Abstract
Duchenne muscular dystrophy (DMD), the most common lethal heritable childhood disease, is caused by mutations in the DMD gene that result in the absence of functional dystrophin protein. Exon skipping mediated by antisense oligonucleotides has recently emerged as an effective approach for the restoration of dystrophin, and skipping of exon 51 of DMD has received accelerated approval. Identifying antisense sequences that can provide the highest possible skipping efficiency is crucial for future clinical applications. Herein, we systematically tested two-step antisense oligonucleotide walks along human DMD exon 53 in order to define sequence-dependent effects of antisense oligonucleotide binding sites in human rhabdomyosarcoma cell lines. The first rough whole-exon 53 walk enabled the identification of a target region, and a second walk of this region was used to determine an optimal antisense oligonucleotide sequence (NS-065/NCNP-01) for exon 53 skipping. This oligonucleotide strongly promoted exon 53 skipping in a dose-dependent manner during pre-mRNA splicing in rhabdomyosarcoma and DMD patient-derived cells, and it restored dystrophin protein levels in patient-derived cells. NS-065/NCNP-01, a phosphorodiamidate morpholino oligomer, appears to be a promising candidate for treating exon 53 skipping, and it is potentially applicable to 10.1% of patients with DMD.
Keywords: Duchenne muscular dystrophy, dystrophin, exon 53, exon skipping, antisense therapeutics, morpholino
Introduction
Duchenne muscular dystrophy (DMD) affects 4.78 per 100,000 males and is the most common muscular disorder.1 It is a severe disease with onset in early childhood. Ambulation difficulty occurs in most cases by 12 years of age, leading to death in the late teens or early 20s owing to respiratory failure or cardiac dysfunction. However, significant research attention to respiratory care and various forms of assisted ventilation have extended the life expectancy to the late 20s and beyond.2 Similar to Becker muscular dystrophy (BMD), DMD is an X-linked recessive allelic disorder caused by mutations in the DMD gene, which encodes the dystrophin protein. The symptoms of BMD are typically milder than those of DMD because, in BMD, the DMD gene reading frame is not disrupted by the mutation, allowing the expression of a partially functional dystrophin protein. A novel and promising approach to the treatment of DMD is that of exon skipping as mediated by antisense oligonucleotides (AONs), in which splicing is modified to restore the DMD gene reading frame, thereby inducing the expression of a partially functional BMD-type dystrophin protein.3 Consequently, exon skipping is expected to cause the conversion of DMD symptoms to milder ones, similar to those of BMD.
The exon-skipping approach has been successfully tested in mice and dogs, and it is currently under clinical trial in patients with DMD. Given that different patients show different mutations in DMD, antisense nucleic acid therapeutic agents need to be specifically designed for each type of mutation. Exon 51 skipping is applicable to the largest proportion of patients with DMD. To date, two AON drugs for DMD exon 51 skipping have been developed. Drisapersen, a 2′-O-methyl phosphorothioate AON, and eteplirsen, a morpholino AON, have shown promising results in clinical trials,4 and a new drug application (NDA) has been filed for each drug with the US Food and Drug Administration (FDA).5 However, in January 2016, the FDA rejected the drisapersen NDA owing to insufficient clinical efficacy and concerns regarding its safety profile.6 Moreover, in April 2016, the FDA expressed negative opinions on eteplirsen approval in the advisory committee. The FDA raised questions about the efficacy of eteplirsen and the appropriateness of its clinical study design.7
However, eteplirsen received accelerated approval in September 2016 based on an increase in dystrophin levels in skeletal muscles observed in some patients treated with eteplirsen, making it the first and only FDA-approved drug for treating DMD. The clinical benefit of eteplirsen has not been established, and continued approval may be contingent upon verification of a clinical benefit in confirmatory trials.8 Regardless of its limitations, the development and subsequent accelerated FDA approval of eteplirsen was a landmark event for DMD therapy. While the discussions during the regulatory review did not deny the concept of exon skipping, the importance of thorough evaluations of AON potency at an early stage of development was highlighted, including mRNA-skipping activity and the recovery effect of the dystrophin protein, which are the underlying mechanisms of exon-skipping therapy. Thus, we believe that new approaches in AON sequence design and evaluation are essential for the resultant AON development for DMD treatment.
Exon 53 skipping is applicable to patients with deletions in the DMD gene consisting of exons 45–52, 47–52, 48–52, 49–52, 50–52, or exon 52 alone. Approximately 10.1% of patients with DMD may be treated by exon 53 skipping.9 To date, approximately 30 AONs for exon 53 skipping of the DMD gene have been reported. These sequences were designed on the basis of the results of in silico analyses for predicting RNA structure,10, 11, 12 exonic splicing enhancers,13 or hybridization.12 Because these analyses are not by themselves adequate to predict the bioactivity of the designed AONs, empirical studies are still needed.14, 15 Therefore, in this study, we performed a non-clinical pharmacology study of NS-065/NCNP-01, a phosphorodiamidate morpholino oligomer (PMO), in human rhabdomyosarcoma (RD) cells and DMD patient-derived cells. Based on the results of the present study, exon 53-skipping clinical trials for patients with DMD have begun.
