Abstract
Objective:
Several factors lead to memory loss, the most important of which is brain aging that is caused mostly by neuroinflammation and oxidative stress. The need of finding preventive treatments of memory impairment in elderly encouraged authors to assess the effect of Acorus calamus on memory loss, anxiety, and antioxidant indices on neuroinflammation rat models.
Materials and Methods:
Different fractions of A. calamus were prepared. The subject rats were grouped in 11 groups of 10 each. In the nine treated groups, the extract gavage began 1 week before intraperitoneal (i.p.) injection of lipopolysaccharide (LPS) and continued for 2 weeks after the last injection of LPS. Behavioral tests, including passive avoidance and elevated plus-maze (EPM) tests, were run on days 24, 25, and 26 and the subjects were sacrificed on the day after the last behavioral test, and their hippocampus was isolated to measure the oxidative stress markers.
Results:
Assessment of oxidative stress markers in hippocampus samples revealed that the amounts of endogenous antioxidant enzymes (superoxide dismutase, glutathione peroxidase, and total antioxidant activity) in the groups that received different fractions were less than their equivalent figures in LPS-control group, and levels of malondialdehyde (MDA) in treatment groups were less than MDA level in LPS-control group. Moreover, the treatment groups with different fractions of A. calamus revealed better performance compared to LPS-control group in shuttle-box test. In EPM test, the groups with different fractions revealed lower stress level in comparison with LPS-control group. The best performance in memory test and the lowest level of stress in EPM was observed in the group with aqueous fraction at 600 mg/kg dose, and the least figures of oxidative stress markers were of the group with aqueous fraction at 600 mg/kg dose.
Conclusion:
The oral administration of different fractions of A. calamus, especially aqueous fraction, prevented from memory deficits and stress through controlling oxidative stress and inflammation processes.
Keywords: Acorus calamus L., memory impairment, neuroinflammation, oxidative stress, stress and anxiety
Introduction
Neuroinflammation is a neurological disorder that could lead to neurodegenerative diseases. Alzheimer's disease is one of the most common neurodegenerative diseases and one of the most common causes of mental decline in healthy people. It is the fourth leading cause of death in developed countries after heart disease, cancer, and trauma. Considering the statistically significant increase in patients with Alzheimer's disease, it is estimated that the number of affected people will triple by 2050.[1] Pathological signs of Alzheimer's disease include accumulation of beta-amyloid plaques in and out of the neurons and the formation of neurofibrillary tangles within the neuronal cell made by Tau proteins.[2]
Oxidative stress is involved in the initial levels of Alzheimer's pathological cascade and several studies have found that oxidative stress compared to Alzheimer's pathological symptoms are premier and considered as an upstream cause.[3] There is also evidence which proposes that beta-amyloid and Tau protein accumulation constitute compensatory response to oxidative stress.[4] It is found that oxidative stress causes behavioral and memory impairment.[5,6] Moreover, studies have also revealed that oxidative stress and neuroinflammation induce stress and anxiety disorder.[7,8] In addition, in some studies, neuroinflammation is considered as a leading cause of initiating pathological symptoms of Alzheimer's disease (beta-amyloid and Tau protein).[9,10,11,12,13,14] Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and antioxidant defense system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Antioxidant defense system includes several enzymes that catalyze reactions to neutralize free radicals and reactive oxygen species. Superoxide dismutases (SODs) and glutathione peroxidase (GPx) constitute endogenous antioxidant enzymes. SODs are a class of enzymes that catalyze the superoxide anion breakdown into oxygen and hydrogen peroxide and are present in almost all aerobic cells and in extracellular fluids. GPx is a selenium-containing antioxidant enzyme that effectively reduces H2O2 and lipid peroxides to water and lipid alcohols, respectively. The lipid peroxidation results in the production of toxic aldehydes; one of the most toxic ones, the malondialdehyde (MDA), is a colorless compound and a final product of fatty oxide decomposition that increases the permeability of the blood–brain barrier and is currently considered as an indicator of lipid peroxidation. Therefore, the extent of damage to cellular lipids caused by oxidation can be determined by measuring the amount of MDA.[15]
Neural tissue due to its high amounts of phospholipid and high level of unsaturated fatty acids together with high metabolism is very sensitive to oxidative stress-induced damage. It is assumed that any increase in oxidative stress constitute one of the major factors affecting brain structure and function.[16] Considering that processes of inflammation and oxidative stress are pathophysiologically very close occurrences,[17] targeting these two processes in a simultaneous manner can be considered as a preventive measure and a therapeutic procedure for the Alzheimer's disease.
