Abstract
Hepatocellular carcinoma (HCC) is a major cause of cancer-associated mortality worldwide and is expected to rise. Patients with early-stage disease may have a good prognosis with a 5-year survival rate of greater than 70%. However, the majority of patients are diagnosed with late-stage disease with a dismal overall survival rate of less than 16%. Therefore, there is a great need for advances in the treatment of advanced HCC, which for approximately the past decade, has been sorafenib. Immunotherapy is an evolving cancer treatment and has shown promise in treating patients with advanced HCC. In this review, we discuss the current standard of care for advanced HCC and then discuss the evolving role of immunotherapies.
Keywords: hepatocellular carcinoma, immune checkpoint inhibitor, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the second leading cause of cancer-associated mortality with an average life expectancy of 6–9 months.1,2 By 2030, these numbers are expected to rise, primarily due to increased rates of hepatitis C virus (HCV)-related cirrhosis.3 In the United States (US), there were 42,220 new cases and 30,200 deaths related to liver cancer in 2018.4 In addition, nonalcoholic fatty liver disease (NAFLD) has been shown to be a major risk factor associated with an increased risk of HCC.5 In fact, NAFLD and other metabolic disorders contribute more to the risk of HCC than any other risk factor in the US, likely in the setting of chronic inflammation, a known catalyst for the development of HCC.6
In early-stage disease, treatment has traditionally comprised of surgery (partial resection or transplantation) or locoregional therapies such as ablation or chemoembolization.6 Patients with early-stage disease have a good prognosis with a 5-year survival rate of greater than 70%, however, the majority of patients are diagnosed with late-stage disease with an overall survival rate of less than 18%.4
In the last few years, immune-based approaches have shown great promise in the treatment of solid tumor malignancies.7 Both anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and anti-programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) therapy enhances antitumor immunity by blocking tumor-induced immune suppression of cytotoxic T-cells. This leads to an exaggerated immune activation and is thought to be the result of tumor neoantigens that are produced. Studies to evaluate targeting CTLA-4 or the PD-1/PD-L1 axis in melanoma, lung, bladder and kidney cancers are associated with survival benefit and long-term disease control.8–10 In HCC, the recent second-line approval of the anti-PD-1 drug, nivolumab, shows the potential role of immunotherapy in this difficult-to-treat disease.11 In this review, we summarize the current standard of care treatment for HCC. We then examine the role of immune-based approaches and discuss currently available and ongoing clinical trials.
In advanced disease, systemic therapy with sorafenib is the standard first-line treatment. Sorafenib is a potent oral multikinase inhibitor that prevents tumor cell growth and angiogenesis. It is approved for inoperable or metastatic HCC based on two randomized phase III clinical trials. In the SHARP trial, sorafenib improved median overall survival (OS) by 3 months compared with best supportive care (BSC); 10.7 months in the sorafenib arm and 7.9 months in the BSC arm [hazard ratio (HR) 0.69; 95% confidence interval (CI) 0.55–0.87, p < 0.001].12 In a similarly designed study by Cheng and colleagues, Asian patients were enrolled to receive sorafenib versus placebo. Median OS improved by approximately 2 months, 6.5 months in the sorafenib arm versus 4.2 months with placebo, (HR 0.68, 95% CI 0.50–0.93, p = 0.014).13
Recently, lenvatinib was shown to be noninferior to sorafenib as a first-line treatment for unresectable or advanced HCC in the REFLECT trial.14 Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR) 1–3, fibroblast growth factor receptor (FGFR) 1–4, platelet-derived growth factor receptor (PDGFR)-α, RET, and KIT. Compared with sorafenib, the median OS for lenvatinib was 13.6 months (HR 0.92; 95% CI 0.79–1.06) which met the criteria for noninferiority. It had a slightly different side-effect profile, causing more hypertension and proteinuria. Lenvatinib is currently undergoing US Food and Drug Administration (FDA) review for approval. Cabozantinib, which inhibits MET, VEGFR and AXL, has also shown some activity based on the recent phase III CELESTIAL trial when compared with placebo.15