Results
Screening of Sequences Targeting Exon 53 of the Human DMD Gene
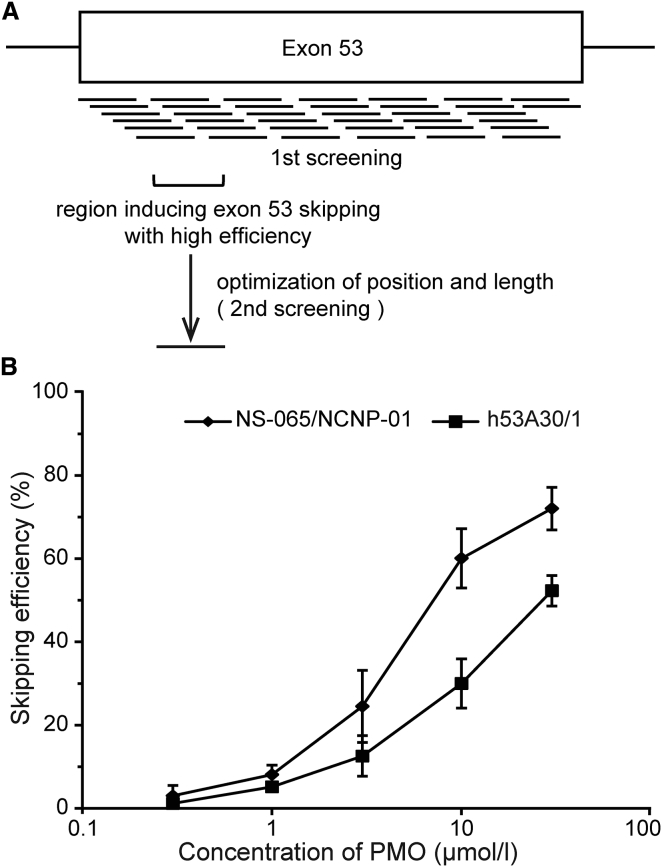
To determine the region of exon 53 in the human DMD gene associated with the highest skipping efficiency, exon 53 oligonucleotides were screened in two stages, and their exon-skipping activity was measured in RD cells (Figure 1A). In the first stage, 2′-O-methyl phosphorothioate oligonucleotides complementary to exon 53 were synthesized as an overlapping series of 38 25-nt oligomers. AONs of 25–31 nt are generally more effective at inducing exon skipping than are their shorter counterparts.16 These oligomers provided coverage of this 212-nt exon in 5-nt increments from the 3′ end to the 5′ end (positions 1–210). The highest skipping efficiency (as defined in the Materials and Methods on RT-PCR) was observed for oligomers complementary to sequences between positions 31 and 65 of the exon (data not shown).
Figure 1.
Exon 53 Skipping Induced by NS-065/NCNP-01 in RD Cells
(A) Strategy for screening exon-skipping sequences. (B) Exon-skipping efficiency of NS-065/NCNP-01 and h53A30/1 as a function of concentration. NS-065/NCNP-01 or h53A30/1 with Nucleofector was used to transfect RD cells, and exon skipping was measured using RT-PCR after 3 days. Each point represents the mean ± SD (n = 3). PMO, phosphorodiamidate morpholino oligomer; RD, rhabdomyosarcoma.
In the second stage, position and length were optimized by screening a series of 25 PMOs that were 15–25 nt in length and complementary to sequences between positions 31 and 65 (data not shown). The activity of the 15-nt oligomer was examined to investigate the lower limit of length that could be used, without decreased activity. This information is of interest because shorter AONs are advantageous in terms of the cost and time required for their production. Of these, NS-065/NCNP-01, a 21-mer complementary to the sequence between positions 36 and 56, displayed the highest level of exon 53 skipping. NS-065/NCNP-01 demonstrated a half-maximal effective concentration (EC50) of 8.6 μmol/L (95% confidence interval, 6.8–10.7 μmol/L; Figure 1B). The sequence between positions 30 and 65 is also effective for h53A30/1 (PMO-G), which specifically targets the sequence from positions 30 to 59 and is a potential clinical trial reagent for the targeted skipping of exon 53 in the DMD gene.14 The skipping activity of h53A30/1 demonstrated an EC50 value of 26.7 μmol/L (95% confidence interval, 22.6–31.6 μmol/L; Figure 1B). Thus, based on the EC50 values, NS-065/NCNP-01 was three times as effective as h53A30/1 at inducing exon 53 skipping.