“Acorus calamus L.” (Vej, or yellow Lily) is a wild plant growing along the rivers. Its roots decoction is consumed in Iranian ethnomedicine as a memory enhancer. This plant was referred to as an effective medication for increasing memory in popular sources of Iranian traditional medicine.[18] There exits articles suggesting this plant for treatment of Alzheimer's disease.[19,20] A. calamus extract contains various compounds such as flavonoids, alpha-asarone, beta-asarone, alkaloids, mucilages, lectins, phenols, quinones, saponins, etc.[21]
This plant has been consuming to treat insomnia, melancholia, epilepsy, hysteria, remittent fevers and neurosis.[22,23] Some studies revealed antioxidant properties of this plant.[22,24,25] Due to the presence of alpha- and beta-asarone, this plant is endowed with antiinflammatory properties which reduce inflammatory cytokines.[26,27]
Attempt is made in the study to assess the preventive and protective features of the traditional medicinal plant on memory loss, anxiety, and oxidative stress markers.
Materials and Methods
Plant material and extraction
A. calamus dried roots (Agire torki in Persian) were collected from traditional medicine bazar in Isfahan and confirmed by Department of the Pharmacognosy, Pharmacy Faculty, Isfahan University of Medical Sciences, Iran. The dry roots were chopped into small pieces, powdered, and a volume of 4 kg was added in a maceration tank of 20 L ethanol: water (70:30) and kept for 3 days. The filtrated extract was concentrated by a rotary evaporator at 40°C at reduced pressure of 70 mbar. The dried extract of 237 g equal to 6% (w/w) of dried plant materials was kept in refrigerator before consumption. By applying a separating funnel, a total extract of 140 g was partitioned between aqueous and ethyl acetate phases. Both parts were separated and concentrated as aqueous and ethyl acetate fractions.
Extract standardization by determination of total polyphenols
Total phenolic content was assessed by a colorimetric method named Folin-Ciocalteau and quantified as the mg of gallic acid equivalents (GAEs).[28] Calibration standard curve was plotted against different concentrations of gallic acid. For illustration of calibration curve and determination of phenolic content, 20 μL of each concentration of standard solution was mixed with 1.58 mL water and 100 μL of Folin–Ciocalteau reagent. After 10 min shaking, 300 μL saturated sodium bicarbonate was added to the mixture. After 2 h, the absorbance was measured at 765 nm through spectrophotometry. After plotting calibration curve, total extract and blank were analyzed as mentioned above in triplicates.[29]
Animals
In this study, 110 adult, male Wistar rats weighing 250–200 g were prepared from the laboratory of experimental animals of Pasteur Institute, Tehran. They were kept in a room at 20°C, and 40–50% humidity, subject to 12 h dark/light conditions. All animals were freely allowed to consume standard water and food on their own. One week before the beginning of the study, all animals were transferred to the lab. The study was run in the animal house of the medical school of Isfahan University of Medical Sciences.
Experimental method
Rats were randomly grouped in 11 groups 10 in each. The first group includes the rats which are treated by vehicle (distilled water as a solvent of the fractions) and known as Sham-control. The second group known as lipopolysaccharide (LPS)-control includes intraperitonially (i.p.)injected rats at 250 μg/kg dose for 7 days, which are treated by the vehicle. Other groups received LPS, and were treated with aqueous, ethanolic, and ethyl acetate fractions at different doses of 200, 400, and 600 mg/kg. In the treated groups, the extract gavage began 1 week before i.p. injection of LPS and continued until 2 weeks after the last injection of LPS. The behavioral tests were performed on days 24, 25, and 26 and subjects were sacrificed on the day after the last behavioral test, and their hippocampus was isolated to measure the oxidative stress markers.
LPS-induced neuroinflammation model
Neuroinflammation model was induced by the i.p. injection of LPS, which leads to cognitive and behavioral disorders similar to those observed in Alzheimer's patient. This i.p. injection can lead to progressive neurodegenerative diseases, as a result of neuroinflammatory effect of this substance.[30,31] Some studies revealed that LPS-induced neuroinflammation can enhance beta-amyloid generation and can accelerate the tau phosphorylation.[31,32]
In this study, animals were transferred to the workroom one week before the study. LPS was prepared daily in glass vials in an amount of 250 μg/kg per body weight. Sterile saline solution 0.9% was added to each 0.5 mL LPS vial. In seven consecutive days, LPS was injected i.p. to normal animals at 250 dose μg/kg per day.
