Abstract
Object:
This study aimed to clarify the outcomes of stereotactic body radiotherapy for spinal metastases with a uniform dose fractionation schedule in our institution.
Materials and Methods:
Patients treated with spine stereotactic body radiotherapy were retrospectively reviewed. The prescribed dose was 24 Gy in 2 fractions. End points were local control, pain control, and adverse events. Local control was defined as elimination, shrinkage, or stable disease in the tumor on imaging evaluations. Pain status was measured on a scale of 0 to 10 by patients’ self-reports, and pain response was defined as the time at which pain scale score decreased by 2 or more from the baseline score without increase in analgesics. In addition, various treatment- and tumor-specific factors were evaluated to determine predictive values for local and pain control.
Results:
This study included 134 lesions in 131 patients, with: lesion histopathology, lung/colorectal/thyroid/renal/breast/prostate/sarcoma/other cancer, 24/22/18/14/12/10/6/25; reirradiation stereotactic body radiotherapy, 82 (61.2%) cases; and postoperative stereotactic body radiotherapy for epidural spinal cord compression, 45 (33.6%) cases. Median follow-up after stereotactic body radiotherapy was 9 months. The 1-year local control rate was 72.3%. Seventy (79.5%) of the 88 cases with pain from spinal metastases achieved pain response. The 1-year pain progression-free rate was 61.7%. Regarding metastases from colorectal cancer, local and pain control rates at 1 year were significantly lower compared with other cancer types (local control rate, 34.1% vs 81.8%; P < .01; pain progression-free rate, 36.9% vs 69.9%; P = .02). On multivariate analysis, colorectal cancer metastases and radiation history were identified as independent predictors of lower local and pain control rates. Radiation-induced myelopathy, radiculopathy, and vertebral compression fractures were observed in 0, 2 (1.5%), and 16 (11.9%) cases, respectively.
Conclusions:
This study showed that spine stereotactic body radiotherapy achieved good local and pain control, with a clinically acceptable safety profile. However, stereotactic body radiotherapy may be less effective against spinal metastases from colorectal cancer.
Keywords: spine SBRT, spinal metastases, radioresistant tumor, colorectal cancer, local and pain control
Introduction
Spinal metastases can cause pain, spinal cord compression, hypercalcemia, and pathologic fracture.1 Conventional external beam radiotherapy (EBRT) has been a standard-of-care management option and provides successful palliation of painful bone metastases with very few side effects.2
However, this treatment has a limitation; the long-term local and pain control rates are low. One study reported local tumor progression in as many as 70% of patients at 1 year as evaluated by radiographic findings after conventional radiotherapy.3 Conventional EBRT has been found in randomized trials to result in progressively higher rates of pain failure with longer follow-up.4 With innovations in systemic therapy extending life expectancy for patients with metastatic disease, the need for long-term pain and tumor control of spinal metastases is growing.
Stereotactic body radiotherapy (SBRT) with intensity-modulated radiation therapy and an image-guidance technique has emerged as a new treatment option for spinal metastases.1 Stereotactic body radiotherapy can deliver high-dose radiation to the target volume, while sparing adjacent at-risk organs. Spine SBRT could therefore achieve high local and pain control rates. The purpose of this study was to clarify the outcomes of spine SBRT in our institution.
Materials and Methods
Patients and Data Acquisition
This retrospective review of a single Japanese institution was performed to identify patients treated with spine SBRT between April 2013 and December 2017. Patients were included if they met the following criteria: (1) spinal metastases diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI); and (2) spine lesion treated with SBRT. We have conducted spine SBRT for cases with oligometastases or reirradiation. However, among patients meeting the indications for SBRT, patients with spinal instability or spinal cord compression underwent decompression and fixation surgery before spine SBRT. Patients with a radiation history of spine SBRT or particle beam therapy to the same spinal level were excluded from the present study. Radioresistant tumor was defined as renal cell carcinoma, thyroid cancer, malignant melanoma, sarcoma, or colorectal cancer.
This study was approved by our institutional ethical review boards (number 2035), and written informed consent was obtained from all patients.
