Abstract
Insulin secretion from pancreatic β-cells is initiated through channel-mediated depolarization, cytoskeletal remodeling, and vesicle tethering at the cell membrane, all of which can be regulated through cell surface receptors. Receptor tyrosine kinases (RTKs) promote β-cell development and postnatal signaling to improve β-cell mass and function, yet their activation has also been shown to initiate exocytotic events in β-cells. This review examines the role of RTK signaling in insulin secretion, with a focus on RTKs c-Kit and insulin receptor (IR). Pathways that control insulin release and the potential interplay between c-Kit and IR signaling are discussed, along with clinical implications of RTK therapy on insulin secretion.
The endocrine cells of the pancreatic islets of Langerhans secrete hormones that are responsible for the minute-to-minute regulation of glucose homeostasis, with the β-cells composing the majority of islet cell mass in mammals (1, 2). Upon stimulation, β-cells release insulin that binds to insulin receptors (IRs) in peripheral tissues (skeletal muscle, adipose tissue, liver) to stimulate glucose uptake and storage (3). Biphasic insulin release from β-cells is initiated with glucose-stimulated closure of ATP-sensitive KATP channels, increased intracellular calcium, and insulin granule exocytosis. The activation of select membrane receptors found on β-cells is an additional factor that can influence insulin secretion. The G protein–coupled receptor (GPCR) glucagon-like peptide-1 receptor (GLP-1R) is one of the most extensively examined β-cell receptors, with a well-established role increasing insulin secretion through increased levels of intracellular calcium and reduced voltage-gated potassium ion channel activity (4–6). Receptor tyrosine kinases (RTKs) are another class of membrane receptors present on cells throughout the islet. Although RTKs are known to maintain β-cell proliferation and survival, the activation of these receptors has also been reported to promote insulin release. This review will examine the role of β-cell RTKs, with a focus on c-Kit and IR, in regulating the trafficking and exocytosis of insulin granules.
Islet RTKs and Their Role in Islet Maintenance and Hormone Secretion
The islet microenvironment expresses various RTKs that regulate β-cell proliferation, function, and insulin synthesis and secretion (Fig. 1). Pancreatic development is one process that relies on the expression of multiple RTKs to achieve islet maturation. Epidermal growth factor receptor (EGFR) expression is necessary for embryonic β-cell maturation, islet migration, and maintenance of β-cell mass and proliferation (7, 8). Other members of the ErbB receptor family were also identified during murine pancreatic development studies (9). The receptor for nerve growth factor (NGF), Trk-A, follows a specific pattern of expression within endocrine cells that depends on the stage of embryonic development (10). Discoidin domain receptor tyrosine kinase 1 (DDR1) has recently been identified during early pancreatic development in emerging endocrine cells and in injury-induced ductal ligated pancreata (11). Platelet-derived growth factor receptor (PDGFR), which shares structural homology to c-Kit, regulates islet proliferation and has been found to decrease in islets with advancing age (12). Both EGFR and c-Met, the receptor for hepatocyte growth factor (HGF), promoted β-cell proliferation during pregnancy (13, 14). The contributions of c-Kit and IR to β-cell development and postnatal function are discussed in subsequent sections.
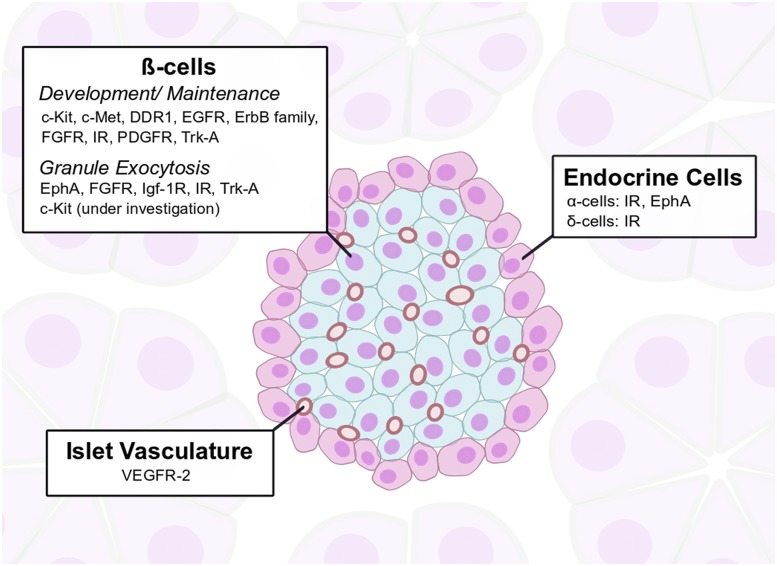
Figure 1.