Passive avoidance: Behavior test
The shuttle box was equipped with two dark and light cubicles separated by a space separator. Rat was placed inside the light room; after that the separator pulled up 10 s later. After the rat enters the dark room, the separator was pushed down and an electric shock of 1 mA was applied to its foot for 3 s. Then the animal was removed from the dark room and placed in a cage. The second step is to evaluate the rat memory. 24 h after receiving the electric shock, once more it was placed inside the light room and the separator was pulled up. Its move toward the dark room takes time, which was considered as a delayed time or transfer latency, and the maximum time which was considered for each step of this test was 300 s. A longer delayed time for the entry into the darkroom in the second day indicates that animal has a good memory and can remember that dark room could be dangerous.[33]
Elevated plus-maze: Behavior test
The elevated plus maze (EPM) consists of two open arms and two closed arms opposite each other, connected by a central plate,[34] with a setup 50 cm from the lab ground. The experiment was run in a dark and silent room. To begin the test, the mouse is placed on the central portion of the device in a gentle and cautious manner.[35] The number of entries into the open arm and the amount of elapsed time in the open arms are recorded for 5 min. When a rat spends more time in open arms and has more number of entries to open arms compared to the other rat, then it indicates that it has a lower level of stress. It is worth mentioning that the criterion for entering the open arms is the full crossing of the four feet of rat, from the junction of central portion to open arm. The appliance was cleaned with clean cloth and alcohol after each test.
Preparing tissue samples
To sacrifice, a rat was anesthetized through ketamine i.p. injection (100 mg/kg). Next, its head was separated and the brain was extracted immediately and the hippocampus was removed and rinsed with 0.9% cold sterile saline. The isolated hippocampus was immediately frozen in liquid nitrogen and stored at −80°C. In order to prepare the homogeneous component in each sample, 1.15% KCl solution (w/v 1:10) was added to the tissue. The hippocampal tissue was prepared and then centrifuged in 20,000g by means of homogeneous mechanical homogenizer for 10 min at 4°C. The supernatant was extracted for further chemical analysis. Total protein content in supernatant was measured through the Bradford method, where bovine albumin serum was used as a standard.[36]
Assessing glutathione peroxidase and superoxide dismutase
To assess cytosolic enzyme activity, the hippocampus samples were homogenized in 1.15% KCl solution. GPx enzyme activity was determined through the Paglia and Valentine method and measured by the Randox kit (UK). In this method, the oxidation of glutathione (GSH) is catalyzed through GPx. In presence of glutathione reductase and nicotinamide adenine dinucleotide phosphate (NADPH), the oxidized glutathione is immediately converted into its reduced form through concomitant oxidation of NADPH into NADP+. A decrease in absorbance at 340 nm is measured.[37] Tissue SOD was measured through Woolliams method by Ransod kit. In this method, xanthine and xanthine oxidase are applied in order to generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to yield a red formazan dye. The SOD activity is then determined by the degree of inhibition of this reaction. One unit of SOD causes a 50% inhibition of INT reduction rate subject to conditions of the assay.[38]
Assessing total antioxidant activity
Total antioxidant activity (TAC) was assessed through ABTS assay based on Miller's method. In this method, where ABTS (2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]) is incubated with a peroxidase (metmyoglobin) and H2O2 to produce the radical cation ABTS. This has a relatively stable blue-green color, which is measured at 600 nm. Antioxidants in the added sample cause suppression of this color production to a degree which is proportional to their concentration.[39]
Malondialdehyde assessment
Malondialdehyde (MDA) was assessed based on its reaction with thiobarbituric acid, followed by adding normal butanol as an extractor and its absorbance was measured at 532 nm through spectrophotometry. The absorbance rate was compared with standard curve.[40]
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) and repeated measures. Data were presented as mean ± standard deviation (SD). In all calculations, the significance level of the difference was P < 0.05.
Results
Extract standardization through total phenolics measurement
As observed in Figure 1, the calibration curve was plotted by linear regression in the range of 0.5–5 μg/mL. The regression equation was expressed as: y = 0.065x + 0.0167. y-axis shows absorption where x-axis shows GAE phenol concentration in sample (μg/mL) with the correlation factor of r2 = 0.9921. The content of total extract is 6.71 ± 0.64% GAE (mg/100 mg).
Figure 1.