Stereotactic Body Radiotherapy
Patients were immobilized using a full-body evacuated cushion (CIVCO Medical Solutions, Kalona, Iowa) in a stable supine position. Planning CT simulation was performed with a slice thickness of 1 mm, and all patients underwent MRI for delineation of the tumor and spinal canal. The clinical target volume (CTV) included the gross tumor and immediately adjacent bony anatomic compartments at risk of microscopic disease extension, as described by contouring guidelines for spine SBRT.5,6 The spinal cord and cauda equina were contoured with T1- or T2-weighted MRI. Other organs at risk (OARs) were contoured based on simulation CT images. A 2-mm margin was added to the CTV to create the planning target volume (PTV). A 1.5-mm margin was added to the spinal cord and defined as planning OAR volume of the cord (PRVcord). For the cauda equina, the thecal sac was contoured with no additional margin. The prescribed dose was 24 Gy in 2 fractions, and all planning goals were to maximize the PTV irradiated 100% of the prescribed dose, note however, that the 95% of the PTV should be irradiated the 70% of the prescribed dose even if the PTV was in proximity to normal tissues (D 95% ≥ 70% × prescribed dose). In addition, we set 2 constraints for the PTV: dose to the 50% of the volume to be between 105% and 107% of the prescribed dose (105% ≤ D 50% ≤ 107% × prescribed dose), and maximum dose was limited as 135% of the prescribed dose (D max ≤ 135% × prescribed dose). Dose constraints were set for PRVcord and cauda equina so that the maximum point dose (the point indicated as 0.035 cc7) was less than 17 Gy for radiation-naïve patients and less than from 11.0 to 12.2 Gy for irradiated patients, based on the report by Sahgal et al (Figure 1).8,9
Figure 1.

Images obtained from a 68-year-old man with metastatic ninth thoracic metastases from lung cancer. A and B, Axial and sagittal CT images with contouring for planning SBRT. C and D, Axial and sagittal T1-weighted MRI with contouring for planning SBRT. E and F, Axial and sagittal CT images with dose distribution of SBRT. CT indicates computed tomography; SBRT, stereotactic body radiotherapy.
Evaluation
End points in the present study were local control, pain control, and adverse events. Tumor response was evaluated in 4 steps of elimination/shrinkage/stable disease/tumor progression, and local control was defined as elimination, shrinkage, or stable disease of the tumor on CT or MRI obtained basically every 3 months after SBRT. Pain status at the treated index spine was measured on a scale of 0 to 10 using the Numerical Rating Pain Score (NRPS) to note the worst score for the previous 3 days at baseline and at 1 to 3 months, 4 to 6 months, 7 to 9 months, and 10 to 12 months after SBRT. In cases showing pain at SBRT, pain response was evaluated as complete response (CR) or partial response according to the International Consensus Pain Response Endpoints guideline using the NRPS,10 and we noted best response during follow-up. Pain failure was defined as the time at which NRPS increased by 2 or more from the scale at the preceding examination or analgesic requirements (convert to oral morphine-equivalent dose) increased by >25% from baseline. Some factors were selected as potential predictors, and their impacts on local and pain control rates were assessed using univariate and multivariate modeling. These factors included Karnofsky performance status, lesion histopathology, activity of other systemic disease, number of spinal levels, radiation history, decompression surgery, degree of spinal cord compression as classified by the Bilsky grade,11 dosimetric data of spine SBRT, and systemic therapy after SBRT. The systemic therapy was defined as following agents of cytotoxic chemotherapy, molecular target drug, immune checkpoint inhibitor, and hormone therapy, and as following duration from SBRT to the local failure or for 1 year after SBRT if local control was maintained. Adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 412 and the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer radiation morbidity scoring system.13 Vertebral compression fracture (VCF) events were defined as the development of a new VCF or the progression of an existing VCF in vertebral bodies treated with SBRT based on imaging evaluations.