RTKs in the islet microenvironment. The mature murine islet is composed of insulin-secreting β-cells in the core of the islet (blue) and other endocrine hormone–secreting cells along the islet’s periphery (red). β-cells express multiple RTKs that influence their growth and development. The direct effect of RTK activation on insulin secretion has been demonstrated to date in select RTKs. β-cells regulate and are influenced by RTKs expressed on the islet vasculature.
The current literature regarding RTK expression on the remaining islet endocrine cells (α-, δ-, PP-, and ε-cells) is limited. Unlike the receptor for insulin, the receptors for other islet hormones belong to the GPCR family (15, 16). The presence of IR has been confirmed on α-cells and is necessary for cell proliferation and the regulation of glucagon secretion through the Akt/P70S6K1 axis (17, 18). IR expression is also found in isolated single δ-cells from human islets and in the somatostatin-secreting TGP52 cell line (19, 20). Recent findings demonstrated that glucagon release from α-cells can be manipulated through the erythropoietin-producing hepatoma A (EphA) receptor, an RTK class that is unique due to forward and reverse signaling through β-cell/β-cell and β-cell/α-cell membrane receptor–membrane-bound ligand interactions (21, 22). However, the presence of RTKs and their activity in non–β-cell endocrine cells warrants further investigation.
RTKs are not only limited to hormone-secreting cells but are crucial for integrin–extracellular matrix (ECM) communication and islet vascularization. One study identified that β-cell fibroblast growth factor receptor 1 (FGFR1) promoted ERK phosphorylation, but its activation depended on the binding of α6β1 integrin to specific ECM ligands produced from endothelial cells (23), which increased both islet microvasculature remodeling and β-cell insulin secretion and survival (24). Vascular endothelial growth factor (VEGF) is secreted from β-cells and binds to VEGF receptors (VEGFRs) on islet endothelial cells to promote angiogenesis. Reduced islet vascularization, decreased β-cell mass, and impaired insulin and glucagon secretion were noted in mice with β-cell Vegf-a loss during embryonic development (25–27). Although inducing Vegf-a inactivation in the postnatal adult β-cell also lowered islet vascularization 3 months after its initial loss, these mice developed only mild glucose intolerance and unchanged β-cell mass (26), which indicates that islet endothelial cell regulation of β-cell mass occurs during select developmental time points. Hypervascularization of the islets through increased VEGFR expression can also detrimentally affect β-cell survival and lead to the development of hyperglycemia, stressing the importance of maintaining an optimal range for islet vascularization. Overexpression of Vegf-a during islet development or induced in adult β-cells led to increased endothelial cell density, but this expansion impaired the formation of islet clusters and decreased β-cell proliferation and mass in both mouse models (28, 29). Nonobese diabetic mice also had increased Vegf-a production from β-cells, which increased the expression of VEGFR on endothelial cells and subsequently led to islet inflammation and T-cell–mediated β-cell destruction (30).
In addition to controlling overall islet function, the activation of certain RTKs can directly regulate insulin granule release. NGF signaling through Trk-A maintained glucose-stimulated insulin secretion in mouse islets, and Trk-A internalization initiated F-actin reorganization and led to insulin exocytosis (31–33). Signaling through the c-Met receptor has also been observed to regulate secretion. Its disruption in murine β-cells resulted in impaired insulin release, with a minimal effect on islet morphology (34). IGF-1 receptor (IGF-1R), which shares structural homology with IR, was found to be necessary for glucose-stimulated insulin release and glucose sensing through the maintenance of Glut2 expression (35, 36). