20
Antivirals
RBV obtained from Tokyo Chemical Industry (Portland, Oregon); FAV from MedKoo Biosciences Inc. (Morrisville, North Carolina) and Human IFN-α subtype 2a from PBL assay science (Piscataway, New Jersey). Drug stocks were prepared as previously described.21
Drug assays
A549, HUH-7, or Vero cells were plated on six-well plates. Confluent monolayers were infected with CHIKV at a multiplicity of infection (MOI) of 0.1 for A549 and HUH-7 cells, and 0.0001 for Vero cells. Since Vero cells are more permissive to infection compared to the other two cell lines, a lower MOI was necessary to ensure viral replication kinetics remained similar in all cell lines. The virus was allowed to adsorb onto cells for 1 h, then viral inoculum was removed from the wells, and wells were washed twice with phosphate-buffered saline to remove unbound virus; 3 mL of drug containing medium (RBV, IFN-α, or FAV) at concentrations ranging from 0 to 1000 µg/mL for RBV; 0 to 10,000 IU/mL for IFN-α; and 0 to 157.10 μg/mL for FAV were added to the wells. For FAV experiments, a final concentration of 1% DMSO was maintained in cell culture media to ensure drug solubility. Plates were incubated at 37°C, 5% CO2 for three days. Viral supernatant was collected daily for three days, clarified by high-speed centrifugation, and frozen at −80°C until the end of the study. Infectious virus was quantified by plaque assay on Vero cells as previously described.20
Cytotoxicity assays
Cytotoxicity assays were performed with the commercially available Viral ToxGlo Assay (Promega; Madison Wisconsin) as per the manufacturer’s instructions. Luminescence was measured using a GloMax 96 Microplate Luminometer (Promega; Madison, Wisconsin).
Statistical analysis
EC50 values were determined over the entire three-day study by calculating the area under the viral burden-time curves (AUCVB) for all regimens using Prism software version 7.02 (GraphPad Software, Inc., La Jolla, CA). AUCVB values were graphed against the corresponding drug concentration and an inhibitory sigmoid Emax model was fitted to the data using Prism software. CC50 values were determined by graphing relative light unit values against drug concentration at the 72 h time point and fitting an inhibitory sigmoid Emax model to the data using Prism software.
Results
RBV
For these studies, we aimed to closely match viral replication kinetics between cell lines to allow for fair comparison of antiviral effect in each cell type. Thus, different MOIs were employed for each cell line to account for variability in permissiveness to infection. Vero cells were infected at an MOI of 0.0001 PFU/cell, whereas HUH-7 and A549 cells were infected at an MOI of 0.1 PFU/cell. In the absence of treatment, viral burden reached a peak of approximately 108 PFU/mL at day 2 in all cell lines indicating the replication kinetics were well matched between cell types (Figure 1).
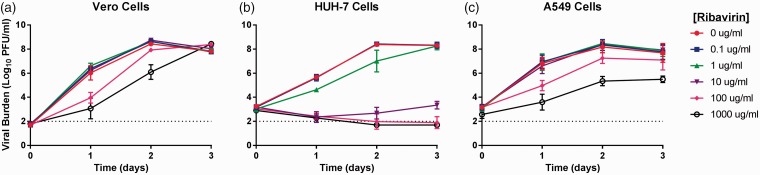
Figure 1.
Antiviral effect of ribavirin (RBV) against chikungunya virus (CHIKV) in Vero (a) HUH-7 (b), and A549 (c) cells. Vero cells were inoculated with CHIKV at a multiplicity of infection of 0.0001 and HUH-7 and A549 cells were inoculated at a MOI of 0.1. Extracellular infectious CHIKV, reported as Log10 plaque forming units per ml (PFU/ml), was quantified from clarified cell culture supernatants by plaque assay on Vero cells. Data points represent the mean of three independent samples and error bars correspond to one standard deviation. The dashed line signifies the assay limit of detection.