Statistical Analysis
Local control was calculated in months from the start date of SBRT to the tumor progression date for the treated vertebral segment or the last follow-up imaging study if local control was maintained; death was not included as an event in terms of local control. Pain progression-free duration was calculated in months from the start date of SBRT to pain failure in the treated area or last follow-up physical examination in cases suffering pain at SBRT. Overall survival was defined as the time from the start date of SBRT until death from any cause. Local control rates, pain progression-free rates, and overall survival rates were estimated using the Kaplan-Meier method, and logrank tests were used to evaluate correlations between control rates and potential predictors of interest as univariate analysis. For the multivariate analysis of local and pain control rates, a Cox proportional hazards model was developed by forward, stepwise regression for all predictor variables identified as significant or trend (P < .20) in univariate analysis. Results were considered significant for values of P < .05. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).14
Results
Patient Characteristics
From more than 180 patients treated with bone SBRT at our institution, 131 patients (134 lesions) satisfied the eligibility criteria. Patient and tumor characteristics are summarized in Table 1. Eighty-two (61.2%) cases had a history of conventional EBRT. Twelve cases received intraoperative radiotherapy (IORT). We used to conduct IORT with megavolt electron beam delivery using a conventional linear accelerator for epidural spinal cord compression at decompression surgery. Although 20 Gy were delivered with a posterior port, the irradiated dose to the spinal cord was kept to less than 2 Gy by positioning a lead plate over the spinal cord.15 However, concurrent therapy with IORT and SBRT was not conducted. Forty-five (33.6%) cases underwent decompression surgery for epidural spinal cord compression prior to SBRT. Sixty-two (46.3%) cases were radioresistant tumors including renal cell carcinoma, thyroid cancer, sarcoma, and colorectal cancer. Fifty-six (41.8%) cases underwent systemic therapy after SBRT.
Table 1.
Patient and Tumor Characteristics.
| Characteristic | 134 Lesions in 131 Patients |
|---|---|
| Sex | |
| Male/female | 81/50 |
| Mean age (years) | 65 (range: 29-82) |
| Karnofsky performance status | |
| ≥80/<80 | 105/26 |
| Lesion histopathology | |
| Lung | 24 (18.3%) |
| Colorectal | 22 (16.8%) |
| Thyroid | 18 (13.7%) |
| Renal cell | 14 (10.7%) |
| Breast | 12 (9.2%) |
| Prostate | 10 (7.6%) |
| Sarcoma | 6 (4.6%) |
| Other | 25 (19.1%) |
| Systemic disease | |
| Controlled/active | 71/60 |
| Levels treateda | |
| Cervical/thoracic/lumbar/sacral | 21/65/41/24 |
| Number of spinal levels | |
| 1/2/≥3/uncountable (sacral) | 61/24/31/18 |
| With radiation historyb | 82 (61.2%) |
| 30 Gy/10 fr. | 35 |
| 20 Gy/1 fr. (IORT) | 12 |
| 40 Gy/20 fr. | 7 |
| 35 Gy/14 fr. | 7 |
| 8 Gy/1 fr. | 7 |
| 20 Gy/5 fr. | 6 |
| Others | 26 |
| Surgical decompression before SBRT | |
| +/− | 45 (33.6%)/89 |
| Bilsky grade at SBRT | |
| 0/I (no/dural compression) | 98 |
| II/III (cord compression) | 36 |
| Systemic therapy after SBRT | |
| +/−/Unknown | 56/74/4 |
Abbreviations: fr., fration; IORT, intraoperative radiotherapy; PS, performance status; SBRT, stereotactic body radiotherapy.
a Some cases had lesions across areas.
b Eighteen lesions had 2 irradiation histories.
Clinical Outcomes
Median follow-up after spine SBRT was 9 months (range: 1-50 months). Fifty-three (40.5%) patients died, at a median of 9 months (range: 1-50 months) from the time of spine SBRT. The overall survival rate at 1 year was 65.0% and median survival time was 23 months. The local control rate at 1 year was 72.3% (Figure 2A). Among the 111 cases that underwent image evaluations, best tumor response was elimination in 3 (2.7%) cases, shrinkage in 29 (26.1%), stable disease in 70 (63.1%), and tumor progression in 9 (8.1%). The local control rate at 1 year for metastases from colorectal cancer only was significantly lower compared with other cancer types (34.1% vs 81.8%; P < .01; Figure 2B). On univariate analysis, histopathology (colorectal cancer), radiation history (Figure 2C), and dosimetric data of delivered maximum dose were significant prognostic factors. However, on multivariate analyses, spinal metastases from colorectal cancer and radiation history were independent prognostic factors (Table 2).
Figure 2.
Kaplan-Meier curves of local control after spine stereotactic body radiotherapy in all patients (A), patients with metastases from colorectal cancer and other origins (B), and patients irradiated de novo and reirradiated (C).
Table 2.