Effect of NS-065/NCNP-01 in Cells Derived from Patients with DMD
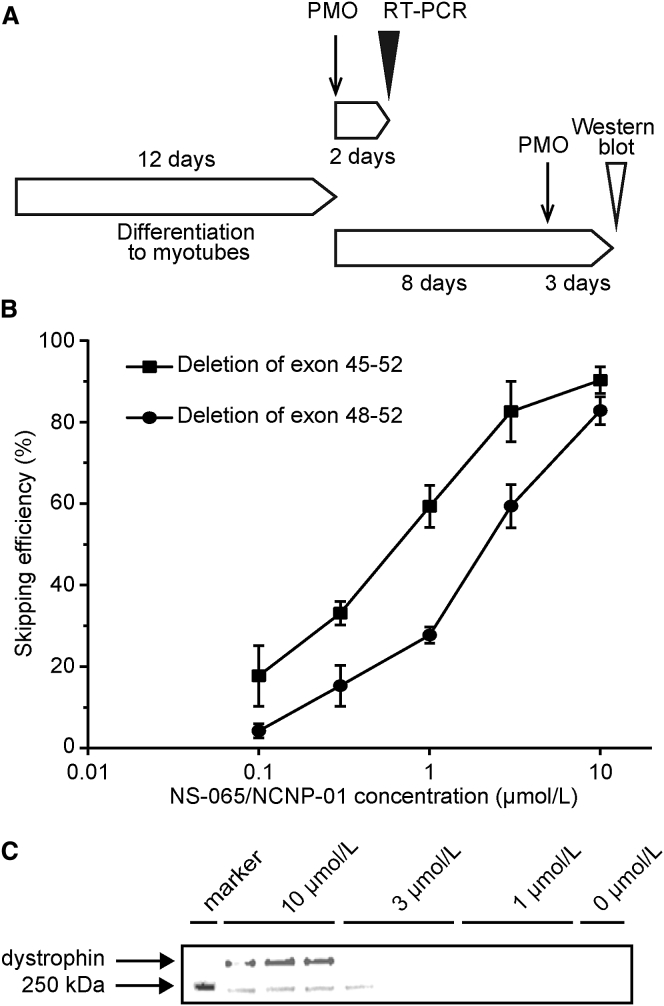
To verify the exon-skipping activity of NS-065/NCNP-01 in cells derived from patients with DMD, primary fibroblasts were obtained from two patients with DMD amenable to exon 53 skipping. After patient fibroblasts were transfected with the human MYOD gene to induce differentiation into myotubes, the myotubes were treated with NS-065/NCNP-01 in the presence of Endo-Porter as a delivery agent, and the effects of exon 53 skipping were measured by RT-PCR 2 days after the start of treatment (Figure 2A). NS-065/NCNP-01 induced exon 53 skipping in cells from a patient with a deletion of exons 45–52 (patient A) with an EC50 value of 0.63 μmol/L (95% confidence interval, 0.53–0.74 μmol/L; Figure 2B) and in cells from a patient with a deletion of exons 48–52 (patient B) with an EC50 value of 2.3 μmol/L (95% confidence interval, 2.0–2.6 μmol/L; Figure 2B). Dystrophin protein was detected by western blotting in cells from patient B (deletion of exons 48–52) after 3 days of treatment with NS-065/NCNP-01 at a final concentration of 10 μmol/L (Figure 2C).
Figure 2.
Effect of NS-065/NCNP-01 in Cells Derived from Patients with DMD
(A) Schedule of cell differentiation, PMO transfection, RT-PCR, and western blot. (B) Exon 53 skipping induced by NS-065/NCNP-01 in cells derived from patients with deletion of either exons 45–52 (n = 4) or exons 48–52 (n = 3). Each point represents the mean ± SD. (C) Dystrophin protein expression in cells derived from a patient with DMD involving deletion of exons 48–52 transfected with NS-065/NCNP-01 at final concentrations of up to 10 μmol/L. DMD, Duchenne muscular dystrophy; PMO, phosphorodiamidate morpholino oligomer.
Sustainability of Exon 53 Skipping
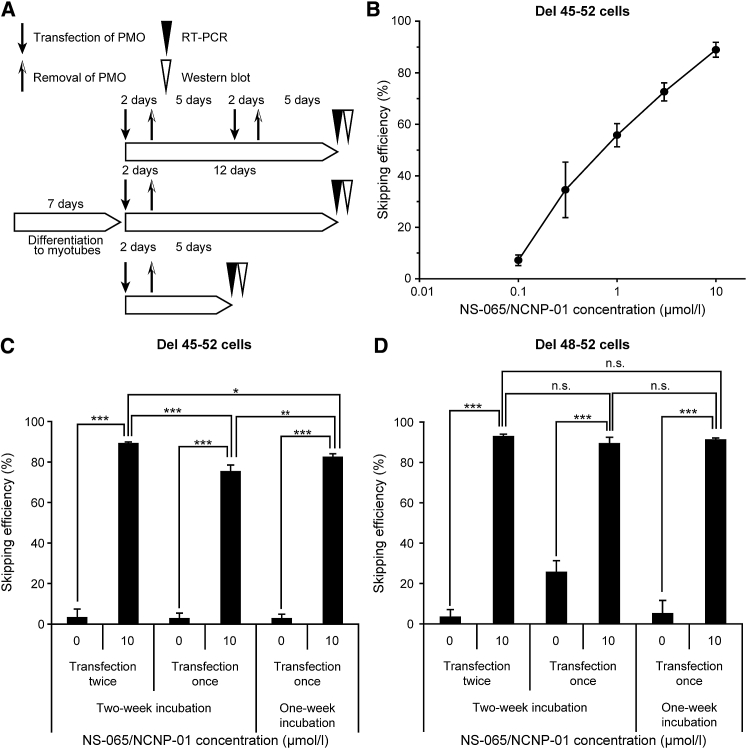
To investigate the sustainability of exon 53 skipping, NS-065/NCNP-01 was transfected into myotubes derived from fibroblasts from patient A (deletion of exons 45–52) with Endo-Porter over a period of 2 days, and exon 53 skipping was measured 1 week after the start of transfection. Specifically, NS-065/NCNP-01 was removed by replacement of the medium with NS-065/NCNP-01-free medium, and exon 53 skipping was measured by RT-PCR 1 week after the start of transfection and 5 days after the end of transfection (Figure 3A). The results demonstrated that exon 53 skipping was sustained for 1 week after the start of transfection; the efficiency of skipping was dose dependent with an EC50 value of 0.82 μmol/L (95% confidence interval, 0.67–1.00 μmol/L; Figure 3B).
Figure 3.
Sustainability of Exon 53 Skipping Induced by NS-065/NCNP-01 in Cells Derived from a Patient with DMD
(A) Schedule of cell differentiation, PMO transfection, RT-PCR, and western blot. (B) Exon 53 skipping induced by NS-065/NCNP-01 in cells derived from a patient with deletion of exons 45–52 at 1 week after the start of transfection. Each point represents the mean ± SD (n = 4). Exon 53 skipping induced by NS-065/NCNP-01 in cells from a patient with deletion of (C) exons 45–52 or (D) exons 48–52 at 1 and 2 weeks after transfection with NS-065/NCNP-01, either once or twice over a period of 2 days. The bars show the mean and SD (n = 4). n.s., no significant difference; *p < 0.05, **p < 0.01, ***p < 0.001 (Tukey’s multiple comparison test). DMD, Duchenne muscular dystrophy; PMO, phosphorodiamidate morpholino oligomer.
