Abstract
Acrylamide (ACR), formed during the Maillard reaction induced by high temperature in food processing, is one of the main causes of neurodegenerative diseases. Taurine, a free intracellular β-amino acid, is characterized by many functions, including antioxidation, anti-inflammatory, and neuroprotective properties. This promotes its application in the treatment of neurodegenerative diseases. In this study, the neuroprotective effects of taurine against ACR-induced neurotoxicity and the potential underlying mechanisms were explored. Rats were intoxicated with ACR and injected with taurine in different groups for totally 2 weeks between January and July 2017. Electron microscopic analysis was used to observe the changes in tissues of the rats. Meanwhile, the levels of proteins including p-Akt, p-GSK3β, SIM312, and MBP were detected by Western blot. Furthermore, the GSK3β phosphorylation in taurine-treated dorsal root ganglion (DRG) with ACR was examined in the presence of the Akt inhibitor, MK-2206. The analysis of behavioral performances and electron micrographs indicated that taurine treatment significantly attenuated the toxic manifestations induced by ACR and stimulated the growth of axons and the medullary sheath, which was associated with the activation of the Akt/GSK3β signaling pathway. Mechanistically, it was found that taurine activated GSK3β, leading to significant recovery of the damage in ACR-induced sciatic nerves. Furthermore, MK-2206, an inhibitor of Akt, was applied in DRG cells, suggesting that taurine-induced GSK3β phosphorylation was Akt dependent. Our findings demonstrated that taurine attenuated ACR-induced neuropathy in vivo, in an Akt/GSK3β-dependent manner. This confirmed the treatment with taurine to be a novel strategy against ACR-induced neurotoxicity.
Keywords: acrylamide, Akt/GSK3β-dependent pathway, axonal and myelinated damage, taurine
Introduction
Acrylamide (ACR), as a water-soluble vinyl monomer, has been widely applied in chemical industries,1 including oil extraction and paper pulp production. Its neurotoxicity has helped it gain increasing attention on its application in scientific studies.2 The major approaches of exposure to ACR for humans are dietary meals and occupational exposure. As a food contaminant, ACR can be formed during thermal processing of carbohydrate-rich foods, such as deep-frying, oven-baking, and roasting.3,4 This induces its possible neurotoxic and carcinogenic effect.5 ACR has been proved to be able to cause neuropathy in both animals and humans. It has been proved that ACR does not only impede the development of children, but also cause birth defects including the digestive system, nervous system, and the immune system.6 The obvious symptoms of ACR-intoxicated rats include gait disorders and impaired behavioral performance.7 Meanwhile, humans exposed to ACR display a series of symptoms such as sweating hands, numbness, peeling skin, and limb pain.8 Therefore, further studies on the recovery of nerve function induced by taurine in ACR-treated rats are of great significance.
Neurodegenerative diseases induced by ACR have been demonstrated in the literature to be mediated via the damage of axons and medullary sheath in the peripheral nervous system.9,10 The structural integrity of axons and the medullary sheath are necessary for the function of the sciatic nerve. It is verified by electron microscopy that intravenous injection of calpeptin or nerve growth factor contributes to the significant recovery of ACR-intoxicated rats by repairing axons and the medullary sheath. In this research, the authors hypothesized that promoting the recovery of injured neurons may be an effective way to attenuate the neuropathy associated with ACR.
Taurine, 2-aminoethanesulfonic acid, as a free intracellular β-amino acid has been greatly applied for the treatment of many neurodegenerative diseases because of its neuroprotective properties.11 A number of studies have suggested that the neuroprotective effect of taurine observed in spiral ganglion neurons in vitro and the peripheral nervous system plays a role in the regulation of various cellular and tissue functions.12,13 They significantly stimulate neurite outgrowth, including axons and the medullary sheath.14 Furthermore, some studies indicate that the anti-depressant-like effect of taurine is attributed to the activation of the Akt–cAMP response element binding protein (CREB) signaling pathway,12 and taurine treatment brings the increase in myocardial Akt/protein kinase B (PKB) phosphorylation. In this way, myocardial function and heart oxidant status undergo improvement.15 Akt, which is an important upstream regulator of glycogen synthase kinase 3β (GSK3β), increases the level of GSK3β phosphorylation, leading to its inactivation. Boosting of the central nervous system (CNS) axon regeneration may be achieved by harnessing the antagonistic effects of the GSK3β activity.16 Moreover, small-molecule GSK3 inhibitors have been shown to rescue apoptosis and neurodegeneration in dorsal root ganglion (DRG) neurons injured by anesthetics.17 Thus, it was assumed that the taurine-mediated stimulation of the growth of axons and the medullary sheath occurred through the activation of the Akt/GSK3β signaling pathway, acting against the ACR-induced decrease in phosphorylated GSK3β.