In Vero cells, RBV concentrations ranging from 0.1 to 10 µg/mL were not found to effectively inhibit viral replication, as viral burden in these treatment arms was nearly identical to the no treatment control (Figure 1(a)). RBV delayed viral replication in Vero cells but did not ultimately suppress it since viral burden in the 1000 µg/mL arm was similar to that of the no treatment control at day 3 (Figure 1(a)); the EC50 value in Vero cells was 99.56 µg/mL. CHIKV was most susceptible to the antiviral effect of RBV in HUH-7 cells, as concentrations of RBV ≥10 μg/mL led to a marked decline (>5-log10 PFU/ml) in CHIKV replication that was sustained throughout the three-day course of treatment (Figure 1(b)); the EC50 in this cell line was 2.575 µg/mL. RBV concentrations ≤ 10 µg/mL did not effectively suppress viral replication in A549 cells. Like in Vero cells, viral burden in these treatment arms was nearly identical to the no treatment control. RBV concentrations ≥100 µg/mL were required to inhibit viral replication in A549 cells (Figure 1(c)); the EC50 in this cell line was 117.1 µg/mL.
HUH-7 cells were the most susceptible to cytotoxic effects of RBV (CC50 = 11.95 µg/mL) (Table 1), suggesting antiviral activity at RBV concentrations exceeding 10 µg/mL is likely due to a combination of antiviral activity and cytotoxicity. Effective concentrations exceed the CC50 values of both Vero and A549 cells (65.01 µg/mL and 50.21 µg/mL, respectively) (Table 1) suggesting that like in HUH-7 cells, cytotoxicity contributes to the observed antiviral effect.
Table 1.
Fifty percent effective concentration (EC50) and 50% cytotoxic concentration (CC50) values for RBV, FAV, and IFN-α on Vero, HUH-7, and A549 cells.
| Vero cells |
HUH-7 cells |
A549 cells |
||||
|---|---|---|---|---|---|---|
| Drug | EC50 | CC50 | EC50 | CC50 | EC50 | CC50 |
| Ribavirin (µg/ml) | 99.56 | 65.01 | 2.575 | 11.95 | 117.1 | 50.21 |
| Favipiravir (µg/ml) | 28.99 | >1000 | 20.00 | >1000 | 38.51 | >1000 |
| Interferon (IU/ml) | 1344.6 | >10,000 | 26.58 | >10,000 | 4.235 | >10,000 |
FAV
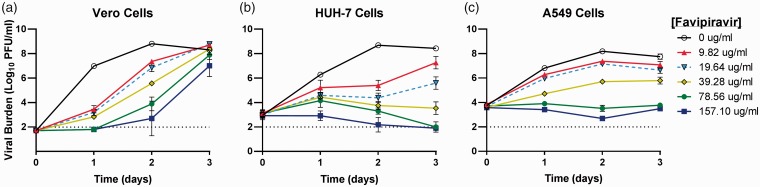
FAV had greatest antiviral effect in HUH-7 cells, followed by Vero cells, then A549 cells (Figure 2). A clear dose-response effect was observed when CHIKV-infected Vero cells were treated with FAV. Antiviral effect was not maintained in this cell line, as viral burden steadily increased throughout the three-day course of treatment (Figure 2(a)). The EC50 value for FAV against CHIKV was 28.99 μg/mL in Vero cells. In HUH-7 cells, all concentrations of FAV resulted in a decline in viral burden that was maintained throughout the duration of the study. At the lowest concentration (9.82 μg/mL), treatment with FAV led to a 3.3-log10 PFU/ml reduction in viral burden at day 2 when peak viral titers were achieved in the no-treatment control (Figure 2(b)). Further reductions in viral replication were observed as FAV concentrations increased, with the highest concentration (157.10 μg/mL) completely suppressing viral production (Figure 2(b)). FAV exhibited an EC50 value equivalent to 20.00 μg/mL in HUH-7 cells. Treatment of A549 cells with FAV led to a sustained dose-dependent suppression of viral replication (Figure 2(c)). FAV at 39.28 μg/mL resulted in a 2.5-log10 PFU/ml reduction in viral burden on day 2, whereas concentrations of 78.56 μg/mL and 157.10 μg/mL completely suppressed the production of infectious virus from baseline (Figure 2(c)). The EC50 value of FAV in A549 cells was 38.51 μg/mL. Cytotoxicity was not observed at any concentration in all cell lines (Table 1).