For patients who progress following first-line treatment, regorafenib and recently nivolumab, are approved as second-line agents. Regorafenib is a multikinase inhibitor targeting tumor growth and angiogenesis. In a study comparing regorafenib with placebo in patients with advanced HCC who progressed through sorafenib, regorafenib improved OS by approximately 4 months (HR 0.63, 95% CI 0.50–0.79, p < 0.001), and progression-free survival (PFS) (HR 0.46, 95% CI 0.37–0.56, p < 0.001).16 Nivolumab was approved based on the CHECKMATE-040 study and is discussed in further detail below.11
Ramucirumab, a VEGFR 2 inhibitor, was not associated with a survival benefit compared with placebo as a second-line treatment option based on the REACH trial (HR 0.80; 95% CI 0.63–1.02; p = 0.06).17 In a subset analysis, patients with alpha-fetoprotein (AFP) > 400 ng/ml did reach a survival benefit with a Child–Pugh score (CPS) of 5 (HR 0.61; 95% CI 0.43–0.87; p = 0.01) and a CPS of 6 (HR 0.64; 95% CI 0.42–0.98; p = 0.04). Based on these findings, REACH-2 was conducted with the goal of evaluating ramucirumab specifically in patients with AFP > 400 ng/ml (AFP-high). The median OS was recently reported to be 8.5 months (HR 0.71; 95% CI 0.53–0.95; p = 0.02) reaching statistical significance compared with placebo. The PFS also improved to 2.8 months with ramucirumab compared with 1.6 months with placebo (HR 0.45; 95% CI 0.34–0.60; p < 0.001).18 Although ramucirumab is not currently US FDA-approved for HCC, it demonstrates promise for biomarker-based therapy. Cabozantinib, which inhibits MET, VEGFR and AXL, has also shown some activity based on the recent phase III CELESTIAL trial when compared with placebo.15 Cabozantinib resulted in an OS benefit of 10.2 months (HR 0.76; 95% CI 0.63–0.92; p = 0.0049). Final reported data are pending but based on the survival benefit, cabozantinib is undergoing US FDA review for approval. Additionally, the c-MET inhibitor, tepotinib, has shown some promising results in early-phase clinical trials.19
Despite the few successes of treating HCC as shown above, the majority of clinical trials have failed to prove a survival advantage. The approval of the immune checkpoint inhibitor, nivolumab, however, represents an alternative and promising treatment strategy in immunotherapy.
Immune landscape of HCC
The liver plays an important role in filtering environmental and bacterial agents from the gastrointestinal tract. As a result, the liver is under constant antigen exposure from portal–venous blood flow. In order to prevent widespread immune activation from these antigens, the liver has developed intrinsic tolerogenic mechanisms within the innate and adaptive immune system.20 This intrinsic tolerance often goes unrecognized and no harm is rendered from ignoring the large majority of antigens. However, this unbiased tolerance is potentially detrimental, since it fails to recognize and act upon tumor-associated antigens (TAAs) and other stimulants leading to HCC growth and progression.21 Additionally, as most cases of HCC occur in the setting of chronic liver disease, chronic inflammation promotes immune suppression through the continuous production of cytokines and recruitment of immunosuppressive cells to the liver.21
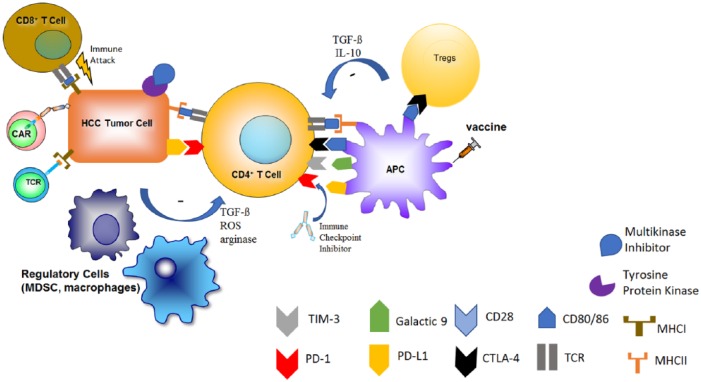
In addition to the immune-tolerant nature of the liver, the tumor cells take advantage of the intrinsic suppressive abilities of the immune system to avoid detection. Strategies include the upregulation of immune checkpoints such as PD-1/PD-L1 and CTLA-4 as well as immune inhibitory factors like arginase-1 and galasctin-922 (Figure 1). PD-L1 overexpression in HCC is associated with more aggressive tumors and increased postoperative recurrences.23 Recruitment of certain immune cells into the microenvironment further suppresses antitumor immunity in HCC. Regulatory T-cells (Tregs) inhibit the immune response by competing for crucial costimulatory receptors. Tregs have been shown to accumulate in patients with HCC where an increase in Tregs has been linked to a worse outcome.24 Myeloid-derived suppressor cells (MDSCs), a heterogeneous group of immature and immunosuppressive myeloid cells, have also been found to be increased in patients with HCC, and elevated counts often correlate with tumor progression.25–27
Figure 1.
Current landscape of systemic HCC therapy.