Next, we tested (1) the sustainability of the exon-skipping activity of NS-065/NCNP-01 for 2 weeks, and (2) the cumulative effect of repeated treatments. NS-065/NCNP-01 at a final concentration of 10 μmol/L in Endo-Porter was used to transfect myotubes derived from fibroblasts from both patients, either once or twice over a period of 2 days (Figure 3A). The skipping efficiency in NS-065/NCNP-01-transfected cells was substantially higher than that in cells not transfected with NS-065/NCNP-01 (Figures 3C and 3D). In cells from patient A (deletion of exons 45–52), the skipping efficiency was 82.7% at 1 week after the start of transfection and 75.6% at 2 weeks after the start of transfection (Figure 3C). When NS-065/NCNP-01 was used to transfect cells a second time 1 week after the start of the first transfection, the skipping efficiency at 1 week after the start of the second transfection was 89.4%. In cells from patient B (deletion of exons 48–52), the corresponding skipping efficiencies were 91.5%, 89.6%, and 93.1%, respectively (Figure 3D).
Thus, in cells from both patients, exon skipping was sustained for 2 weeks after a single transfection. Although exon skipping was slightly lower at 2 weeks after a single transfection compared to that at 1 week after a single transfection, exon skipping was slightly higher at 2 weeks after two transfections compared to that at 1 week after a single transfection.
Dystrophin Protein Expression after the Administration of NS-065/NCNP-01
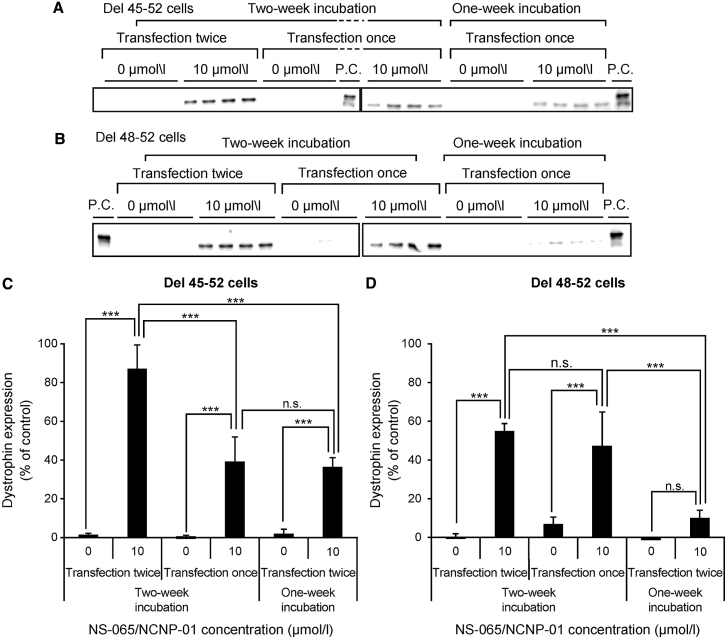
Dystrophin protein expression was investigated in NS-065/NCNP-01-treated cells from patients with DMD. NS-065/NCNP-01 at a final concentration of 10 μmol/L in Endo-Porter was used to transfect cells derived from both patients, either once or twice over a period of 2 days. Western blotting clearly demonstrated that dystrophin was expressed in NS-065/NCNP-01-transfected cells and was either undetectable or barely detectable in cells that had not been treated with NS-065/NCNP-01 (Figures 4A and 4C). Densitometric analysis showed that, in cells derived from patient A (deletion of exons 45–52), dystrophin expression was 36.4% of that in normal human cells at 1 week after the start of transfection and 39.2% at 2 weeks after the start of transfection. Dystrophin expression 1 week after the second weekly transfection reached 87.1% of that in normal human cells (Figure 4B). Similarly, in cells derived from patient B (deletion of exons 48–52), the corresponding percentages of dystrophin expression were 10.1%, 37.3%, and 55.0%, respectively (Figure 4D). Thus, dystrophin expression was induced by NS-065/NCNP-01, and the expression was sustained for 2 weeks after a single transfection, showing increased protein levels following a second transfection.
Figure 4.
Sustainability of Dystrophin Protein Expression Induced by NS-065/NCNP-01
Western blot analysis of dystrophin protein expression in cells derived from patients with DMD involving deletion of (A) exons 45–52 and (B) exons 48–52 1 and 2 weeks after transfection with NS-065/NCNP-01, either once or twice over a period of 2 days. The positive control (P.C.) was a cell lysate of myotubes differentiated from normal human fibroblasts by transduction with MYOD. Densitometric analysis of the western blots of cells from patients with DMD involving deletion of (C) exons 45–52 and (D) exons 48–52 relative to each P.C. on the same membrane is shown. The bars show the mean and SD (n = 4). n.s., no significant difference; ***p < 0.001 (Tukey’s multiple comparison test). DMD, Duchenne muscular dystrophy.