Local Control Rate on Univariate and Multivariate Analyses.
| Characteristics (Number of Cases) | 1-Year Radiographic Local Control Rate | Univariate P Value | Multivariate Analysis | ||
|---|---|---|---|---|---|
| P Value | HR | 95% CI | |||
| Karnofsky PS | |||||
| ≥80 (105) | 73.90% | .27 | ND | ND | ND |
| <80 (26) | 64.30% | ||||
| Histopathology | |||||
| Colorectal (22) | 34.10% | <.01a | <.01a | 3.35 | 1.49-7.51 |
| Thyroid (20) | 80.80% | ||||
| Renal cell (14) | 88.9% 81.8% | ||||
| Sarcoma (6) | Not reached | ||||
| Other (72) | 82.50% | ||||
| Systemic disease | |||||
| Controlled (71) | 75.60% | .48 | ND | ND | ND |
| Active (63) | 68.30% | ||||
| Number of spinal levels | |||||
| 1 (61) | 72.90% | .7 | ND | ND | ND |
| 2 (24) | 83.30% | ||||
| ≥3 (31) | 67% | ||||
| Radiation history | |||||
| Radiation-naïve (52) | 84.70% | .02a | .03a | 0.41 | 0.18-0.92 |
| Prior spine radiation (81) | 61.60% | ||||
| Surgical decompression | |||||
| −89 | 71.40% | .57 | ND | ND | ND |
| +45 | 73.30% | ||||
| Bilsky grade at SBRT | |||||
| 0/I (98) | 77.50% | .28 | ND | ND | ND |
| II/III (36) | 60.60% | ||||
| D 95% | |||||
| ≥82% × PD (64) | 76.90% | .85 | ND | ND | ND |
| <82% × PD (70) | 65.00% | ||||
| D 50% | |||||
| ≥106% × PD (55) | 62.50% | .21 | ND | ND | ND |
| <106% × PD (79) | 80.50% | ||||
| D max | |||||
| ≥125% × PD (64) | 55.30% | <.01a | .052 | 2.28 | 0.97-5.38 |
| <125% × PD (70) | 88.20% | ||||
| Systemic therapy after SBRT | |||||
| +56 | 79.30% | .29 | ND | ND | ND |
| −74 | 64.50% | ||||
| Unknown (4) | 100% | ||||
Abbreviations: CI, confidence index; DX %, dose to the X% of the PTV; HR, hazard ratio; ND, no data; PD, prescribed dose; PS, performance status; RT, radiotherapy; SBRT, stereotactic body radiotherapy.
a Significant prognostic variable.
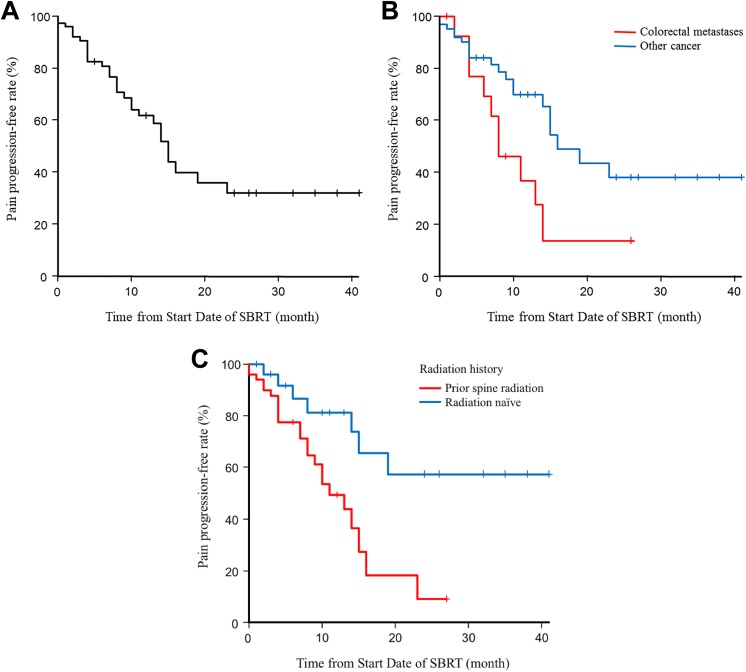
Regarding pain control, 70 (79.5%) cases of the 88 cases suffering pain at SBRT achieved pain response after SBRT, and 44 (50.0%) cases achieved CR. Mean NRPS at baseline, 1 to 3 months, 4 to 6 months, 7 to 9 months, and 10 to 12 months after SBRT were 3.94, 1.47, 1.34, 1.29, and 1.03, respectively. Median pain progression-free duration was 14 months and the 1-year pain progression-free rate was 61.7% (Figure 3A). The 1-year pain progression-free rate was significantly lower for metastases from colorectal cancer than for other cancer types (36.9% vs 69.9%; P = .02; Figure 3B). On univariate analysis, histopathology (colorectal cancer) and radiation history (Figure 3C) were significant prognostic factors, while Karnofsky performance status showed a trend. On multivariate analysis, metastases from colorectal cancer and radiation history were independent prognostic factors (Table 3). Among the 46 cases without pain, appearance of pain was observed in 1 case, and the pain progression-free rate at 1 year was 97.5%.