Figure 2.
Antiviral effect of favipiravir (FAV) against chikungunya virus (CHIKV) in Vero (a) HUH-7 (b), and A549 (c) cells. Vero cells were inoculated with CHIKV at a multiplicity of infection of 0.0001 and HUH-7 and A549 cells were inoculated at a MOI of 0.1. Extracellular infectious CHIKV was quantified by plaque assay on Vero cells and reported as Log10 plaque forming units per ml (PFU/ml). Data points represent the mean of three independent samples and error bars correspond to one standard deviation. The dashed line signifies the assay limit of detection.
IFN-α
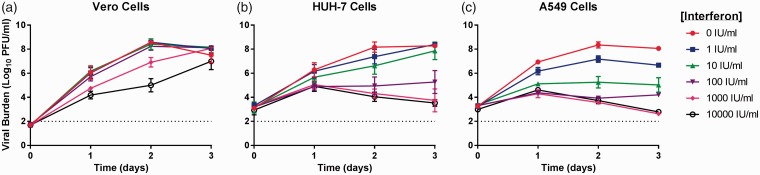
IFN-α was the least effective against CHIKV on Vero cells, resulting in an EC50 value of 1344.6 IU/ml (Figure 3(a)). Concentrations of ≥1000 IU/mL were required to reduce viral burden in this cell line, resulting in a 1.5-log10 PFU/ml decrease in the 1000 IU/ml regimen and a 3.4-log10 PFU/ml decrease in the 10,000 IU/ml arm on day 2 (Figure 3(a)). Ultimately IFN-α did not provide sustained protection, as viral titers steadily increased at all concentrations evaluated (Figure 3(a)). HUH-7 cells were more responsive to IFN-α treatment compared to Vero cells, yielding an EC50 value of 26.58 IU/ml (Figure 3(b)). Antiviral effect was most apparent at concentrations ≥100 IU/mL, with IFN-α at 1000 IU/ml and 10,000 IU/ml resulting in maximal viral suppression (Figure 3(b)). Finally, IFN-α was most effective on A549 cells and resulted in an EC50 value of 4.24 IU/ml (Figure 3(c)). Viral replication was markedly reduced at concentrations ≥10 IU/ml and maximum inhibitory effect was achieved at 1000 IU/ml. Cytotoxic effects were not observed at any IFN-α concentration evaluated in this study (Table 1).
Figure 3.
Antiviral effect of interferon-alpha (IFN-α) against chikungunya virus (CHIKV) in Vero (a) HUH-7 (b), and A549 (c) cells. Vero cells were inoculated with CHIKV at a multiplicity of infection of 0.0001 and HUH-7 and A549 cells were inoculated at a MOI of 0.1. Infectious CHIKV, reported as Log10 plaque forming units per ml (PFU/ml), was quantified by plaque assay on Vero cells. Data points represent the mean of three independent samples and error bars correspond to one standard deviation. The dashed line signifies the assay limit of detection.
Discussion
As there is currently a lack of approved antivirals or vaccines for the treatment of CHIKV infection, our study aimed to determine the antiviral effect of commercially available broad-spectrum antivirals against CHIKV. As a second objective, we sought to evaluate the influence of host cell line selected for preclinical drug evaluations on the observed antiviral effect against CHIKV. Together, these findings can be used to select the best repurposed candidate for the treatment of CHIKV infections to move forward in further preclinical evaluations.
RBV had the greatest antiviral activity against CHIKV in HUH-7 cells (EC50 = 2.575 µg/mL) followed by Vero cells (EC50 = 99.56 µg/mL) then A549 cells (EC50 = 117.1 µg/mL). Increased antiviral effect in HUH-7 cells may be due to a variety of factors. For example, HUH-7 cells were more susceptible to RBV toxicity relative to Vero and A549 cells. Our cytotoxicity assays demonstrated that exposure to RBV did not lead to cell death, but instead had a cytostatic effect at higher concentrations (≥100 µg/ml). This observed cytostatic effect likely contributed to decreased viral burden at the highest concentrations of RBV as virus replication is dependent on actively proliferating cells.