Methods
Chemicals
ACR (purity > 99%) was purchased from Glenview (Naples, FL). Taurine, Akt, and p-Akt were obtained from Sigma-Aldrich (St Louis, MO); GSK3β and p-GSK3β were from Cell Signaling Technology (Sigma-Aldrich); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin were from Santa Cruz Biotechnology (OR) (Washington, USA).18,19 ACR was diluted in 0.9% saline water for a final concentration of 5 mg/mL as adopted in many studies. The dosing volume for the ACR solution was determined based on the body weight of each animal.
Animal treatment and tissue preparation
In total, 40 adult male Sprague Dawley rats (210–230 g) were obtained from the Experimental Animal Center of Dalian Medical University. From January to July 2017, these rats were housed in polycarbonate boxes, with sufficient food and water, maintained in a 12-h light/dark cycle, with temperature at 20°C–24°C and relative humidity at 50% in Dalian Medical University, China. The rats were randomly divided into four groups (n = 10 for each group):
Group I. Rats were regarded as control and received normal food for 14 days.
Group II. Rats were fed normal food and water for 4 days and then injected with ACR (50 mg/kg dry body weight bw/d, intragastrically (i.g.)) for 10 days.
Group III. Rats received taurine (250 mg/kg bw/d, tail vein injection) for 4 days and then were co-treated with ACR (50 mg/kg bw/d i.g.) for 10 days.
Group IV. Rats received taurine (250 mg/kg bw/d, tail vein injection) for 14 days.
At the end of the treatment period, all the rats were sacrificed by cervical dislocation. The sciatic nerves were immediately dissected and frozen in liquid nitrogen before being stored at −80°C for further processing. All experiments complied with the Animal Guideline of Dalian Medical University and obtained approval from the Ethical Committee of Dalian Medical University.
Electron microscopy
As reported previously,20–22 fixed sciatic nerve samples were prepared by a series of processes, including dehydration, embedment, and slicing with an ultramicrotome. The specimens were stained by lead citrate and uranyl acetate. The pathological changes in axon and medullary sheath were observed using a transmission electron microscope (H/7500; Hitachi, Tokyo, Japan).
Western blotting
Sciatic nerves were homogenized on ice using lysis buffer for 5 min and then centrifuged at 12,000×g for 15 min at 4°C. Afterwards, the protein concentration of each supernatant was determined by ristocetin-induced platelet agglutination (RIPA) of Tissue Protein Extraction Reagent (Beyotime, Shanghai, China). The tissue extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Haverhill, MA). The membranes were incubated with the appropriate primary antibodies overnight at 4°C: anti-Akt (1:1000; Sigma-Aldrich), anti-p-Akt (ser-473) (1:1000; Sigma-Aldrich), anti-SIM312 (1:500; Sigma-Aldrich), anti-MBP (1:500; Sigma-Aldrich), anti-β-actin (1:500; Sigma-Aldrich), rabbit anti-GSK3B antibody (1:500; Abcam), rabbit polyclonal anti-GSK3β antibody (1:500; Abcam, California, USA), and anti-GAPDH antibody (1:1000; Sigma-Aldrich). Horseradish peroxidase–conjugated secondary antibody was employed to visualize immunoreactivity using enhanced chemiluminescence; UVP BioSpectrum Multispectral Imaging System (Ultra-Violet) was used for densitometric analysis.
DRG neuronal cultures
The DRG neurons were dissected from three adult male rats. Briefly, DRG neurons were maintained in Dulbecco’s modified Eagle’s medium with 0.3% collagenase type IA (Sigma-Aldrich), 0.25% trypsin acid (Thermo Fisher Scientific, New York, USA), and then were mechanically dissociated.23–25 The cells were supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 500 U/mL penicillin/streptomycin (ZS-Bio, Shanghai, China) in poly-L-ornithine-coated 96-well plates with 20 μg/mL laminin (Sigma-Aldrich). Cells were grown at 37°C in a humidified incubator with 5% CO2.
Statistical analysis
All results were represented as mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance, followed by the least significant difference (LSD) test for the comparison of group differences. All tests were conducted using SPSS 19.0 statistical software. The P-values < 0.05 were considered as statistically significant.