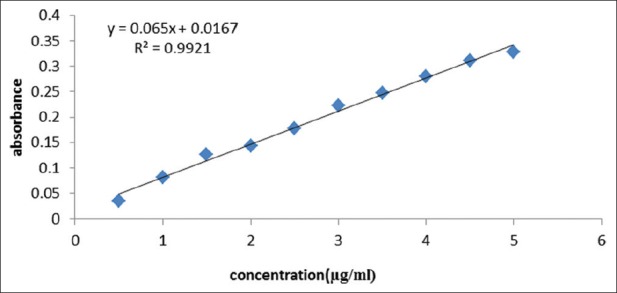
Calibration curve of gallic acid for Acorus Calamus L
Passive avoidance behavioral tests
As observed in Figure 2, the one-way ANOVA did not reveal any significant difference between transfer latency in the experimental groups on the first day (F = 0.53, P = 0.85). However, there was a significant difference between the groups in the second day (F = 3.81, P = 0.002). Repeated-measures ANOVA indicated that mean of transfer latency in the first day is significantly different from second day (P = 0.000). Also, the average of the second day (198.13 ± 12.04 s) is higher than the average in treatment groups with fractions on the first day (11.00 ± 1.02 s). The highest figure of transfer latency was observed in the group that received aqueous fraction at 600 mg/kg dose, indicating the best performance with the least memory impairment.
Figure 2.
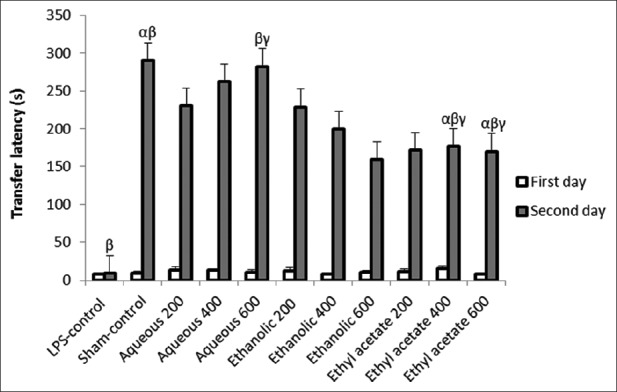
Effect of total extract, aqueous, and ethyl acetate fractions of A. calamus on transfer latency time. Through Dunnett's test, significant differences were observed among following groups: (1) α indicating the Sham-control group with groups of ethyl acetate at 400 and 600 mg/kg doses. (P < 0.05), (2) β indicating the LPS-control group with Sham-control group, aqueous group at 600 mg/kg dose, and groups of ethyl acetate at 400 and 600 mg/kg doses (P < 0.001), and (3) γ indicating the group of aqueous at dose of 600 mg/kg with groups of ethyl acetate at 400 and 600 mg/kg doses (P < 0.05)
Elevated plus-maze behavioral tests
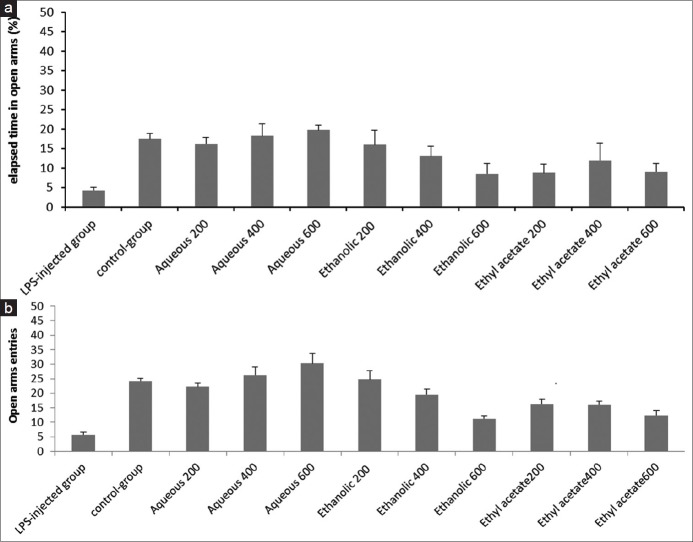
As observed in Figure 3a, one-way ANOVA test revealed significant differences in elapsed time by the groups treated with the total extract and fractions, compared to the LPS-control group. The most elapsed time in the open arms was observed in groups that received aqueous fraction at 600 and 400 mg/kg doses. As observed in Figure 3b, the groups treated with the different fractions made more entries to open arms in comparison with LPS-control group. A significant difference was observed between LPS-control group and groups with aqueous fraction at 200 and 600 mg/kg doses (P = 0.000). The highest entry numbers in open arms and time spent there were observed in the group with aqueous fraction at 600 mg/kg dose, indicating the least level of stress against the other experimental groups.