Figure 3.
Kaplan-Meier curves of pain control after spine stereotactic body radiotherapy in all patients (A), patients with metastases from colorectal cancer and other origins (B), and patients irradiated de novo and reirradiated (C).
Table 3.
Pain Progression-Free Rate on Univariate and Multivariate Analyses.
| Characteristics (Number of Cases) | 1-Year Pain Progression-Free Rate | Univariate P Value | Multivariate Analysis | ||
|---|---|---|---|---|---|
| P Value | HR | 95% CI | |||
| Karnofsky PS | |||||
| ≥80 (105) | 64.70% | .17 | .24 | 0.98 | 0.95-1.01 |
| <80 (26) | 41.00% | ||||
| Histopathology | |||||
| Colorectal (22) | 36.90% | .02a | .02a | 2.42 | 1.10-5.31 |
| Thyroid (20) | 50.0% | ||||
| Renal cell (14) | 80.8% 69.9% | ||||
| Sarcoma (6) | Not reached | ||||
| Other (72) | 74.50% | ||||
| Systemic disease | |||||
| Controlled (71) | 69.50% | .31 | ND | ND | ND |
| Active (63) | 56.30% | ||||
| Number of spinal levels | |||||
| 1 (61) | 69.80% | .62 | ND | ND | ND |
| 2 (24) | 43.90% | ||||
| ≥3 (31) | 77.90% | ||||
| Radiation history | |||||
| Radiation-naïve (52) | 81.10% | <.01a | .01a | 0.31 | 0.12-0.77 |
| Prior spine radiation (82) | 49.40% | ||||
| Surgical decompression | |||||
| −89 | 60.80% | .41 | ND | ND | ND |
| +45 | 62.30% | ||||
| Bilsky grade at SBRT | |||||
| 0/I (98) | 67.30% | .22 | ND | ND | ND |
| II/III (36) | 66.50% | ||||
| D 95% | |||||
| ≥82% × PD (64) | 54.60% | .42 | ND | ND | ND |
| <82% × PD (70) | 71.00% | ||||
| D 50% | |||||
| ≥106% × PD (55) | 72.10% | .24 | ND | ND | ND |
| <106% × PD (79) | 51.40% | ||||
| D max | |||||
| ≥125% × PD (64) | 71.40% | .33 | ND | ND | ND |
| <125% × PD (70) | 49.60% | ||||
| Systemic therapy after SBRT | |||||
| +56 | 62.60% | .59 | ND | ND | ND |
| −74 | 56.40% | ||||
| Unknown (4) | Not reached | ||||
Abbreviations: CI, confidence index; DX %, dose to the X% of the PTV; HR, hazard ratio; ND, no data; PD, prescribed dose; PS, performance status; RT, radiotherapy; SBRT, stereotactic body radiotherapy.
a Significant prognostic variable.
Radiation-induced myelopathy, radiculopathy, and VCF were observed in 0, 2 (1.5%) cases, and 16 (11.9%) cases, respectively. Both cases with radiculopathy had radiation history. Eleven cases with VCF were radiation-naïve and the other 5 cases had undergone reirradiation SBRT. No other grade 3 or greater toxicities were encountered.
Discussion
We have shown the outcomes of spine SBRT using 24 Gy in 2 fractions from a single Japanese institution. Spine SBRT achieved high local and pain control rates for the long term with few toxicities, even though many reirradiation cases and cases with radioresistant tumor were included. Metastases from colorectal cancer and radiation history were identified as independent predictors of lower local and pain control rates.