The mainstay of treatment for advanced HCC over the past decade has been the multikinase inhibitor, sorafenib. Within the last few years, more of these multikinase inhibitors have been found to have success in treating HCC. Unlike typical chemotherapy, immunotherapies rely on activating a person’s own immune system or the transfer of immune cells to elicit tumor eradication. The tumor microenvironment may create an immunosuppressive milieu through recruitment of Tregs, MDSCs, and upregulation of immune checkpoints. These immunosuppressive factors lead toward carcinogenesis and tumor progression. Therapies such as immune checkpoint inhibitors, cancer vaccines, cytokine-based therapies, and adoptive cell transfer have attempted to promote antigen recognition and subsequent tumor eradication.
HCC, hepatocellular carcinoma; MDSC, myeloid-derived suppressor cell; Treg, regulatory T-cell.
In order for an immune response to be mounted against a tumor, CD4+ T-cells must be able to recognize its antigen.22 In an attempt to promote antigen recognition, incomplete tumor ablation has been tried in combination with immunotherapies based on the assumption that radiofrequency ablation (RFA) or transarterial chemoembolization (TACE) promotes immunogenic cell death.28 This cell death leads to a systemic release of antigens resulting in a global immune response which is enhanced by immunotherapy.29 Therefore, studies have been performed and more are underway combining ablative and immunotherapies.
Immunotherapy in HCC
The tumor microenvironment creates an immunosuppressive milieu, promoting tumor formation as well as limiting the capacity of the host to mount a proper immune response. Investigations are underway to create immune-based therapies to promote tumor recognition and ultimately tumor eradication. In the following we review various strategies and treatments tested in HCC.
Cytokines
The first immunotherapy studied in patients with HCC was interferon (IFN). The use of IFN appeared as a logical first choice for treatment of HCC and it may show both antiviral and antitumor functions.22 However, the use of IFN has been met with limited success. For patients with advanced disease, the tumor response rates to IFN-α therapy was poor with no OS benefit and a partial response rate of 6% (2 of 30 patients). In addition, IFN was not well tolerated resulting in nearly half of the patients discontinuing treatment due to intolerance or adverse events.30 The use of intratumoral delivery of interleukin (IL)-12 has also been tested, where, in two phase I trials, patients with advanced gastrointestinal (GI) tumors displayed feasibility and safety but did not show promising HCC tumor response rates, although the studies were underpowered.31,32
Transforming growth factor (TGF)-β activity has an important role in maintaining a favorable microenvironment for tumor cell growth in HCC. Studies have shown that TGF-β is involved in the accumulation of the extracellular matrix in the tumor microenvironment, which is associated with prolonged inflammation, remodeling, and eventual destruction of the liver architecture.33 In addition, TGF-β is also involved in the regulation of several signaling pathways including Wnt, MAPK, PI3K, and NOTCH.34,35 A study by Faivre and colleagues evaluated the TGF-β1 receptor inhibitor, Galunisertib (LY2157299), in a phase II study in patients with advanced HCC who had progressed on sorafenib.36 The median OS was 36 weeks. Interestingly, patients with elevated AFP > 200 ng/ml who experienced >20% reduction in AFP at any time during treatment had an improved median OS 93.1 weeks. Several clinical trials are ongoing in HCC with Galunisertib (i.e. ClinicalTrials.gov identifiers: NCT01246986, NCT02423343).
Immune checkpoint inhibitors
After the success of treating patients with melanoma and non-small cell lung cancer with immune checkpoint inhibitors, these agents sparked interest in treating patients with advanced HCC. From 2013 to 2018, the results of four clinical trials utilizing immune checkpoint inhibitors in patients with advanced HCC have been reported (Table 1). Sangro and colleagues reported the first clinical trial of immune checkpoint inhibitors in patients with advanced HCC.37 In this phase II multicenter trial, patients with advanced HCC and chronic hepatitis C viral infection were treated with what is now considered to be a suboptimal dose of tremelimumab, anti-CTLA-4, and evaluated for safety and tumor response. Of the 17 evaluable patients, there were 3 partial responses (17.6%) and an additional 10 patients (58.8%) were found to have stable disease. Despite suboptimal dosing, the time to progression was 6.48 months and the OS reached 8.2 months.
Table 1.
Results from clinical trials in advanced hepatocellular carcinoma using checkpoint inhibitors.