Results
Body weight and clinical observations after taurine treatment
Rats received normal food and water or taurine (250 mg/kg bw/d, tail vein injection) for 4 days and then were treated with ACR (50 mg/kg dry body weight bw/d i.g.) or co-treated with ACR (50 mg/kg bw/d i.g.) for another 10 days. From the beginning of taurine treatment, the body weights of all the groups were measured every 2 days. As shown in Figure 1, the body weights of the control and taurine control groups showed a consistent increase, while ACR-intoxicated rats showed an attenuated increase in body weight and even lost weight during the last 4 days. The weight of the taurine-treated rats increased more quickly than that of ACR-intoxicated rats. On day 12, the body weights of taurine-treated rats were significantly higher (P < 0.05) than ACR-intoxicated rats (Table 1).
Figure 1.
(a) Group assignments were drawn as a time sequence diagram. (b) Clinical performance of each group on day 14. Control group: rats received normal feeding; ACR group: rats were received ACR (50 mg/kg/day i.p.); ACR + TAU group: rats were pretreated with ACR (50 mg/kg/day i.p.) for 14 days and then administered TAU (250 mg/kg/day i.g.) for 14 days; TAU group: rats were treated with TAU (250 mg/kg/day i.g.) for 14 days. (c) A gait score was assigned in the range from 1 to 4, where 1—a normal, unaffected gait, 2—a slightly affected gait (tip-toe walking, slight ataxia, and hindlimb weakness), 3—a moderately affected gait (obvious movement abnormalities characterized by dropped hocks and tail dragging), and 4—a severely affected gait (frank hindlimb weakness and inability to rear).
aP < 0.05, compared with the ACR group.
Table 1.
Effect of taurine (TAU) against acrylamide (ACR) on the body weight of rats.
| Group | Body weight | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 4 | Day 8 | Day 10 | Day 12 | Day 14 | |
| Control | 289.45 ± 15.66 | 295.34 ± 18.23 | 312.66 ± 23.65 | 325.43 ± 25.12 | 339.74 ± 27.68 | 356.24 ± 30.93 |
| TAU | 282.36 ± 16.86 | 297.98 ± 17.78 | 315.35 ± 21.43 | 330.24 ± 23.51 | 341.75 ± 26.62 | 354.75 ± 28.77 |
| ACR | 285.06 ± 17.97 | 294.84 ± 16.08 | 285.24 ± 13.57 | 277.09 ± 10.04 | 265.78 ± 7.83 | 254.45 ± 6.73 |
| ACR + TAU | 281.25 ± 16.24 | 298.37 ± 16.24 | 305.89 ± 17.07 | 310.89 ± 20.46* | 316.21 ± 22.40* | 325.02 ± 25.13* |
Data were shown as mean ± S.E.M. There were 10 animals in each group at each time point to show the effect of taurine against ACR on body weight after taurine treatment. Control group: rats received normal feeding for 14 days; ACR group: rats were fed normal food and water for 4 days and then injected with ACR (50 mg/kg dry body weight bw/d i.g.) for 10 days; ACR + TAU group: rats received taurine (250 mg/kg bw/d, tail vein injection) for 4 days and co-treated with ACR (50 mg/kg bw/d i.g.) for 10 days; and TAU group: rats received TAU (250 mg/kg/day i.g.) in the whole process of animal treatment.
P < 0.05 compared with the ACR group.
Similar to the body weights, the behavioral performance of rats in each group displayed the beneficial influence of taurine. Rats treated with taurine alone presented healthy and quick reactions, which exhibited no difference from normal rats. In contrast, ACR-intoxicated rats exhibited reduced activity and were anorexic and listless. In addition, abnormal symptoms induced by ACR intoxication also experienced a mitigation when ACR-intoxicated rats were treated with taurine (Figure 1).
Taurine attenuates the damage to axons and the medullary sheath induced by ACR in vivo and in vitro
The results showed that the structures of the axons and the medullary sheath were clearly visible in the control and taurine-treated groups only. In myelinated axons from the control group, a compact lamellar sheath closely encompassed an axon, and organized intermediate filaments completely filled each axon. In contrast, structural abnormalities in myelinated axons were clearly visible in samples from the ACR-intoxicated group, as shown by loosening of the myelin sheath and irregular wrinkling of axons, which was significantly mitigated once the ACR-intoxicated rats were injected with taurine (Figure 2(a)). Meanwhile, taurine simulated the growth of axon and medullary sheath by enhancing their protein. Our results indicated that taurine significantly increased the levels of SIM312 and MBP reduced by ACR, which was blocked in the presence of MK-2206 as shown in Figure 2(b) and (c).