Figure 3.
(a) Effect of total extract, aqueous, and ethyl acetate fractions of A. calamus on the percentage of time spent in the open arms in EPM tests. Tukey's test revealed that there are significant differences among LPS-control group and groups of aqueous fractions at 200 and 600 mg/kg doses (P= 0.002). (b) Effect of total extract, aqueous, and ethyl acetate fractions of A. calamus on the numbers of open arm entries in EPM during 5 min. By applying Tukey's test, significant differences were seen between the following groups (P < 0.05): (1) Δ indicating the LPS-control group with all groups except the group of total extract at 600 mg/kg dose, (2) ε indicating the Sham-control group with groups of ethyl acetate at 600 mg/kg dose and total extract at a dose of 600 mg/kg, (3) θ indicating the group of aqueous at 200 mg/kg dose with groups of total extract at 600 mg/kg dose and ethyl acetate at 600 mg/kg dose, (4) λ indicating the group of aqueous at 600 mg/kg dose with groups of total extract at 400 and 600 mg/kg doses and the groups of ethyl acetate at 200, 400, and 600 mg/kg doses, and (5) ϕ indicating the group of aqueous at a dose of 400 mg/kg with the group of ethyl acetate at 200 mg/kg dose
Antioxidant activity
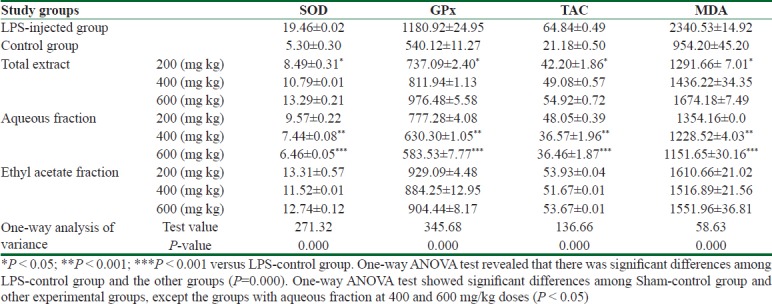
As observed in Table 1, the LPS-control group gained the highest figures of oxidative stress biomarkers (SOD, GPx, TAC, and MDA), compared to Sham-control group. In the treatment group with different fractions, the levels of oxidative stress markers are close to equivalent figures in Sham-control group. The lowest numbers in oxidative stress markers' levels are related to the group with aqueous fraction of 600 mg/kg dose.
Table 1.
Effect of total extract, aqueous and ethyl acetate fractions of Acorus calamus on endogenous antioxidant enzymes including superoxide dismutase (SOD), and glutathione peroxidase (GPX), malondialdehyde (MDA) as lipid peroxidation marker, and total antioxidant capacity (TAC) in hippocampus of rats
Discussion
Many studies have suggested that neuroinflammation is the cause of pathological signs (beta-amyloid accumulation and Tau protein) in Alzheimer's disease.[9,10,11,12,13,14] In this context, activated phagocytosis cells release the reactive species at the site of inflammation, causing cellular damage and oxidative stress. Oxidative stress acts as an endogenous stimulus, inducing an in situ inflammation process. Studies indicated that in pathological conditions, oxidative stress and inflammation processes may occur consequently. In other words, when one of them (oxidative stress and inflammation) appears, the possibility of occurrence of another process is intensified.[17] Regarding the above mentioned, inhibition and control of inflammation and oxidative stress processes in a simultaneous manner can prevent pathological occurrences associated with Alzheimer's disease, such as beta-amyloid and Tau protein accumulation, lipid peroxidation, nerve cell membrane destruction, and nerve cell death.
Several studies claimed that i.p. injection of LPS causes learning and memory impairment. Likewise, here it is revealed that rats in LPS-control group developed memory loss.
It should be noted that in LPS-control group endogenous antioxidant enzymes (SOD, GPx, and MDA) increased significantly compared to normal values in Sham-control group; consequently, these changes could be considered a defensive reaction as a result of processes of inflammation and oxidative stress. Moreover, the amount of MDA was more than twice in LPS-control group compared with that of Sham-control group. Due to the high amount of MDA, high level of lipid peroxidation and cell damage is evident. It is considered that an increase in endogenous antioxidant enzymes could not compensate destructive effects of oxidative stress.