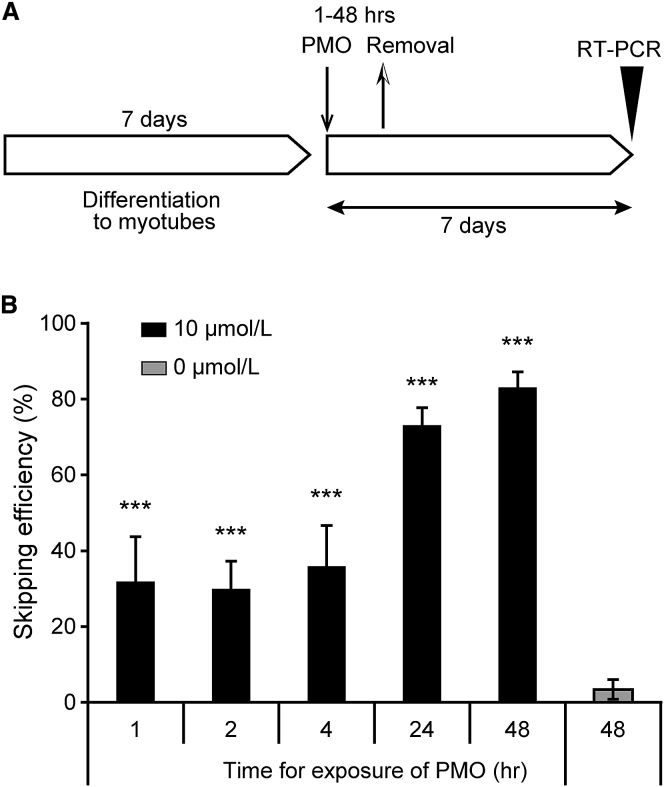
Sustainability of Exon 53 Skipping after Transfection for Short Incubation Times
To investigate the influence of the duration of incubation with NS-065/NCNP-01 on exon 53 skipping, NS-065/NCNP-01 was transfected into cells for periods of 1–48 hr (Figure 5A). When NS-065/NCNP-01 was used to transfect cells derived from patient A (deletion of exons 45–52) at a final concentration of 10 μmol/L with Endo-Porter over periods of 1, 2, 4, 24, and 48 hr, the skipping efficiencies were 31.9%, 29.9%, 35.9%, 73.1%, and 83.1%, respectively, which were substantially higher than the skipping efficiencies observed in cells that had not been treated with NS-065/NCNP-01 (Figure 5B). Although the skipping efficiency was lower with shorter incubation times, exon skipping was nonetheless sustained for 1 week when the cells were transfected with NS-065/NCNP-01 for at least 1 hr.
Figure 5.
Sustainability of Exon 53 Skipping Induced by NS-065/NCNP-01 after Transfection for Short Incubation Times
(A) Schedule of cell differentiation, PMO transfection, and RT-PCR. (B) Exon 53 skipping induced by NS-065/NCNP-01 was measured in cells derived from a patient with deletion of exons 45–52 at 1 week after the start of transfection. NS-065/NCNP-01 was transfected for the indicated times. The bars show the mean and SD (n = 4). ***p < 0.001 compared to 0 μmol/L by Dunnett’s multiple comparison test. PMO, phosphorodiamidate morpholino oligomer.
Discussion
In the present study, a series of AONs was synthesized to cover the entirety of exon 53 in the DMD gene, and the optimal sequence was selected by examining their exon 53-skipping activity in RD cells. Although RD cells are useful for determining the exon-skipping activity of AONs, they cannot be used to detect the restoration of the dystrophin protein, because the reading frame of the DMD gene is disrupted by exon 53 skipping. Therefore, exon 53-skipping activity and the consequent expression of dystrophin were subsequently determined for the candidate sequence in cells derived from patients with DMD amenable to exon 53 skipping. The optimal sequence inducing exon 53 skipping in RD cells was found to be that of NS-065/NCNP-01, which showed an adequate effect that could be sustained for 2 weeks at an efficiency of over 70% in DMD patient-derived cells.
The AON h53A30/1 also induces exon 53 skipping strongly and persistently.14 In the present study, the exon 53-skipping activity of NS-065/NCNP-01 was directly compared to that of h53A30/1 in RD cells, and NS-065/NCNP-01 was found to be three times as potent as h53A30/1 (i.e., a given activity level of NS-065/NCNP-01 was achieved at one-third of the concentration of h53A30/1 required to produce the same activity). Because NS-065/NCNP-01 is a 21-mer whereas h53A30/1 is a 30-mer, and because the concentrations used in the in vitro assay are given in molar terms (μmol/L), the dose of NS-065/NCNP-01 in mass terms (mg/kg body mass) required for a patient with DMD is correspondingly lower than the dose of h53A30/1 required due to the lower molecular mass of the 21-mer. This would be an advantage in the development of a new drug because the toxicity of a PMO is potentially reduced when the therapeutic dose is lower.
In addition, the influence of oligonucleotide length on bioactivity has been previously investigated.14, 16 Popplewell et al.14 reported that all tested h53A30/1 30-mers are more bioactive than their 25-mer counterparts, and, therefore, they concluded that AON length is the most important factor in determining bioactivity. However, in the current study, a 21-mer AON, NS-065/NCNP-01, was identified as exhibiting the highest skipping activity of all sequences tested, and it was also the shortest sequence tested.
In preclinical studies, various therapeutic effects have been observed for exon-skipping PMOs intravenously administered once a week to dystrophic mice17, 18 and dogs.19 In a clinical trial, eteplirsen was intravenously administered to a patient with DMD once a week, and it was found to restore dystrophin levels in the patient’s muscles, which was associated with the maintenance of ambulatory function.20 In the present study, the efficiency of exon 53 skipping induced by NS-065/NCNP-01 over a period of 1 week was investigated. The skipping efficiency was 82.7%–91.5%, and it showed only a slight decrease at 5 days following the removal of NS-065/NCNP-01. In cells from two patients with two different types of DMD amenable to exon 53 skipping, exon skipping was sustained for 2 weeks after transfection with NS-065/NCNP-01.