Figure 2.
Taurine attenuates the damage to axons and the medullary sheath induced by ACR in vivo and in vitro. (a) Electron microscopic analysis was performed in the spinal cord of rats and the representative images were shown. DRG was treated with ACR (0.1 mM) or saline for 24 h and then with taurine (5 mM) in the presence or absence of MK-2206 1 h pretreatment for additional 24 h. Expression of SIM312 (b) and MBP (c) were detected with Western blot.
aP < 0.05, compared with the control group; bP < 0.05, compared with the ACR group; cP < 0.05, compared with the TAU + ACR group.
Taurine enhanced the level of Akt phosphorylation in vivo and in vitro
To demonstrate whether taurine stimulated Akt phosphorylation, the phosphorylation status of Akt was measured through immunoblot analyses. No significant difference was observed between the control and taurine-treated control groups, indicating that taurine had no influence on the levels of phosphorylated Akt in control rats. Taurine enhanced the activation of Akt by stimulating its phosphorylation that was inhibited in ACR-intoxicated rats, as shown in Figure 3(a). Consistent with the influence induced by taurine in vivo, it also stimulated the Akt signaling pathway, even when performed after ACR intoxication as shown in Figure 3(b).
Figure 3.
Effect of taurine on ACR-induced Akt activation in vivo and in vitro. (a) In the in vivo experiment, Akt and p-Akt levels were detected with Western blot. (b) The effects of TAU on the levels of Akt and p-Akt in the spinal cord of ACR-intoxicated rats were detected with Western blot and the density of blots was quantified. DRG was treated with ACR (0.1 mM) or saline for 24 h and then with or without taurine (5 mM).
aP < 0.05, compared with the control group; bP < 0.05, compared with the ACR group.
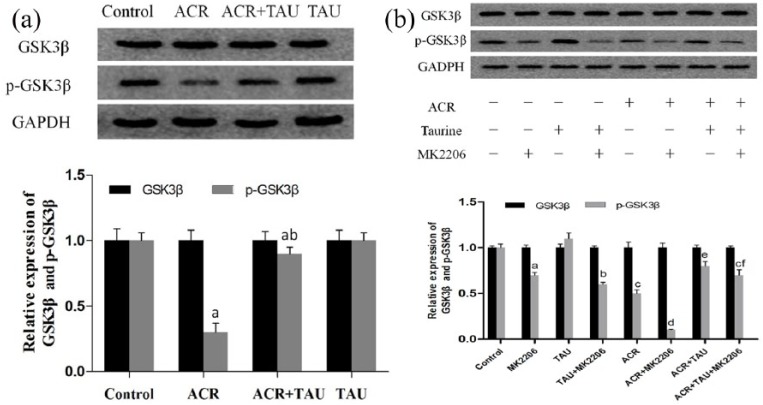
Taurine inhibited ACR-activated GSK3β
The results of this study confirmed that ACR increased the activation of GSK3β by reducing the levels of phosphorylated GSK3β, without the impact on the total GSK3β levels. By contrast, taurine attenuated this phenomenon by increasing the phosphorylation of GSK3β, with no changes in the total GSK3β levels, as shown in Figure 4(a). No obvious differences between the levels of phosphorylated GSK3β and total GSK3β levels were observed in the control and ACR-intoxicated rats. Treatment with MK-2206, which is an inhibitor of Akt, significantly mitigated the elevation of phosphorylated GSK3β levels induced by taurine. This demonstrated that the taurine-mediated GSK3β phosphorylation was Akt dependent (Figure 4(b)).
Figure 4.
Taurine enhanced the p-GSK3β level decreased by ACR in an Akt-dependent manner. (a) The effects of TAU on the levels of p-GSK3β in the spinal cord of ACR-intoxicated rats were detected with Western blot and the density of blots was quantified. (b) DRG was treated with ACR (0.1 mM) or saline for 24 h and then with taurine (5 mM) in the presence or absence of MK-2206 1 h pretreatment for additional 24 h. The p-GSK3β levels were detected with Western blot. GSK3β and p-GSK3β levels were detected with Western blot in (a) (the in vivo experiment) and (b) (the in vitro experiment).