Most patients with spinal metastases will be offered palliative conventional EBRT, which has been associated with only short-term tumor and pain control.3,4 Patients with metastatic disease including spinal lesions have achieved extended life expectancies thanks to improvements in systemic agents, and the needs for long-term tumor and pain control of spinal metastases are growing as a result. Spine SBRT carries the possibility of satisfying these needs, since SBRT can provide ablative doses of radiation to a tumor while creating a steep dose gradient surrounding the spinal cord. A prospective nonrandomized study of spine SBRT reported an overall long-term improvement rate for pain and an overall long-term tumor control rate of 86% and 90%, respectively.16 Stereotactic body radiotherapy was used for oligometastases due to the good tumor control, and some clinical trials have shown promising results about SBRT for oligometastases.17,18 In addition, a systematic review article about spine SBRT as reirradiation reported radiation-induced myelopathy and VCF in 1.2% and 12% of patients, respectively.19 Based on these features and outcomes, we consider that spine SBRT has absolute indications for patients with solitary spinal metastasis with curative intent or for patients who have previously undergone irradiation.
Some papers have reported that spine SBRT achieved good local control even for the so-called radioresistant histopathologies, including sarcoma,20 thyroid cancer,21 renal cell carcinoma,22 and melanoma.16 The biological rationale for these observations may be explained by experimental data suggesting that only high-dose-per-fraction SBRT, typically exceeding 8 to 10 Gy per fraction, activates pathways to enhance cell death, particularly via the sphingomyelinase pathway within tumor endothelium.23 However, the current results suggest that control of colorectal cancer metastases was difficult despite SBRT.
A phase I/II trial of chemoradiotherapy for local recurrent rectal cancer shows the pathological CR rate is only 9.9%,24 while neoadjuvant chemoradiotherapy for primary rectal cancer achieved pathological CR rate of 17.8% to 20.7% in some clinical trials.25,26 Regarding lung metastases, some retrospective data have shown that the local control rate with SBRT for lung metastases from colorectal cancer is lower compared with that for other primaries.27,28 Given the findings, including the current results, colorectal cancer metastases or recurrences may have resistance even to high-dose irradiation. Although the reasons for worse outcomes are unclear, some speculations have been reported. One is that metastases from colorectal cancer contain larger proportions of hypoxic cells compared with other tumor types,29 and hypoxia leads to a decrease in radiosensitivity. Another speculation is that microscopic extension of metastases from colorectal cancer may be more than those of other cancers. Romero et al reported that, in 10% of colorectal liver metastases, microscopic extension was located at a distance of more than 6 mm.30 We are preparing additional detailed examinations, including investigation of tumor parameters and treatment parameters of SBRT for spinal metastases from colorectal cancer.
To the best of our knowledge, comprehensive reports of spine SBRT from a Japanese institution have not previously been published. Although the present study is the first to show the clinical outcomes of spine SBRT, prospective clinical trials to confirm the safety and efficacy of spine SBRT in Japan are required. Publication of results from a clinical trial (University hospital Medical Information Network, number UMIN000013428) that our institution also participated in is awaited.