Others have demonstrated that RBV accumulation in the host cell is linked to antiviral activity.9,22 This accumulation may be host cell-dependent as some cell lines (Vero and A549 included) have been found to be more resistant to RBV treatment. Accumulation of RBV is potentially more efficient in HUH-7 cells compared to the other cell lines. Another possible cause of variability in antiviral effect involves activation of RBV. Since several proposed mechanisms of action involve phosphorylation by the host cell to an active form, it is plausible that variability in host cell kinases between cells of different species and tissues leads to varying intracellular concentrations of active RBV.10,13,23 Resistance to RBV antiviral effect in Vero and A549 cell lines may therefore be due to variability in the rate of intracellular metabolism of active drug.12
RBV shows no clinical promise as single agent therapy, as effective concentrations (≥100 µg/ml) are supratherapeutic due to toxicity and greatly exceed clinically achievable concentrations of approximately 1 µg/ml.24,25 However, RBV may have a therapeutic role as part of combination therapy with other antivirals, as RBV has been shown to have a synergistic effect with IFN-α when used for the treatment of CHIKV in vitro.20
FAV antiviral effect was greatest in HUH-7 cells (EC50 = 20.00 μg/mL), followed by Vero cells (EC50 = 28.99 μg/mL), then A549 cells (EC50 = 38.51 μg/mL). Like RBV, FAV is also tri-phosphorylated to an active form by host cell kinases.26 Accumulation and slow catabolism of the active metabolite, FAV-RTP, may contribute to its observed antiviral effect.17 Variation in the ability of host cell kinases to convert FAV to its active form, FAV-RTP, or in the rate of catabolism of the active triphosphate metabolite may have contributed to the variation in antiviral activity that was observed in all three cell lines.
In humans, systemic FAV exposures can reach levels corresponding to a static concentration of up to 61.27 μg/mL.21,27,28 FAV substantially reduced viral titers in both human-derived cell lines (4.9-log10 PFU/ml on HUH-7 and 2.5-log10 PFU/ml on A549) at 39.28 μg/mL, a clinically achievable concentration. This, coupled with its oral bioavailability, makes FAV the most promising agent investigated in our study. It is important to note that CHIKV resistance to FAV has been described, as a variant harboring a mutation in the nsP4 protein (the RNA dependent RNA polymerase) leading to reduced FAV susceptibility emerged during FAV treatment in vitro.29 Thus, combination therapy with a second antiviral agent with a distinct mechanism of action may be required to suppress the emergence of FAV resistance. We will continue to evaluate FAV as monotherapy as well as combination therapy for its usefulness against CHIKV.
Antiviral activity of IFN-α was greatest in A549 cells (EC50 = 4.235 IU/mL), followed by HUH-7 (EC50 = 26.58 IU/mL), then Vero (EC50 = 1344.6 IU/mL). In these studies, IFN-α was effective in the human-derived cell lines but produced the least antiviral effect against CHIKV in Vero cells. Previous studies have shown Vero cells infected with CHIKV were resistant to treatment with IFN-α when therapy was initiated after infection.30 This is likely due to the fact that Vero cells are IFN-α deficient, as they lack the genes responsible for the synthesis of endogenous interferons.30–32 Vero cells do possess IFN-α receptors and are thus able to respond to exogenous IFN-α when added to tissue culture medium.30–32 Therefore, the inability of Vero cells to produce endogenous IFN-α likely prevents them from mounting a robust antiviral response to CHIKV infection, as observed for HUH-7 and A549 cells.
A549 cells responded best to treatment with IFN-α. In addition to stimulation of host antiviral response, others have shown IFN-α also exerts antitumor properties. IFN-α inhibits proliferation of A549 cells without causing cell death or cell cycle arrest at a specific phase of the cell cycle.33 As viruses need actively proliferating cells to replicate, this decrease in cell proliferation may contribute to the antiviral effect observed in this cell line. Further investigation of IFN-α’s antiviral potential against CHIKV is warranted due to its inhibitory effect at clinically achievable concentrations (∼200 IU/mL) in human-derived cell lines.34 Because IFN-α is administered through injection, it is less optimal as a therapeutic agent relative to FAV, which is available orally.