In (a), aP < 0.05, compared with the control group; bP < 0.05, compared with the ACR group; cP < 0.05, compared with the ACR + TAU group.
In (b), aP < 0.05, compared with the control group; bP < 0.05, compared with the TAU group; cP < 0.05, compared with the TAU + MK-2206 group; dP < 0.05, compared with the ACR group; eP < 0.05, compared with the ACR + MK-2206 group; fP < 0.05, compared with the ACR + TAU group.
Discussion
This study demonstrated taurine treatment to be an effective strategy for neuroprotection in a rat model of ACR-induced neuropathy. The salient results of this study are as follows: (1) taurine treatment stimulated the growth of sample rats which was inhibited by ACR; (2) taurine injection alleviated the ACR-induced symptoms, indicating that ACR-intoxicated rats could be cured by taurine; (3) taurine protected the nerves by stimulating the growth of axons and the medullary sheath in the sciatic nerve that was damaged by ACR; (4) taurine enhanced the level of Akt phosphorylation, which was lowered by ACR; and (5) taurine-induced GSK3β phosphorylation was Akt/GSK3β dependent, resulting in the decreased GSK3β activation.
As a reactive water-soluble chemical, ACR impairs the growth and leads to central-peripheral distal axonopathy and myelinopathy in ACR-intoxicated rats.26–28 It was found that ACR-intoxicated rats exhibited weight loss and behavioral impairment compared with the control group. This effect was attenuated once the rats were treated with ACR, as shown in previous studies.28,29 Toxin accumulation and neurodegenerative diseases were caused by long exposure to ACR,30 suggesting the objectiveness and effectiveness of our model. It has been shown that taurine can not only repair the damage to optic nerve,31 but also be applied for the treatment of several CNS diseases, including Alzheimer’s disease and Parkinson’s disease.32 In contrast with the intoxicated group, taurine promoted the gradual recovery of lost weight and normal behavioral performance. This proved that taurine treatment was successful in rats intoxicated by ACR.
Based on previous studies, the damage to the central and peripheral nervous systems induced by ACR can cause axonal damage.33 Moreover, ACR has an impact on the nervous system, causing axonopathy and impairment of neurotransmitter release, as shown by damaging the terminal axon34 of the distal nerve. Due to its neurotrophic effects and anti-neurotoxicity, taurine has gained considerable attention for the treatment of neurodegenerative diseases.14,35 In this study, exposure to ACR impaired the structure of axons and the medullary sheath, which was attenuated by taurine, leading to functional rescue of the nervous system.
The Akt signaling pathway plays an important role in cell survival.36 Thus, the phosphorylated Akt levels were measured to determine whether the Akt signaling pathway was involved in the neuroprotection conferred by taurine. Notably, taurine inhibited prenatal stress–decreased phosphorylation of Akt by activating the Akt–CREB–PGC1α pathway, which significantly improved cognitive function.37 Furthermore, the PI3K–Akt–Bad pathway activated by taurine played a critical role in protecting against myocardial toxicity due to doxorubicin.38 Taurine treatment ameliorated myocardial function through the increase of myocardial Akt/PKB phosphorylation.15 In contrast, ACR induced mitochondrial dysfunction and apoptosis in BV-2 microglial cells by suppressing Akt activation, increasing JNK and p38 activation. This posed an indirect proinflammatory influence.39 This study established the protective effect of taurine against the ACR-induced decrease in Akt activation by demonstrating the increased levels of phosphorylated Akt.
Consistent with the Akt activation in taurine-treated ACR-intoxicated rats, taurine also increased the level of GSK3β phosphorylation in the sciatic nerve. Previous studies have reported that ACR inhibits neurogenesis through the activation of the GSK3β signaling pathway.40 By comparison, taurine has been shown to exert an antidepressant-like effect, influencing depression-related signaling cascades in the hippocampus by altering the levels of phosphorylated GSK3β.41 In addition, taurine has an impact on various signaling pathways and the gene expression of many proteins, including those involved in the Akt/PKB and PI3K/Akt signaling pathways, especially GSK3β phosphorylation.42 In our study, taurine significantly decreased the activation of GSK3β, as shown by the increased levels of GSK3β phosphorylation which was reduced by taurine.