Furthermore, exon-skipping efficiency and dystrophin expression levels were compared under three sets of conditions: 2-week incubation and two transfections, 2-week incubation and one transfection, and 1-week incubation and one transfection. Cells from patient A showed a significant increase in exon-skipping efficiency with 2-week incubation and two transfections compared to that under the other conditions. Cells from patient B also showed an increase in exon-skipping efficiency with 2-week incubation and two transfections, although the increase was not significant. In cells from patient B, skipping efficiency was almost saturated under all three conditions. With regard to dystrophin protein levels, cells from both patients showed significant increases in protein expression with 2-week incubation and two transfections compared to that with 1-week incubation and one transfection. Given that exon skipping was sustained for at least 2 weeks, the production of dystrophin protein over this period was cumulative. This result is similar to the findings in mdx mice, where dystrophin protein expression induced by AON treatment was sustained for 2 weeks.21 Overall, these results suggest that NS-065/NCNP-01 may have a therapeutic effect when injected once weekly.
PMOs are rapidly cleared from the plasma with half-lives ranging from 1 to 20 hr following intravenous injection.20, 22 In the current study, to estimate the efficiency of exon 53 skipping in muscle cells exposed to NS-065/NCNP-01 for a short duration, cells derived from DMD patients were treated with NS-065/NCNP-01 for at least 1 hr. The results demonstrated that exon 53 skipping was sustained for 1 week at an efficiency of over 30%. PMOs for mouse exon 51 skipping, intravenously injected into mdx52 mice at a single dose of 320 mg/kg, showed a skipping efficiency of approximately 30% in the gastrocnemius muscle, and recovery of muscle function was observed with weekly recurrent injections at this dose over a period of 7 weeks.18 Thus, an exon-skipping efficiency of 30% may be sufficient for a therapeutic effect.
Based on these findings, NS-065/NCNP-01 could be expected to show a similar exon-skipping efficiency and consequent therapeutic effect when intravenously administered to a patient with DMD over similar periods. Thus, we planned an investigator-initiated clinical trial of NS-065/NCNP-01, which was a phase I, open-label, dose-escalation study (ClinicalTrials.gov: NCT02081625). The primary endpoint of this clinical trial was safety, and the secondary endpoints were pharmacokinetics and efficacy. NS-065/NCNP-01 induced exon 53 skipping in dystrophin-encoding mRNA in a dose-dependent manner, and it significantly increased the dystrophin:spectrin ratio in some of the patients. No severe adverse drug reactions were observed, and no treatment discontinuation occurred.23 On the basis of these results, NS-065/NCNP-01 is expected to exhibit therapeutic efficacy in DMD. NS-065/NCNP-01 is the first AON discovered in Japan to be granted a Fast Track Designation, Orphan Drug Designation, and Rare Pediatric Disease Designation by the FDA. A phase 2 clinical study (ClinicalTrials.gov: NCT02740972) in the United States and a phase 1/2 study in Japan began recruitment in December 2016 and July 2016, respectively.
In conclusion, the induction of exon 53 skipping and dystrophin protein expression by transfection with NS-065/NCNP-01 was successfully demonstrated in cells derived from patients with DMD amenable to exon 53 skipping. Our results suggest that NS-065/NCNP-01 is a highly promising exon 53-skipping candidate that is expected to show a high and sustainable therapeutic effect in clinical trials.
Materials and Methods
Antisense Oligomers
2′-O-Methyl phosphorothioate oligonucleotides were purchased from Japan Bio Services (Saitama, Japan). PMOs were synthesized as previously described.24 All-P-ambo-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secocytidylyl-(5′→(′a)-P,3′-dideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secothymidylyl-(5′→(′a)-P,3′-dideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secothymidylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secoguanylyl-(5′→(′a)-P,3′-dideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secothymidylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secoguanylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secoguanylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secoadenylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secoadenylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secoguanylyl-(5′→(′a)-P,3′-dideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secothymidylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secocytidylyl-(5′→(′a)-P,3′-dideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secothymidylyl-(5′→(′a)-P,3′-dideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secothymidylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secoguanylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secoguanylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secocytidylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secocytidylyl-(5′→(′a)-P,3′-dideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secothymidylyl-(5′→(′a)-P,2′,3′-trideoxy-P-dimethylamino-2′,3′-imino-2′,3′-secocytidylyl-(5′→(′a)-2′,3′-dideoxy-2′,3′-imino-2′,3′-secocytidine was synthesized as NS-065/NCNP-01.
Cells
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of the National Center of Neurology and Psychiatry (approval 22-3-7) and the ethics committee of Nippon Shinyaku (approval A100901). RD cells were obtained from the Health Science Research Resources Bank and cultured under 5% CO2 at 37°C in Eagle’s minimum essential medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum. Fibroblasts from patients with DMD involving deletion of exons 45–52 or exons 48–52 of the DMD gene were prepared with informed consent from skin biopsies using the migration method. Specifically, each skin biopsy was minced, and four or five skin tissue fragments were placed on the bottom of a 10-cm2 dish. DMEM/F-12 1:1 (Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum (Life Technologies) and 1% penicillin/streptomycin (Sigma-Aldrich) was added, and the skin pieces were allowed to adhere to the dish surface for 7 days under 5% CO2 at 37°C. After 1 week of culture, cells began to appear around the skin pieces, and the medium was changed until the cell culture became confluent. The differentiation medium utilized was DMEM/F-12 1:1 containing 2% horse serum (Life Technologies), 1% penicillin/streptomycin, and insulin-transferrin-selenium liquid media supplement (Sigma-Aldrich). After transfection of the MYOD gene, fibroblasts were sorted by fluorescence-activated cell sorting and induced to differentiate into myotubes, as previously described.25
Transfection of NS-065/NCNP-01 into Cells
NS-065/NCNP-01 was dissolved in distilled water, and it was transfected into RD cells using the Amaxa cell line Nucleofector kit L and a Nucleofector II device (Lonza, Basel, Switzerland) with program T-030 or into cells from a patient with DMD using Endo-Porter Aqueous (Gene Tools, Philomath, OR, USA), according to the manufacturer’s protocols.