| Drug, dose | Sorafenib exposure | ORR | DCR | TTP | OS | Reference |
|---|---|---|---|---|---|---|
| Tremelimumab 15 mg/kg q90 days |
Naïve, intolerant, or progressed | 3/17 (17.6%) PR | 13/17 (76.4%) | 6.48 months | 8.2 months | Sangro and colleagues37 |
| Tremelimumab 10 mg q28 days + ablation |
Progressed | 5/19 (26.3%) PR | NR | 7.4 months | 12.3 months | Duffy and colleagues28 |
| Nivolumab 0.1–10 mg/kg q14 days (escalation) |
Naïve, intolerant, or progressed | 2/48 (4.2%) CR 4/48 (8.3%) PR |
28/48 (58%) | 3.4 months | 15 months | El-Khoueiry and colleagues11 |
| Nivolumab 3 mg/kg q14 days (expansion) |
Naïve, intolerant, or progressed | 3/214 (1.4%) CR 39/214 (18.2%) PR |
139/214 (64.5%) | 4.1 months | 83% alive at 6 months | El-Khoueiry and colleagues11 |
| Pembrolizumab 200 mg q3 weeks for about 2 years |
Intolerant, or progressed | 1/104 (1%) CR 17/104 (16%) PR |
46/104 (44%) | 4.9 months | 54% alive at 12 months | Zhu and colleagues38 |
CR, complete response; DCR, disease control rate; NR, not reported; ORR, overall response rate; OS, overall survival; PR, partial response; TTP, time to progression.
The next trial used an optimal dose of tremelimumab in combination with incomplete tumor ablation utilizing RFA or TACE.28 This was a phase I/II study where, of the 19 evaluable patients, 5 patients (26%) had a partial tumor response and 12 patients (63%) had stable disease. Following the promising results of the tremelimumab trials, nivolumab was studied in a multicenter, open-label trial conducted in patients with HCC and Child–Pugh A cirrhosis who progressed on, or were intolerant to, sorafenib. The study included patients with and without chronic viral hepatitis, including active hepatitis B virus (HBV) (31%) and HCV (21%) but not those with active co-infection with HBV and HCV or with hepatitis D virus infection. In the dose-expansion cohort, patients received nivolumab 3 mg/kg by intravenous infusion every 2 weeks. The objective response rate was 20% (95% CI 15–26) with 3 complete responses and 39 partial responses. Response duration ranged from 3.2 to 38.2+ months; 91% of responders had responses lasting 6 months or longer and 55% had responses lasting 12 months or longer.11 Recently, the results from KEYNOTE-224 were published utilizing pembrolizumab, anti-PD-1, in patients with advanced HCC who had progressed on sorafenib. Pembrolizumab produced an objective response in 18 of 104 patients with one complete response and 16 partial responses while 44% of patients were deemed to have stable disease.38
There are several noteworthy points from the above immune checkpoint inhibitor trials. The immune checkpoint inhibitor studies were performed in patients with more advanced liver disease than those patients in the SHARP trial. In Sangro and colleagues’ trial, 43% of patients had Child–Pugh stage B and in Duffy and colleagues’ trial, 14% of patients were Child–Pugh class B compared with the Llovet and colleagues’ trial, utilizing sorafenib, which only consisted of patients with Child–Pugh stage A.12,28,37 The dose-escalation phase of El-Khoueiry’s study included patients with Child–Pugh A or B7 while the dose-expansion phase only included patients with Child–Pugh A liver disease.11 The recent study with pembrolizumab only consisted of patients with Child–Pugh A liver disease.38 Therefore, the survival results may be skewed between the studies on the basis of confounding liver disease but despite this, all three studies demonstrated a survival benefit. In addition, active hepatitis does not seem to be affected by immunotherapy. The above studies demonstrate immune checkpoint inhibitors are well tolerated in patients with advanced HCC.39 Clinical trials using checkpoint inhibitors in combination with other strategies are ongoing.
Vaccine therapy
Utilizing the principles of immune recognition to promote an adaptive immune response against specific antigens, vaccines are now being applied not only for cancer prevention but cancer treatment. The basic principle underlying cancer vaccines is to increase immune recognition of tumor-specific neoantigens that result from genetic mutations producing altered proteins to create neoepitopes.40 For example, AFP is not typically expressed on normal adult tissue but is produced by HCC. AFP was the first TAA to be targeted for vaccine-based trials in HCC but was met with limited success. In early studies utilizing AFP peptides or AFP-pulse dendritic cells, a T-cell response was detectable but there was no observed clinical benefit.41,42 In a more recent phase I clinical trial, 15 patients with HCC received an AFP-derived peptide vaccine resulting in T-cell stimulation. This led to one complete tumor response and suppressed tumor growth in eight patients with no serious adverse events.43
Clinical trials have also been performed using the targeted oncolytic poxvirus, JX-594 (Pexa Vec) which is designed to replicate in and destroy cancer cells. JX-594 was found to be well tolerated and displayed promising results with an intrahepatic disease control rate of 46%.44,45 Currently, there is an ongoing phase III clinical trial evaluating JX-594 followed by sorafenib versus sorafenib alone in patients with advanced HCC (ClinicalTrials.gov identifier: NCT02562755).