Recent studies have determined GSK3β to be an important factor in axon formation, by utilizing pharmacological approaches.43 And GSK3β inhibitor was proved to be able to stimulate the growth of axons and upregulate the expression of myelin genes.44 Maslinic acid facilitated axonal regeneration by regulating the Akt/GSK3β signaling pathway to provide neuroprotection in an animal model of middle cerebral artery occlusion.45 What is more, the PI3K–GSK3β signaling pathway was involved in sensory axon regeneration, as shown by the increased level of GSK3β phosphorylation that was validated by an acute depletion of Smad1.46 Since many microtubule-associated proteins (MAPs) including MAP1B are regarded as GSK3β substrates, GSK3β is an important regulator of neuronal microtubules.47,48 Consistent with the decrease in GSK3β phosphorylation, the phosphorylation of MAP1B induced by GSK3β decreased its affinity for microtubules and enhanced its microtubule-stabilizing ability.49,50
In summary, this study provides compelling evidence for the significant role of taurine treatment in attenuating the damage to axons and the medullary sheath induced by ACR in the sciatic nerve in an Akt/GSK3B-dependent manner. The taurine-mediated recovery of nerve function following the onset of neuronal damage proved that taurine treatment could be a novel candidate to cure ACR-induced neuropathy. It was believed that our results could pave the way for new approaches in the clinical treatment of nerve injuries and establish the molecular mechanism underlying the neuroprotective effect of taurine.
Footnotes
Authors’ Note: G.S and S.Q. have contributed equally to this work.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jingsong Sun  https://orcid.org/0000-0002-8030-2062
https://orcid.org/0000-0002-8030-2062
References
- 1. Han JY, Zhang CW. (2006) Toxicity study for acrylamide. Wei Sheng Yan Jiu 35: 513–515. [PubMed] [Google Scholar]
- 2. Parzefall W. (2008) Minireview on the toxicity of dietary acrylamide. Food and Chemical Toxicology 46: 1360–1364. [DOI] [PubMed] [Google Scholar]
- 3. Tardiff RG, Gargas ML, Kirman RC, et al. (2010) Estimation of safe dietary intake levels of acrylamide for humans. Food and Chemical Toxicology 48: 658–667. [DOI] [PubMed] [Google Scholar]
- 4. Haase NU, Grothe KH, Matthäus B, et al. (2012) Acrylamide formation and antioxidant level in biscuits related to recipe and baking. Food Additives and Contaminants. Part A, Chemistry, Analysis, Control, Exposure and Risk Assess 29: 1230–1238. [DOI] [PubMed] [Google Scholar]
- 5. Xie J, Terry KL, Poole EM, et al. (2013) Acrylamide hemoglobin adduct levels and ovarian cancer risk: A nested case-control study. Cancer Epidemiology, Biomarkers and Prevention 22: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kadawathagedara M, Botton J, de Lauzon-Guillian B, et al. (2018) Dietary acrylamide intake during pregnancy and postnatal growth and obesity: Results from the Norwegian Mother and Child Cohort Study (MoBa). Environment International 113: 325–334. [DOI] [PubMed] [Google Scholar]
- 7. Lehning EJ, Balaban CD, Ross JF, et al. Acrylamide neuropathy. III. Spatiotemporal characteristics of nerve cell damage in fore-brain. Neurotoxicology 2003; 24: 125–136. [DOI] [PubMed] [Google Scholar]
- 8. Bachmann M, Myers JE, Bezuidenhout BN. (1992) Acrylamide monomer and peripheral neuropathy in chemical workers. American Journal of Industrial Medicine 21: 217–222. [DOI] [PubMed] [Google Scholar]
- 9. Harry GJ, Morell P, Bouldin TW. (1992) Acrylamide exposure preferentially impairs axonal transport of glycoproteins in myelinated axons. Journal of Neuroscience Research 31: 554–560. [DOI] [PubMed] [Google Scholar]
- 10. LoPachin RM, Castiglia CM, Lehning E, et al. (1993) Effects of acrylamide on subcellular distribution of elements in rat sciatic nerve myelinated axons and Schwann cells. Brain Research 608: 238–246. [DOI] [PubMed] [Google Scholar]
- 11. Das J, Roy A, Sil PC. (2012) Mechanism of the protective action of taurine in toxin and drug induced organ pathophysiology and diabetic complications: A review. Food Function 3: 1251–1264. [DOI] [PubMed] [Google Scholar]