RT-PCR
Total RNA was extracted from RD cells using Isogen (Nippon Gene, Tokyo, Japan) and from DMD patient-derived cells using a QIAshredder spin column (QIAGEN, Valencia, CA, USA) and an RNeasy mini kit (QIAGEN). RNA concentrations were determined by absorbance at 260 nm using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). RT-PCR was performed with 400 ng (RD cells) or 50 ng (DMD patient-derived cells) extracted total RNA using a QIAGEN OneStep RT-PCR Kit (QIAGEN). The primer sets are shown in Table S1.
RT-PCR was performed using an RTC-100 thermocycler (MJ Research, Watertown, MA, USA) for RD cells or an iCycler (Bio-Rad, Hercules, CA, USA) for DMD patient-derived cells. The RT-PCR program for the RTC-100 thermocycler was as follows: reverse transcription at 50°C for 30 min and heat denaturation at 95°C for 15 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min; followed by a final extension at 72°C for 10 min. The RT-PCR program for the iCycler was as follows: reverse transcription at 50°C for 30 min and heat denaturation at 95°C for 15 min; 35 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min; followed by a final extension at 72°C for 7 min. The PCR products were analyzed using a 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany) for RD cells or an Experion automated electrophoresis station (Bio-Rad) for DMD patient-derived cells. The skipping efficiency was determined from the following expression: (PCR products without exon 53) × 100/([PCR products without exon 53] + [PCR products with exon 53]).
Western Blotting
Western blotting was performed on cell lysates (3 μg protein) as previously described.24 The positive control consisted of normal dystrophin (427 kDa) from a cell lysate of myotubes differentiated from normal human fibroblasts (TIG-119) for 20 days. The amount of dystrophin without the segment corresponding to exons 45–53 (372 kDa) or the segment corresponding to exons 48–53 (390 kDa) was determined using an ImageQuant LAS 4000 mini imaging analyzer (FujiFilm, Tokyo, Japan). Protein levels (dystrophin expression) were determined as follows: (dystrophin expression level after exon 53 skipping [372 or 390 kDa]) × 100/(dystrophin expression level of normal control [427 kDa] on the same membrane). Protein levels were normalized to the positive control of each western blotting membrane.
Statistical Analysis
All analyses were performed using SAS software (version [v.]9.1.3; SAS Institute, Cary, NC, USA) and EXSUS (v.7.7.1; CAC Exicare, Tokyo, Japan). EC50 values and 95% confidence intervals were calculated by fitting the skipping-efficiency data to a logistic curve. Tukey’s and Dunnett’s multiple comparison tests were performed using a significance level of 0.05.
Author Contributions
N.W., T.N., Y.S., S.M., and T.S. performed the in vitro experiments. H. Kitagawa synthesized the PMOs. H. Komaki conducted skin biopsies from DMD patients. K.T. and S.T. coordinated and supervised the project. N.W. and T.N. wrote the manuscript.
Conflicts of Interest
The authors have no conflicts of interest.
Acknowledgments
The authors wish to thank Dr. Gerald E. Smyth for assistance in preparing the manuscript. This study was supported by Grants-in-Aid for Research on Nervous and Mental Disorders (25-5); Health and Labour Sciences Research Grants for Translation Research (H24-Translational Research-010); Comprehensive Research on Disability Health and Welfare (H23-Neuromuscular Disease-005) from the Ministry of Health, Labour and Welfare of Japan, Japan; Grants for Promoting Clinical Trials for the Development of New Drugs and Medical Devices and Health and Labour Science Research Grants for Comprehensive Research on Persons with Disabilities from the Japan Agency for Medical Research and Development, Japan, and the Takeda Science Foundation, Japan. The work was performed in Kodaira, Tokyo, and Tsukuba, Ibaraki, Japan. PMO was provided to NCNP by Nippon Shinyaku Co.
Footnotes
Supplemental Information includes one table and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.09.017.
Supplemental Information
References
- 1.Mah J.K., Korngut L., Dykeman J., Day L., Pringsheim T., Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul. Disord. 2014;24:482–491. doi: 10.1016/j.nmd.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Emery A.E. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 3.Leung D.G., Wagner K.R. Therapeutic advances in muscular dystrophy. Ann. Neurol. 2013;74:404–411. doi: 10.1002/ana.23989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendell J.R., Goemans N., Lowes L.P., Alfano L.N., Berry K., Shao J., Kaye E.M., Mercuri E., Eteplirsen Study Group and Telethon Foundation DMD Italian Network Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 2016;79:257–271. doi: 10.1002/ana.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarepta Therapeutics (2015). Sarepta Therapeutics announces FDA has filed eteplirsen NDA for the potential treatment of Duchenne muscular dystrophy for patients amenable to exon 51 skipping. http://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-fda-has-filed-eteplirsen-nda.