Other trials have been conducted utilizing peptide vaccines against the carcinoembryonic antigen glypican-3 (GPC3). GPC3 is a glycosylphosphatidylinositol (GPI)-anchored cell surface protein consisting of a core protein and two heparan sulfate chains and functions as a co-receptor for Wnt and FGF and facilitates signaling pathways.46,47 It has also shown activity in other pathways including TGF-β2, EMT and ERK.48–50 GPC3 is expressed in nearly all HCCs and plays an important role in promoting tumor growth and progression of HCC. Early studies utilizing a GPC3 peptide vaccine found the treatment to be well tolerated and able to induce tumor infiltration of CD8+ T-cells. However, the therapy produced only 1 partial response out of 33 treated patients with a median time to tumor progression of 3.4 months.51 Preclinical studies have shown that utilizing anti-PD1 therapy may result in an increased response to GPC3 peptide vaccines and therefore combination therapy may be warranted.52 The GPC3 vaccine was also tested in the adjuvant setting demonstrating a significantly improved recurrence rate in patients treated with surgery plus vaccine compared with surgery alone at 1 year but was found to be no longer statistically significant at 2 years.53
Besides a vaccine, targeting GPC3 through an anti-GPC3 antibody, GC33, has shown to be well tolerated and may have promise in further phase II trials.54 Other trials utilizing a vaccine-based strategy with dendritic cells pulsed with antigens have failed to demonstrate a significant clinical benefit.21 Additionally, a phase II trial of low dose cyclophosphamide in combination with the telomerase peptide GV1001 in patients with advanced HCC showed no radiologically detectable tumor responses.55
Cell-based therapies: adoptive cell transfer, T-cell receptors and chimeric antigen receptor T-cells
Adoptive cell transfer (ACT) is a highly personalized form of cancer immunotherapy that involves the transfer of host-derived expanded immune cells.56 ACT exploits the natural ability of T-cells to recognize and eliminate its target antigen. In normal conditions, recognition and response to diseased cells is mediated by the T-cell receptor (TCR) and its interaction with the major histocompatibility complex (MHC) molecules on the affected cell. The MHC is comprised of a group of cell surface proteins that are essential for the presentation of TAAs to the immune system. ACT of autologous tumor-infiltrating lymphocytes (TILs) has been shown to produce a complete and durable tumor regression in patients with metastatic melanoma as well as in a select case of cholangiocarcinoma.57,58 There are limited data in the treatment of HCC patients with metastatic or unresectable disease via ACT. Haruta and colleagues evaluated two ACT techniques, lymphokine-activated killer (LAK) therapy and tumor-specific cytotoxic T-cell (CTL) therapy, finding better results in the CTL group which produced 3 complete responses out of 18 patients and 2/18 partial responses.59
An emerging strategy of ACT in HCC is through the use of cytokine-induced killer (CIK) cells. CIK cells are expanded ex vivo from a patient’s peripheral blood mononuclear cells and cultured with a cytokine cocktail producing a cell population with potent antitumor effects.60,61 A nonrandomized evaluation demonstrated CIK cell therapy may improve OS when given with RFA or TACE.62 Additionally, in a phase II randomized trial, it was found that the addition of CIK cell therapy can improve OS and PFS as compared with standard treatment.63
Tumor cells have evolved ways to escape the immune system by downregulating antigen presentation through reduced MHC expression which renders T-cells blind to the presence of cancer cells.64 Through genetic manipulation, we have the ability to produce modified TCRs aimed at specific tumor antigens. The first MHC-restricted tumor antigens targeted by ACT therapy using TILs were found in patients with melanoma including MART1, tyrosinase, and GP100.65 Since TILs could not be isolated from all patients, tumor reactive T-cells could be generated through genetic modification with TCR genes that were isolated from effective TILs.66,67 In virally-related cancers like HCC, TCRs can be generated from viral antigens and be effective as long as these are expressed on tumor cells. As an example, TCR-targeted HBV-infected HCC tumor cells were found to reconstitute virus-specific T-cell immunity directed at HBV-infected HCC cells.68 AFP is being targeted in the ongoing phase I trial evaluating the safety and antitumor activity of AFP-targeted TCRs in patients with advanced HCC (ClinicalTrials.gov identifier: NCT03132792). A limitation to TCR therapy is that it can only be used in a proportion of patients, due to the MHC-restricted nature of TCR function.