- 12. Toyoda A, Iio W. (2013) Antidepressant-like effect of chronic taurine administration and its hippocampal signal transduction in rats. Advances in Experimental Medicine and Biology 775: 29–43. [DOI] [PubMed] [Google Scholar]
- 13. Farah MH, Pan BH, Hoffman PN, et al. (2011) Reduced BACE1 activity enhances clearance of myelin debris and regeneration of axons in the injured peripheral nervous system. Journal of Neuroscience 31: 5744–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang B, Yang X, Gao X. (2010) Taurine protects against bilirubin-induced neurotoxicity in vitro. Brain Research 1320: 159–167. [DOI] [PubMed] [Google Scholar]
- 15. Wang GG, Li W, Lu XH, et al. Taurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic rats. Croatian Medical Journal 2013; 54: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leibinger M, Andreadaki A, Golla R, et al. (2017) Boosting CNS axon regeneration by harnessing antagonistic effects of GSK3 activity. Proceedings of the National Academy of Sciences of the United States of America 114: E5454–E5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu T, Lin W. (2016) Small-molecule GSK-3 inhibitor rescued apoptosis and neurodegeneration in anesthetics-injured dorsal root ganglion neurons. Biomedicine and Pharmacotherapy 84: 395–402. [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Wang E, Chen F, et al. (2013) Potential protective effects of oral administration of allicin on acrylamide-induced toxicity in male mice. Food and Function 4: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 19. Prasad SN, Muralidhara (2013) Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: Behavioral and biochemical evidence. Neurochemical Research 38: 330–345. [DOI] [PubMed] [Google Scholar]
- 20. Lehning EJ, Dyer KS, Jortner BS, et al. Axonal atrophy is a specific component of 2,5-hexanedione peripheral neuropathy. Toxicology and Applied Pharmacology 1995; 135: 58–66. [DOI] [PubMed] [Google Scholar]
- 21. Song F, Yu S, Zhang C, et al. (2008) The reversibility of neurofilaments decline induced by 2,5-hexanedione in rat nerve tissues. Biochemical Pharmacology 75: 737–744. [DOI] [PubMed] [Google Scholar]
- 22. LoPachin RM, Jortner BS, Reid ML, et al. (2003) Gamma-diketone central neuropathy: Quantitative morphometric analysis of axons in rat spinal cord white matter regions and nerve roots. Toxicology and Applied Pharmacology 193: 29–46. [DOI] [PubMed] [Google Scholar]
- 23. Gobrecht P, Leibinger M, Andreadaki A, et al. (2014) Sustained GSK3 activity markedly facilitates nerve regeneration. Nature Communications 5: 4561–4567. [DOI] [PubMed] [Google Scholar]
- 24. Leibinger M, Andreadaki A, Gobrecht P, et al. (2016) Boosting central nervous system axon regeneration by circumventing limitations of natural cytokine signaling. Molecular Therapy 102: 1712–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gobrecht P, Andreadaki A, Diekmann H, et al. (2016) Promotion of functional nerve regeneration by inhibition of microtubule detyrosination. The Journal of Neuroscience 36: 3890–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roberts DG, Johnsonbaugh HB, Spence RD, et al. (2016) Optical clearing of the mouse central nervous system using passive CLARITY. Journal of Visual Experiments 112: 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Passalacqua KD, Charbonneau ME, Donato NJ, et al. Anti-infective activity of 2-cyano-3-acrylamide inhibitors with improved drug-like properties against two intracellular pathogens. Antimicrobial Agents and Chemotherapy 2016; 60: 4183–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He Y, Tan D, Bai B, et al. (2017) Epigallocatechin-3-gallate attenuates acrylamide-induced apoptosis and astrogliosis in rat cerebral cortex. Toxicology Mechanisms and Methods 27: 298–306. [DOI] [PubMed] [Google Scholar]
- 29. Costa LG, Deng H, Calleman CJ, et al. (1995) Evaluation of the neurotoxicity of glycidamide, an epoxide metabolite of acrylamide: Behavioral, neurochemical and morphological studies. Toxicology 98: 151–161. [DOI] [PubMed] [Google Scholar]
- 30. Bondy SC, Tilson HA, Agrawal AK. (1981) Neurotransmitter receptors in brain regions of acrylamide-treated rats. II: Effects of extended exposure to acrylamide. Pharmacology, Biochemistry and Behavior 14: 533–537. [DOI] [PubMed] [Google Scholar]