- 6.BioMarin (2016). FDA issues complete response letter for Kyndrisa™ for Duchenne muscular dystrophy amenable to exon 51 skipping. https://globenewswire.com/news-release/2016/01/14/802009/0/en/FDA-Issues-Complete-Response-Letter-for-KyndrisaTM-for-Duchenne-Muscular-Dystrophy-Amenable-to-Exon-51-Skipping.html.
- 7.Sarepta Therapeutics (2016). Sarepta issues statement on advisory committee outcome for use of eteplirsen in the treatment of Duchenne muscular dystrophy. http://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-issues-statement-advisory-committee-outcome-use.
- 8.Sarepta Therapeutics (2016). Sarepta Therapeutics announces FDA accelerated approval of EXONDYS 51™ (eteplirsen) injection, an exon skipping therapy to treat Duchenne muscular dystrophy (DMD) patients amenable to skipping exon 51. http://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-fda-accelerated-approval-exondys.
- 9.Bladen C.L., Salgado D., Monges S., Foncuberta M.E., Kekou K., Kosma K., Dawkins H., Lamont L., Roy A.J., Chamova T. The TREAT-NMD DMD Global Database: Analysis of More than 7,000 Duchenne Muscular Dystrophy Mutations. Hum. Mutat. 2015;6:395–402. doi: 10.1002/humu.22758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aartsma-Rus A., De Winter C.L., Janson A.A., Kaman W.E., Van Ommen G.J., Den Dunnen J.T., Van Deutekom J.C. Functional analysis of 114 exon-internal AONs for targeted DMD exon skipping: indication for steric hindrance of SR protein binding sites. Oligonucleotides. 2005;15:284–297. doi: 10.1089/oli.2005.15.284. [DOI] [PubMed] [Google Scholar]
- 11.Wee K.B., Pramono Z.A., Wang J.L., MacDorman K.F., Lai P.S., Yee W.C. Dynamics of co-transcriptional pre-mRNA folding influences the induction of dystrophin exon skipping by antisense oligonucleotides. PLoS ONE. 2008;3:e1844. doi: 10.1371/journal.pone.0001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popplewell L.J., Trollet C., Dickson G., Graham I.R. Design of phosphorodiamidate morpholino oligomers (PMOs) for the induction of exon skipping of the human DMD gene. Mol. Ther. 2009;17:554–561. doi: 10.1038/mt.2008.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilton S.D., Fall A.M., Harding P.L., McClorey G., Coleman C., Fletcher S. Antisense oligonucleotide-induced exon skipping across the human dystrophin gene transcript. Mol. Ther. 2007;15:1288–1296. doi: 10.1038/sj.mt.6300095. [DOI] [PubMed] [Google Scholar]
- 14.Popplewell L.J., Adkin C., Arechavala-Gomeza V., Aartsma-Rus A., de Winter C.L., Wilton S.D., Morgan J.E., Muntoni F., Graham I.R., Dickson G. Comparative analysis of antisense oligonucleotide sequences targeting exon 53 of the human DMD gene: Implications for future clinical trials. Neuromuscul. Disord. 2010;20:102–110. doi: 10.1016/j.nmd.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Popplewell L.J., Malerba A., Dickson G. Optimizing antisense oligonucleotides using phosphorodiamidate morpholino oligomers. Methods Mol. Biol. 2012;867:143–167. doi: 10.1007/978-1-61779-767-5_10. [DOI] [PubMed] [Google Scholar]
- 16.Harding P.L., Fall A.M., Honeyman K., Fletcher S., Wilton S.D. The influence of antisense oligonucleotide length on dystrophin exon skipping. Mol. Ther. 2007;15:157–166. doi: 10.1038/sj.mt.6300006. [DOI] [PubMed] [Google Scholar]
- 17.Alter J., Lou F., Rabinowitz A., Yin H., Rosenfeld J., Wilton S.D., Partridge T.A., Lu Q.L. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat. Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- 18.Aoki Y., Nakamura A., Yokota T., Saito T., Okazawa H., Nagata T., Takeda S. In-frame dystrophin following exon 51-skipping improves muscle pathology and function in the exon 52-deficient mdx mouse. Mol. Ther. 2010;18:1995–2005. doi: 10.1038/mt.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokota T., Lu Q.L., Partridge T., Kobayashi M., Nakamura A., Takeda S., Hoffman E. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann. Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendell J.R., Rodino-Klapac L.R., Sahenk Z., Roush K., Bird L., Lowes L.P., Alfano L., Gomez A.M., Lewis S., Kota J., Eteplirsen Study Group Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 2013;74:637–647. doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- 21.Lu Q.L., Mann C.J., Lou F., Bou-Gharios G., Morris G.E., Xue S.A., Fletcher S., Partridge T.A., Wilton S.D. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat. Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- 22.Amantana A., Iversen P.L. Pharmacokinetics and biodistribution of phosphorodiamidate morpholino antisense oligomers. Curr. Opin. Pharmacol. 2005;5:550–555. doi: 10.1016/j.coph.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Komaki H., Nagata T., Saito T., Masuda S., Takeshita E., Sasaki M., Tachimori H., Nakamura H., Aoki Y., Takeda S. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Sci. Transl. Med. 2018;10:eaan0713. doi: 10.1126/scitranslmed.aan0713. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe, N., Satou, Y., Takeda, S., and Nagata, T. August 2012. Antisense nucleic acid. International patent WO/2012/029986.
- 25.Saito T., Nakamura A., Aoki Y., Yokota T., Okada T., Osawa M., Takeda S. Antisense PMO found in dystrophic dog model was effective in cells from exon 7-deleted DMD patient. PLoS ONE. 2010;5:e12239. doi: 10.1371/journal.pone.0012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.