On the other hand, chimeric antigen receptor (CAR)-bound T-cells are MHC unrestricted. CAR-T-cells incorporate chimeric antigen receptors, where an antibody single-chain variable fragment joins with TCRs and T-cell costimulatory receptor signaling domains to recognize cell surface antigens in an MHC-unrestricted approach.69 Simply put, CAR-T-cells work independently of antigen processing and presentation. These can be manipulated to specifically target malignancy-associated antigens. Different types of antigens can be used as potential targets, such as tissue-specific differentiation antigens (e.g. CD19), germ cell antigens (e.g. NY-ESO-1) which is detected in normal testis and a variety of tumor types, overexpression of self-proteins (e.g. HER2), mutational antigens (e.g. BRAF-V600E), and viral antigens (e.g. human papilloma virus in cervical cancer).70
First generation CAR-T-cells contained an intracellular portion of the TCR CD3ζ subunit at the T-cell signaling domain. Later generations now integrate two types of T-cell signaling domains comprising of costimulatory domains derived from costimulatory receptors such as CD28 or 4-1BB and a T-cell activation domain originated from CD3ζ.71 The gene that encodes for the CAR is transfected into the T-cell genome using gene-therapy vectors including a replication-incompetent γ-retrovirus and less commonly, lentivirus or transposon systems.72–75
Recent reports have demonstrated that GPC3-targeted CAR-T-cells induce tumor regression in preclinical models of HCC.76,77 GPC3-targeted CAR-T-cells were found to eradicate HCC xenografts with high levels of GPC expression and suppress the growth of low GPC3-expressing xenografts in vivo using Huh-7 cell lines.76 The tolerability and efficacy was confirmed in a phase I Chinese study using GPC3 CAR-T-cells in relapsed or refractory GPC3-positive HCC. All 13 patients tolerated the treatment well without dose limiting toxicity (DLT) and 1 grade 3 fever was reported. The patients pretreated with a lymphodepletive regimen (n = 8, 2 were nonevaluable) showed a best response of 1 partial response, 3 stable disease, and 2 progression of disease. The CAR-T-cell dose ranged from 0.013 × 107 to 14.68 × 107 cells/kg.78
Combination therapies
As we have seen benefits of combining multiple chemotherapeutic agents in other disease settings, similar results have also been witnessed with immunotherapies. Combining different immunotherapy modalities may improve HCC response rates. The combination of checkpoint inhibitors, adoptive T-cell therapy, cytokines and vaccines have been studied in other malignancies with varying success. In patients with advanced melanoma, the combination of nivolumab and ipilimumab has shown increased efficacy compared with ipilimumab alone.79 The combination of atezolizumab and bevacizumab produced a partial response in 62% of patients with advanced HCC naïve to treatment.80 Clinical trials with the combination of tremelimumab and durvalumab along with ablative therapies (ClinicalTrials.gov identifier: NCT02821754) and nivolumab plus sorafenib (ClinicalTrials.gov identifier: NCT03439891) are currently underway. Combination therapies may prove to be important for tumor response to immune-based therapies, as studies have shown tumors have the capacity to adapt to treatment becoming resistant to therapies and if we are able to target the alternative pathway we may improve tumor response.81,82
Limitations of immunotherapy
There are several limitations with the application of immunotherapy to patients with HCC. The vast majority of patients with HCC have underlying liver disease.6 Patients selected for the majority of trials in HCC had well-preserved liver function and performance status and therefore the results and safety profiles must come into question with the real-world application to patients who do not fit these characteristics.39 However as previously mentioned above, a modest amount of patients with Child–Pugh class B liver function were included in the checkpoint inhibitor trials.
Additionally, although patients with viral hepatitis were included in some immunotherapy trials, the long-term effects of modulating the immune system in patients with active infections remains largely unknown. In early studies, there was a concern that immunotherapies may cause hepatocyte destruction due to an overwhelming immune response against infected hepatocytes.37,39 Furthermore, several immune-based approaches such as ACT and personalized vaccine therapies require specialized centers and these treatments are difficult to make commercially available.
Finally, in addition to surgical resection and RFA as curative treatment options for early-stage HCC, orthotopic liver transplantation offers a curative oncologic procedure while also addresses the underlying liver disease.6 However, immunotherapies are not well studied in patients receiving an organ transplantation, as these patients were excluded from prior clinical trials involving immunotherapies. The overall effects of the combination of systemic immunosuppressive medications, as given to transplant patients, and immunotherapies is also not well known. There have been case reports of patients with advanced melanoma treated with immune checkpoint inhibitors after receiving a liver transplant without going into fulminant liver failure or graft rejection.83,84 However, there are not enough data to support the safety of immunotherapies in the transplant population.
Conclusion
The role of immunotherapy has restructured the treatment approach to numerous malignancies. The application of these therapies is quickly evolving for the treatment of patients with advanced HCC. Nivolumab is the first approved immunotherapy for HCC and we expect to see other immune-based approaches approved as ongoing clinical trials publish their results. A number of combination therapies are also being evaluated. Further basic science, translational and clinical studies are required to better understand the complex interactions between tumor cells, immune cells and immunotherapies in the tumor microenvironment.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Gagandeep Brar, Thoracic and GI Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Tim F. Greten, Thoracic and GI Malignancies Branch, Center for Cancer Research, National Institutes of Health, Building 10, Room 3B43, Bethesda, MD 20892, USA.