The assessment on MDA indicated its low levels in groups treated with fractions; especially the group with aqueous fraction at 600 mg/kg dose allocated the least amount of MDA to itself. The levels of lipid peroxidation and cell damage in groups which received fractions were lower, compared with that of LPS-control group, indicating that the pretreatment method could prevent inflammation and oxidative stress processes can damage cells. Furthermore, the low levels of endogenous antioxidant enzymes in groups treated with fraction against LPS-control group indicate that antioxidant defense system in treatment groups was less activated compared to that of LPS-control group.
In the treated groups with extracts, the figures of oxidative stress markers were close to the normal amounts in the Sham-control group. Likewise, Manikandan et al. also revealed that the ethanolic and ethyl acetate extracts of A. calamus effectively inhibit stress-induced changes in endogenous antioxidant enzymes system and this effect is achieved by sweeping free radicals and modulating levels of enzymes antioxidant system.[41] Muthuraman et al. claimed that the hydroalcoholic extract of A. calamus improved the behavioral and biochemical changes induced in the painful environmental neuropathy model and they thought that the antioxidant and antiinflammatory effects of this extract play the main role in this context.[42] Shukla et al. showed that the hydroalcoholic extract of A. calamus has a neuroprotective effect on ischemic rats (through central cerebrospinal fluid obstruction), and this effect was due to the reduction of lipid peroxidation and modulation of endogenous antioxidant enzymes.[43] Shukla et al. also revealed that the hydroalcoholic extract of A. calamus against neurotoxicity has a protective effect and improves behavioral changes and modulates the enzymes of endogenous antioxidants such as glutathione S-transferase.[44]
The memory test in this study revealed that the groups treated with fractions, especially group with aqueous fraction at 600 mg/kg dose, outperform LPS-control group; therefore, the memory impairment in groups that received fractions was noticeably less compared with that of LPS-control group.
Moreover, Shin et al. revealed that alpha-asarone, as a major component of the A. calamus, improves memory in animals with induced memory impairment.[26] Furthermore, Geng et al. showed that beta-asarone, as an important component of A. calamus, improves cognitive function in rats with memory impairment with intrahippocampal beta-amyloid injection.
Some studies suggested that stress and anxiety could be considered as a result of the inflammation and oxidative stress processes.[7,8] Likewise, stress test in this test revealed the low levels of stress in groups treated with fraction in comparison to LPS-control group, which could be considered due to presence of antiinflammatory and antioxidant substances in A. calamus. In addition, several studies have suggested that extract of A. calamus and its major compounds have the antiinflammatory effect through reducing the preinflammatory cytokines.[26,45,46,47] Due to the close correlation between the inflammatory and the oxidative stress processes, weakness in one would have a direct effect on the other. Because this plant (A. calamus) is endowed with antioxidant, antiinflammatory, and antiacetylcholine esterase properties, it can affect the multiple cellular pathways, leading to Alzheimer's pathogenesis. This plant also acts more successfully than the available chemical drugs which affect only on one cellular pathway. In addition, natural substances have less side effects in comparison to synthetic drugs.
Conclusion
The results of this study revealed that different fractions of A. calamus L. are dose-dependently effective in preventing memory impairment and stress development through controlling oxidative stress and inflammation.
Ethical considerations
The study was approved by the Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran. Ethical issues (including plagiarism, misconduct, data fabrication, falsification, double publication or submission, redundancy) have been completely observed by the authors.
Financial support and sponsorship
Nil.
Conflict of interests
There are no conflicts of interest.
Acknowledgements
This paper is part of thesis of Amir Mokhtarian submitted in partial fulfillment of the requirements for the degree of Master in Anatomical Science. We are grateful to Neda Abrishami in statistical office in Isfahan Faculty of Medicine for help in statistical analysis and interpretation of data.