There were several limitations to this study. First, static drug concentrations were evaluated in these assays. This does not accurately reflect the dynamic concentration-time profiles that occur in humans following administration of an agent due to absorption, distribution, metabolism, and excretion processes. We are currently investigating the antiviral effect of FAV, RBV, and IFN-α when human PK profiles associated with clinical dosage regimens are simulated using the in vitro hollow fiber infection model system. A second limitation involves the selection of host cell lines for this study. To our knowledge, the cellular tropism of CHIKV infection in humans has yet to be clearly defined. In the absence of a true target host cell, we chose to use HUH-7 cells and A549 cells because they are both human-derived cell lines that are permissive to CHIKV infection and yield high viral titers. We will continue to evaluate the antiviral effects of all agents in more relevant host cell lines that support robust CHIKV replication as they are identified. Finally, a third limitation is that we evaluated a single CHIKV strain. Due to the requirement for higher biocontainment to work with non-vaccine CHIKV isolates, we chose to first evaluate the feasibility of these broad-spectrum agents in a biosafety level 2 setting. It should be noted that robust replication was achieved with the vaccine CHIKV strain used in these studies, achieving peak viral titers of ∼108 PFU/ml. Future studies will examine the effectiveness of these agents against other clinical isolates of CHIKV.
The results of this study demonstrate that antiviral effect against CHIKV is influenced by cell line. When selecting cell lines for preclinical drug evaluations, it is crucial to consider the tissue and species of origin of the cell line and the impact variability between cells may have on observed antiviral effect. Our findings showed that FAV and IFN-α substantially inhibit CHIKV replication at clinically relevant concentrations in human-derived cell lines. Further studies will focus on the antiviral potential of these compounds both alone and in combination.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Institute for Therapeutic Innovation, University of Florida.
References
- 1.Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Micro 2010; 8: 491–500. [DOI] [PubMed] [Google Scholar]
- 2.Couderc T, Lecuit M. Chikungunya virus pathogenesis: from bedside to bench. Antiviral Rese 2015; 121: 120–131. [DOI] [PubMed] [Google Scholar]
- 3.Pan American Health Organization. Geographic Spread of Chikungunya in the Americas 2013-2017, http://ais.paho.org/phip/viz/ed_chikungunya_amro.asp (accessed 24 September 2017).
- 4.Centers for Disease Control and Prevention. Chikungunya Virus, www.cdc.gov/chikungunya/symptoms/index.html (2015, accessed 24 April 2017).
- 5.Silva LA, Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest 2017; 127: 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy V, Desai A, Krishna SS, et al. Molecular mimicry between chikungunya virus and host components: a possible mechanism for the arthritic manifestations. PLoS Negl Trop Dis 2017; 11: e0005238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goupil BA, Mores CN. A review of chikungunya virus-induced arthralgia: clinical manifestations, therapeutics, and pathogenesis. Open Rheumatol J 2016; 10: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC;. Chikungunya Virus Symptoms, Diagnosis, & Treatment, www.cdc.gov/chikungunya/symptoms/index.html (2016, accessed 4 October 2018).