Zachary J. Brown, Thoracic and GI Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001; 94: 153–156. [DOI] [PubMed] [Google Scholar]
- 3. Hatzakis A, Chulanov V, Gadano AC, et al. The present and future disease burden of hepatitis C virus (HCV) infections with today’s treatment paradigm - volume 2. J Viral Hepat 2015; 22(Suppl. 1): 26–45. [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 5. Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016; 122: 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016; 2: 16018. [DOI] [PubMed] [Google Scholar]
- 7. Whiteside TL, Demaria S, Rodriguez-Ruiz ME, et al. Emerging opportunities and challenges in cancer immunotherapy. Clin Cancer Res 2016; 22: 1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-Cell carcinoma. N Engl J Med 2015; 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–384. [DOI] [PubMed] [Google Scholar]
- 11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet; 2017; 389: 2492–2502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 13. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 14. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 15. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol 2018; 36: abstract 207. [Google Scholar]
- 16. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 17. Zhu AX, Baron AD, Malfertheiner P, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: analysis of REACH trial results by child-pugh score. JAMA Oncol. Epub ahead of print 22 September 2016. DOI: 10.1001/jamaoncol.2016.4115. [DOI] [PubMed] [Google Scholar]
- 18. Zhu AX, Kang YK, Yen CJ, et al. REACH-2: a randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. J Clin Oncol 2018; 36: abstract 4003. [Google Scholar]
- 19. Faivre S, Blanc J-F, Merle P, et al. PD-020Tolerability and activity of second-line tepotinib, a potent and highly selective c-MET inhibitor, in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Ann Oncol 2016; 27(Suppl. 2): ii109. [Google Scholar]
- 20. Knolle PA, Thimme R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology 2014; 146: 1193–1207. [DOI] [PubMed] [Google Scholar]
- 21. Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, et al. The yin and yang of evasion and immune activation in HCC. J Hepatol 2015; 62: 1420–1429. [DOI] [PubMed] [Google Scholar]
- 22. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2015; 12: 681–700. [DOI] [PubMed] [Google Scholar]
- 23. Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009; 15: 971–979. [DOI] [PubMed] [Google Scholar]
- 24. Ormandy LA, Hillemann T, Wedemeyer H, et al. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 2005; 65: 2457–2464. [DOI] [PubMed] [Google Scholar]
- 25. Arihara F, Mizukoshi E, Kitahara M, et al. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 2013; 62: 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greten TF, Wang XW, Korangy F. Current concepts of immune based treatments for patients with HCC: from basic science to novel treatment approaches. Gut 2015; 64: 842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008; 135: 234–243. [DOI] [PubMed] [Google Scholar]
- 28. Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017; 66: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol 2017; 18. pii: S0168-8278(17)32287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Llovet JM, Sala M, Castells L, et al. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology 2000; 31: 54–58. [DOI] [PubMed] [Google Scholar]
- 31. Mazzolini G, Alfaro C, Sangro B, et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol 2005; 23: 999–1010. [DOI] [PubMed] [Google Scholar]
- 32. Sangro B, Mazzolini G, Ruiz J, et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol 2004; 22: 1389–1397. [DOI] [PubMed] [Google Scholar]
- 33. Giannelli G, Villa E, Lahn M. Transforming growth factor-beta as a therapeutic target in hepatocellular carcinoma. Cancer Res 2014; 74: 1890–1894. [DOI] [PubMed] [Google Scholar]
- 34. de Gramont A, Faivre S, Raymond E. Novel TGF-beta inhibitors ready for prime time in onco-immunology. Oncoimmunology 2017; 6: e1257453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoshida Y, Nijman SM, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res 2009; 69: 7385–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Faivre SJ, Santoro A, Kelley RK, et al. A phase 2 study of a novel transforming growth factor-beta (TGF-β1) receptor I kinase inhibitor, LY2157299 monohydrate (LY), in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2014; 32: abstract LBA173. [Google Scholar]
- 37. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013; 59: 81–88. [DOI] [PubMed] [Google Scholar]
- 38. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 39. Brown ZJ, Heinrich B, Steinberg SM, et al. Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer. J Immunother Cancer 2017; 5: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sahin U, Tureci O. Personalized vaccines for cancer immunotherapy. Science 2018; 359: 1355–1360. [DOI] [PubMed] [Google Scholar]
- 41. Butterfield LH, Economou JS, Gamblin TC, et al. Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients. J Transl Med 2014; 12: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Butterfield LH, Ribas A, Meng WS, et al. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res 2003; 9: 5902–5908. [PubMed] [Google Scholar]