References
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–83. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Nunomura A, Perry G, Pappolla MA, Friedland RP, Hirai K, Chiba S, et al. Neuronal oxidative stress precedes amyloid-β deposition in Down syndrome. J Neuropathol Exp Neurol. 2000;59:1011–7. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- 4.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:631–41. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 5.Fukui K, Omoi NO, Hayasaka T, Shinnkai T, Suzuki S, Abe K, et al. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann N Y Acad Sci. 2002;959:275–84. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 6.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Wang M, Guo YY, Sun T, Li YJ, Yang Q, et al. Systemic inflammation induces anxiety disorder through CXCL12/CXCR4 pathway. Brain Behav Immun. 2016;56:352–62. doi: 10.1016/j.bbi.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Salim S, Asghar M, Taneja M, Hovatta I, Chugh G, Vollert C, et al. Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain Res. 2011;1404:63–71. doi: 10.1016/j.brainres.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B. TNFα plus IFNγ induce the production of Alzheimer β-amyloid peptides and decrease the secretion of APPs. FASEB J. 1999;13:63–8. doi: 10.1096/fasebj.13.1.63. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, et al. Interferon-γ and tumor necrosis factor-α regulate amyloid-β plaque deposition and β-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007;170:680–92. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Guo JT, Yu J, Grass D, de Beer FC, Kindy MS. Inflammation-dependent cerebral deposition of serum amyloid a protein in a mouse model of amyloidosis. J Neurosci. 2002;22:5900–9. doi: 10.1523/JNEUROSCI.22-14-05900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, et al. Anti-inflammatory drug therapy alters β-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. 2003;23:7504–9. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eikelenboom P, Zhan SS, van Gool WA, Allsop D. Inflammatory mechanisms in Alzheimer's disease. Trends Pharmacol Sci. 1994;15:447–50. doi: 10.1016/0165-6147(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 15.Garcia YJ, Rodríguez-Malaver AJ, Peñaloza N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J Neurosci Methods. 2005;144:127–35. doi: 10.1016/j.jneumeth.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Tasset I, Agüera E, Sánchez-López F, Feijóo M, Giraldo AI, Cruz AH, et al. Peripheral oxidative stress in relapsing–remitting multiple sclerosis. Clin Biochem. 2012;45:440–4. doi: 10.1016/j.clinbiochem.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Biswas SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev 2016. 2016 doi: 10.1155/2016/5698931. 5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmadian-Attari MM, Ahmadiani A, Kamalinejad M, Dargahi L, Shirzad M, Mosaddegh M. Treatment of Alzheimer's disease in Iranian traditional medicine. Iranian Red Crescent Medical Journal. 2015;17:1. doi: 10.5812/ircmj.18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dastmalchi K, Dorman HD, Vuorela H, Hiltunen R. Plants as potential sources for drug development against Alzheimer's disease. Int J Biomed Pharm Sci. 2007;1:83–104. [Google Scholar]
- 20.Nandakumar S, Menon S, Shailajan S. A rapid HPLC-ESI-MS/MS method for determination of β-asarone, a potential anti-epileptic agent, in plasma after oral administration of Acorus calamus extract to rats. Biomed Chromatogr. 2013;27:318–26. doi: 10.1002/bmc.2794. [DOI] [PubMed] [Google Scholar]
- 21.Joshi RK. Acorus calamus Linn.: phytoconstituents and bactericidal property. World J Microbiol Biotechnol. 2016;32:164. doi: 10.1007/s11274-016-2124-2. [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Li WG, Zhang XB, Wang L, Xu TL, Wu D, et al. Alpha-asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology. 2013;65:1–1. doi: 10.1016/j.neuropharm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Sharma V, Singh I, Chaudhary P. Acorus calamus (The Healing Plant): A review on its medicinal potential, micropropagation and conservation. Nat Prod Res. 2014;28:1454–66. doi: 10.1080/14786419.2014.915827. [DOI] [PubMed] [Google Scholar]
- 24.Parki A, Chaubey P, Prakash O, Kumar R, Pant AK. Seasonal Variation in Essential Oil Compositions and Antioxidant Properties of Acorus calamus L. Accessions Medicines. 2017;4:81. doi: 10.3390/medicines4040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manikandan S, Srikumar R, Parthasarathy NJ, Devi RS. Protective effect of Acorus calamus LINN on free radical scavengers and lipid peroxidation in discrete regions of brain against noise stress exposed rat. Biol Pharm Bull. 2005;28:2327–30. doi: 10.1248/bpb.28.2327. [DOI] [PubMed] [Google Scholar]