Abbreviations
- CR
complete response
- CT
computed tomography
- CTV
clinical target volume
- EBRT
external beam radiotherapy
- IORT
intraoperative radiotherapy
- MRI
magnetic resonance imaging
- NRPS
Numerical Rating Pain Score
- OAR
organ at risk
- PTV
planning target volume
- SBRT
stereotactic body radiotherapy
- VCF
vertebral compression fracture
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kei Ito, MD, PhD  http://orcid.org/0000-0001-5792-3795
http://orcid.org/0000-0001-5792-3795
Katsuyuki Karasawa, MD, PhD  http://orcid.org/0000-0003-3505-2655
http://orcid.org/0000-0003-3505-2655
References
- 1. Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965–976. [DOI] [PubMed] [Google Scholar]
- 2. Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol). 2012;24(2):112–124. [DOI] [PubMed] [Google Scholar]
- 3. Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien). 1998;140(9):957–967. [DOI] [PubMed] [Google Scholar]
- 4. Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol. 1999;52(2):111–121. [PubMed] [Google Scholar]
- 5. Cox BW, Spratt DE, Lovelock M, et al. International spine radiosurgery consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(5): e597–605. [DOI] [PubMed] [Google Scholar]
- 6. Redmond KJ, Robertson S, Lo SS, et al. Consensus Contouring Guidelines for postoperative stereotactic body radiation therapy for metastatic solid tumor malignancies to the spine. Int J Radiat Oncol Biol Phys. 2017;97(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4101. [DOI] [PubMed] [Google Scholar]
- 8. Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85(2):341–347. [DOI] [PubMed] [Google Scholar]
- 9. Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):107–116. [DOI] [PubMed] [Google Scholar]
- 10. Chow E, Hoskin P, Mitera G, et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):1730–1737. [DOI] [PubMed] [Google Scholar]
- 11. Moulding HD, Elder JB, Lis E, et al. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. 2010;13(1):87–93. [DOI] [PubMed] [Google Scholar]
- 12. Common Terminology Criteria for Adverse Events Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed February 1, 2018. [DOI] [PubMed]
- 13. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. [DOI] [PubMed] [Google Scholar]
- 14. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kondo T, Hozumi T, Goto T, Seichi A, Nakamura K. Intraoperative radiotherapy combined with posterior decompression and stabilization for non-ambulant paralytic patients due to spinal metastasis. Spine. 2008;33(17):1898–1904. [DOI] [PubMed] [Google Scholar]
- 16. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32(2),193–199. [DOI] [PubMed] [Google Scholar]
- 17. Gomez DR, Blumenschein GR, Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trovo M, Furlan C, Polesel J, et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial. Radiother Oncol. 2018;126(1):177–180. [DOI] [PubMed] [Google Scholar]
- 19. Myrehaug S, Sahgal A, Hayashi M, et al. Reirradiation spine stereotactic body radiation therapy for spinal metastases: systematic review. J Neurosurg Spine. 2017;27(4):428–435. [DOI] [PubMed] [Google Scholar]
- 20. Leeman JE, Bilsky M, Laufer I, et al. Stereotactic body radiotherapy for metastatic spinal sarcoma: a detailed patterns-of-failure study. J Neurosurg Spine. 2016;25(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernstein MB, Chang EL, Amini B, et al. Spine stereotactic radiosurgery for patients with metastatic thyroid cancer: secondary analysis of phase I/II Trials. Thyroid. 2016;26(9):1269–1275. [DOI] [PubMed] [Google Scholar]
- 22. Smith BW, Joseph JR, Saadeh YS, et al. Radiosurgery for treatment of renal cell metastases to spine: a systematic review of the literature. World Neurosurg. 2018;109:e502–e509. [DOI] [PubMed] [Google Scholar]
- 23. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89–91. [DOI] [PubMed] [Google Scholar]
- 24. Cai G, Zhu J, Palmer JD, et al. CAPIRI-IMRT: a phase II study of concurrent capecitabine and irinotecan with intensity-modulated radiation therapy for the treatment of recurrent rectal cancer. Radiat Oncol. 2015;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from national surgical adjuvant breast and bowel project trial R-04. J Clin Oncol. 2014;32(18):1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979–989. [DOI] [PubMed] [Google Scholar]
- 27. Agolli L, Bracci S, Nicosia L, Valeriani M, De Sanctis V, Osti MF. Lung metastases treated with stereotactic ablative radiation therapy in oligometastatic colorectal cancer patients: outcomes and prognostic factors after long-term follow-up. Clin Colorectal Cancer. 2017;16(1):58–64. [DOI] [PubMed] [Google Scholar]
- 28. Takeda A, Kunieda E, Ohashi T, Aoki Y, Koike N, Takeda T. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol. 2011;101(2):255–259. [DOI] [PubMed] [Google Scholar]
- 29. van Laarhoven HW, Kaanders JH, Lok J, et al. Hypoxia in relation to vasculature and proliferation in liver metastases in patients with colorectal cancer. Int J Radiat Oncol Biol Phys. 2006;64(2):473–482. [DOI] [PubMed] [Google Scholar]
- 30. Romero AM, Seppenwoolde Y, Verheij J, et al. Macroscopic and microscopic pathologic findings of colorectal liver metastases correlated with magnetic resonance imaging to establish safety margins for stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;78(3):S56. [Google Scholar]