- 43. Nakagawa H, Mizukoshi E, Kobayashi E, et al. Association between high-avidity T-cell receptors, induced by alpha-fetoprotein-derived peptides, and anti-tumor effects in patients with hepatocellular carcinoma. Gastroenterology 2017; 152: 1395–1406.e1310. [DOI] [PubMed] [Google Scholar]
- 44. Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 2013; 19: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol 2008; 9: 533–542. [DOI] [PubMed] [Google Scholar]
- 46. Feng M, Gao W, Wang R, et al. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci U S A 2013; 110: E1083–E1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gao W, Kim H, Feng M, et al. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology 2014; 60: 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009; 119: 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun CK, Chua MS, He J, et al. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-beta2. Neoplasia 2011; 13: 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu Y, Liu H, Weng H, et al. Glypican-3 promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. Int J Oncol 2015; 46: 1275–1285. [DOI] [PubMed] [Google Scholar]
- 51. Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 2012; 18: 3686–3696. [DOI] [PubMed] [Google Scholar]
- 52. Sawada Y, Yoshikawa T, Shimomura M, et al. Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int J Oncol 2015; 46: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sawada Y, Yoshikawa T, Ofuji K, et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 2016; 5: e1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu AX, Gold PJ, El-Khoueiry AB, et al. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2013; 19: 920–928. [DOI] [PubMed] [Google Scholar]
- 55. Greten TF, Forner A, Korangy F, et al. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer 2010; 10: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015; 348: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17: 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014; 344: 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Haruta I, Yamauchi K, Aruga A, et al. Analytical study of the clinical response to two distinct adoptive immunotherapies for advanced hepatocellular carcinoma: comparison between LAK cell and CTL therapy. J Immunother Emphasis Tumor Immunol 1996; 19: 218–223. [DOI] [PubMed] [Google Scholar]
- 60. Schmidt-Wolf IG, Lefterova P, Mehta BA, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol 1993; 21: 1673–1679. [PubMed] [Google Scholar]
- 61. Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015; 148: 1383–1391.e1386. [DOI] [PubMed] [Google Scholar]
- 62. Huang ZM, Li W, Li S, et al. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J Immunother 2013; 36: 287–293. [DOI] [PubMed] [Google Scholar]
- 63. Yu X., Zhao H, Liu L, et al. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J Clin Immunol 34: 194–203. [DOI] [PubMed] [Google Scholar]
- 64. Ma WI, Wu L, Zhou F, et al. T cell-associated immunotherapy for hepatocellular carcinoma. Cell Physiol Biochem 2017; 41: 609–622. [DOI] [PubMed] [Google Scholar]
- 65. Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013; 13: 525–541. [DOI] [PubMed] [Google Scholar]
- 66. Clay TM, Custer MC, Sachs J, et al. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol 1999; 163: 507–513. [PubMed] [Google Scholar]
- 67. Morgan RA, Dudley ME, Yu YY, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol 2003; 171: 3287–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gehring AJ, Xue SA, Ho ZZ, et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol 2011; 55: 103–110. [DOI] [PubMed] [Google Scholar]
- 69. Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev 2014; 257: 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Houot R, Schultz LM, Marabelle A, et al. T-cell-based immunotherapy: adoptive cell transfer and checkpoint inhibition. Cancer Immunol Res 2015; 3: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 71. Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 2018; 15: 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014; 6: 224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015; 33: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011; 121: 1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 2014; 20: 6418–6428. [DOI] [PubMed] [Google Scholar]
- 77. Li W, Guo L, Rathi P, et al. Redirecting T cells to glypican-3 with 4–1BB zeta chimeric antigen receptors results in Th1 polarization and potent antitumor activity. Hum Gene Ther 2017; 28: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhai B, Shi D, Gao H, et al. A phase I study of anti-GPC3 chimeric antigen receptor modified T cells (GPC3 CAR-T) in Chinese patients with refractory or relapsed GPC3+ hepatocellular carcinoma (r/r GPC3+ HCC). J Clin Oncol 2017; 35: abstract 3049. [Google Scholar]
- 79. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stein S, Pishvaian MJ, Lee MS, et al. Safety and clinical activity of 1L atezolizumab + bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC). J Clin Oncol 2018; 36: abstract 4074. [Google Scholar]
- 81. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016; 7: 10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168: 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kuo JC, Lilly LB, Hogg D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: a case report and literature review. Melanoma Res 2018; 28: 61–64. [DOI] [PubMed] [Google Scholar]
- 84. Ranganath HA, Panella TJ. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother 2015; 38: 211. [DOI] [PubMed] [Google Scholar]