- 26.Shin JW, Cheong YJ, Koo YM, Kim S, Noh CK, Son YH, et al. α-Asarone ameliorates memory deficit in lipopolysaccharide-treated mice via suppression of pro-inflammatory cytokines and microglial activation. Biomol Ther. 2014;22:17. doi: 10.4062/biomolther.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim HW, Kumar H, Kim BW, More SV, Kim IW, Park JI, et al. β-Asarone (cis-2, 4, 5-trimethoxy-1-allyl phenyl), attenuates pro-inflammatory mediators by inhibiting NF-κB signaling and the JNK pathway in LPS activated BV-2 microglia cells. Food Chem Toxicol. 2014;72:265–72. doi: 10.1016/j.fct.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Harborne AJ. Phytochemical methods a guide to modern techniques of plant analysis. Springer Science & Business Media; 1998 [Google Scholar]
- 29.Singleton VL, Orthofer R, Lamuela-Raventós RM. Methods in Enzymology. Vol. 299. Academic Press; 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent; pp. 152–78. [Google Scholar]
- 30.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee DC, Rizer J, Selenica ML, Reid P, Kraft C, Johnson A, et al. LPS-induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J Neuroinflammation. 2010;7:56. doi: 10.1186/1742-2094-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishiyama T, Tokuda K, Ishibashi T, Ito A, Toma S, Ohno Y. Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur J Pharmacol. 2007;572:160–70. doi: 10.1016/j.ejphar.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 34.Naplekova PL, Narkevich VB, Voronina TA, Kudryashov NV, Kostochka LM, Kudrin VS. STUDYING ANXIOLYTIC AND ANTIDEPRESSANT PROPERTIES OF 2, 2, 6, 6-TETRAMETHYLPIPERIDONE DERIVATIVE. Eksperimental'nia i klinicheskaia farmakologiia. 2016;79:3–6. [PubMed] [Google Scholar]
- 35.Holmes A, Parmigiani S, Ferrari PF, Palanza P, Rodgers RJ. Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiol Behav. 2000;71:509–16. doi: 10.1016/s0031-9384(00)00373-5. [DOI] [PubMed] [Google Scholar]
- 36.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–9. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Transl Res. 1967;70:158–69. [PubMed] [Google Scholar]
- 38.Woolliams JA, Wiener G, Anderson PH, McMurray CH. Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci. 1983;34:253–6. [PubMed] [Google Scholar]
- 39.Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci. 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 40.Kaya H, Sezik M, Ozkaya O, Dittrich R, Siebzehnrubl E, Wildt L. Lipid peroxidation at various estradiol concentrations in human circulation during ovarian stimulation with exogenous gonadotropins. Horm Metab Res. 2004;36:693–5. doi: 10.1055/s-2004-826018. [DOI] [PubMed] [Google Scholar]
- 41.Manikandan S, Srikumar R, Parthasarathy NJ, Devi RS. Protective effect of Acorus calamus LINN on free radical scavengers and lipid peroxidation in discrete regions of brain against noise stress exposed rat. Biol Pharm Bull. 2005;28:2327–30. doi: 10.1248/bpb.28.2327. [DOI] [PubMed] [Google Scholar]
- 42.Muthuraman A, Singh N. Attenuating effect of Acorus calamus extract in chronic constriction injury induced neuropathic pain in rats: An evidence of anti-oxidative, anti-inflammatory, neuroprotective and calcium inhibitory effects. BMC Complement Altern Med. 2011;11:24. doi: 10.1186/1472-6882-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shukla PK, Khanna VK, Ali MM, Maurya R, Khan MY, Srimal RC. Neuroprotective effect of Acorus calamus against middle cerebral artery occlusion–induced ischaemia in rat. Hum Exp Toxicol. 2006;25:187–94. doi: 10.1191/0960327106ht613oa. [DOI] [PubMed] [Google Scholar]
- 44.Shukla PK, Khanna VK, Ali MM, Maurya RR, Handa SS, Srimal RC. Protective effect of Acorus calamus against acrylamide induced neurotoxicity. Phytother Res. 2002;16:256–60. doi: 10.1002/ptr.854. [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Han TH, Lee SG. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocyte HaCaT cells. J Ethnopharmacol. 2009;122:149–56. doi: 10.1016/j.jep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Lim HW, Kumar H, Kim BW, More SV, Kim IW, Park JI, et al. β-Asarone (cis-2, 4, 5-trimethoxy-1-allyl phenyl), attenuates pro-inflammatory mediators by inhibiting NF-κB signaling and the JNK pathway in LPS activated BV-2 microglia cells. Food Chem Toxicol. 2014;72:265–72. doi: 10.1016/j.fct.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Shi GB, Wang B, Wu Q, Wang TC, Wang CL, Sun XH, et al. Evaluation of the wound-healing activity and anti-inflammatory activity of aqueous extracts from Acorus calamus L. Pak J Pharm Sci. 2014;27:91–5. [PubMed] [Google Scholar]