- 9.Iikura M, Furihata T, Mizuguchi M, et al. ENT1, a ribavirin transporter, plays a pivotal role in antiviral efficacy of ribavirin in a hepatitis C virus replication cell system. Antimicrob Agents Chemother 2012; 56: 1407–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JZ, Larson G, Walker H, et al. Phosphorylation of ribavirin and viramidine by adenosine kinase and cytosolic 5'-nucleotidase II: implications for ribavirin metabolism in erythrocytes. Antimicrobi Agents Chemother 2005; 49: 2164–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crotty S, Cameron C, Andino R. Ribavirin's antiviral mechanism of action: lethal mutagenesis? J Mol Med 2002; 80: 86–95. [DOI] [PubMed] [Google Scholar]
- 12.Shah NR, Sunderland A, Grdzelishvili VZ. Cell type mediated resistance of vesicular stomatitis virus and Sendai virus to ribavirin. PloS One 2010; 5: e11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci U S A 2001; 98: 6895–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graci JD, Cameron CE. Quasispecies, error catastrophe, and the antiviral activity of ribavirin. Virology 2002; 298: 175–180. [DOI] [PubMed] [Google Scholar]
- 15.Vanderlinden E, Vrancken B, Van Houdt J, et al. Distinct effects of T-705 (Favipiravir) and ribavirin on influenza virus replication and viral RNA synthesis. Antimicrob Agents Chemother 2016; 60: 6679–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sissoko D, Laouenan C, Folkesson E, et al. Experimental treatment with favipiravir for ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in guinea. PLoS Med 2016; 13: e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smee DF, Hurst BL, Egawa H, et al. Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells. J Antimicrob Chemother 2009; 64: 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Clercq E, Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev 2016; 29: 695–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange CM, Jacobson IM, Rice CM, et al. Emerging therapies for the treatment of hepatitis C. EMBO Mol Med 2014; 6: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallegos KM, Drusano GL, D Argenio DZ, et al. Chikungunya virus: in vitro response to combination therapy with ribavirin and interferon alfa 2a. J Infect Dis 2016; 214: 1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pires de Mello CP, Tao X, Kim TH, et al. Zika virus replication is substantially inhibited by novel favipiravir and interferon alpha combination regimens. Antimicrob Agents Chemother 2017; 62: pii: e01983–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibarra KD, Pfeiffer JK. Reduced ribavirin antiviral efficacy via nucleoside transporter-mediated drug resistance. J Virol 2009; 83: 4538–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page T, Connor JD. The metabolism of ribavirin in erythrocytes and nucleated cells. Int J Biochem 1990; 22: 379–383. [DOI] [PubMed] [Google Scholar]
- 24.Austin RK, Trefts PE, Hintz M, et al. Sensitive radioimmunoassay for the broad-spectrum antiviral agent ribavirin. Antimicrob Agents Chemother 1983; 24: 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston SL, Drusano GL, Glue P, et al. Pharmacokinetics and absolute bioavailability of ribavirin in healthy volunteers as determined by stable-isotope methodology. Antimicrob Agents Chemother 1999; 43: 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuta Y, Gowen BB, Takahashi K, et al. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013; 100: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pharmaceuticals and Medical Devices Agency . Report on the deliberation results of favipiravir (English version). Review report. Tokyo, Japan: Pharmaceuticals and Medical Devices Agency, 2014. [Google Scholar]
- 28.Madelain V, Guedj J, Mentre F, et al. Favipiravir pharmacokinetics in nonhuman primates and insights for future efficacy studies of hemorrhagic fever viruses. Antimicrob Agents Chemother 2017; 61: e01305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delang L, Segura Guerrero N, Tas A, et al. Mutations in the chikungunya virus non-structural proteins cause resistance to favipiravir (T-705), a broad-spectrum antiviral. J Antimicrob Chemother 2014; 69: 2770–2784. [DOI] [PubMed] [Google Scholar]
- 30.Fros JJ, Liu WJ, Prow NA, et al. Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling. J Virol 2010; 84: 10877–10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emeny JM, Morgan MJ. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J General Virol 1979; 43: 247–252. [DOI] [PubMed] [Google Scholar]
- 32.Osada N, Kohara A, Yamaji T, et al. The genome landscape of the African green monkey kidney-derived vero cell line. DNA Res 2014; 21: 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krejcova D, Prochazkova J, Kubala L, et al. Modulation of cell proliferation and differentiation of human lung carcinoma cells by the interferon-alpha. Gen Physiol Biophys 2009; 28: 294–301. [DOI] [PubMed] [Google Scholar]
- 34.Gutterman JU, Fine S, Quesada J, et al. Recombinant leukocyte A interferon: pharmacokinetics, single-dose tolerance, and biologic effects in cancer patients. Ann Intern Med 1982; 96: 549–556. [DOI] [PubMed] [Google Scholar]