- 31. González-Quevedo A, Obregón F, Urbina M, et al. Effect of chronic methanol administration on amino acids and monoamines in retina, optic nerve, and brain of the rat. Toxicology and Applied Pharmacology 2002; 185: 77–84. [DOI] [PubMed] [Google Scholar]
- 32. Chung MC, Malatesta P, Bosquesi PL, et al. (2012) Advances in drug design based on the amino acid approach: Taurine analogues for the treatment of CNS diseases. Pharmaceuticals (Basel) 5: 1128–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park HR, Kim MS, Kim SJ, et al. (2010) Acrylamide induces cell death in neural progenitor cells and impairs hippocampal neurogenesis. Toxicology Letters 193: 86–93. [DOI] [PubMed] [Google Scholar]
- 34. LoPachin RM, Ross JF, Lehning EJ. (2002) Nerve terminals as the primary site of acrylamide action: A hypothesis. Neurotoxicology 23: 43–59. [DOI] [PubMed] [Google Scholar]
- 35. Ye HB, Wang J, Zhang WT, et al. (2013) Taurine attenuates bilirubin-induced neurotoxicity in the auditory system in neonatal guinea pigs. International Journal of Pediatric Otorhinolaryngology 77: 647–654. [DOI] [PubMed] [Google Scholar]
- 36. Maiese K, Chong ZZ, Wang S, et al. (2012) Oxidant stress and signal transduction in the nervous system with the PI 3-K, Akt, and mTOR cascade. International Journal of Molecular Sciences 13: 13830–13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jia N, Sun Q, Su Q, et al. (2016) Taurine promotes cognitive function in prenatally stressed juvenile rats via activating the Akt-CREB-PGC1α pathway. Redox Biology 10: 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun X, Gu J, Chi M, et al. (2014) Activation of PI3K-Akt through taurine is critical for propofol to protect rat cardiomyocytes from doxorubicin-induced toxicity. Canadian Journal of Physiology and Pharmacology 92: 155–161. [DOI] [PubMed] [Google Scholar]
- 39. Liu Z, Song G, Zou C, et al. (2015) Acrylamide induces mitochondrial dysfunction and apoptosis in BV-2 microglial cells. Free Radical Biology and Medicine 84: 42–53. [DOI] [PubMed] [Google Scholar]
- 40. Song L, Wang J, Zhang W, et al. (2014) Effective suppression of acrylamide neurotoxicity by lithium in mouse. Neurochemical Research 39(11): 2170–2179. [DOI] [PubMed] [Google Scholar]
- 41. Iio W, Matsukawa N, Tsukahara T, et al. (2011) Regulation of taurine transport systems by protein kinase CK2 in mammalian cells. Cell Physiology and Biochemistry 28(6): 1099–1110. [DOI] [PubMed] [Google Scholar]
- 42. Lambert IH, Hansen DB. (2011) Regulation of taurine transport systems by protein kinase CK2 in mammalian cells. Cell Physiology and Biochemistry 28: 1099–1110. [DOI] [PubMed] [Google Scholar]
- 43. Garrido JJ, Simón D, Varea O, et al. GSK3 alpha and GSK3 beta are necessary for axon formation. FEBS Letters 2007; 581: 1579–1586. [DOI] [PubMed] [Google Scholar]
- 44. Chen Y, Weng J, Han D, et al. (2016) GSK3β inhibition accelerates axon debris clearance and new axon remyelination. American Journal of Translation Research 8: 85410–85420. [PMC free article] [PubMed] [Google Scholar]
- 45. Qian Y, Huang M, Guan T, et al. (2015) Maslinic acid promotes synaptogenesis and axon growth via Akt/GSK-3β activation in cerebral ischemia model. European Journal of Pharmacology 764: 298–305. [DOI] [PubMed] [Google Scholar]
- 46. Saijilafu Hur EM, Liu CM, et al. (2013) PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nature Communications 984: 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goold RG, Owen R, Gordon-Weeks PR. (1991) Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. Journal of Cell Science 112: 3373–3384. [DOI] [PubMed] [Google Scholar]
- 48. Lucas FR, Goold RG, Gordon-Weeks PR, et al. (1998) Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. Journal of Cell Science 111: 1351–1361. [DOI] [PubMed] [Google Scholar]
- 49. Pedrotti B, Islam K. (1996) Dephosphorylated but not phosphorylated microtubule associated protein MAP1B binds to microfilaments. FEBS Letters 388: 131–133. [DOI] [PubMed] [Google Scholar]
- 50. Ulloa L, Avila J, Diaz-Nido J. (1993) Heterogeneity in the phosphorylation of microtubule-associated protein MAP1B during rat brain development. Journal of Neurochemistry 61: 961–972. [DOI] [PubMed] [Google Scholar]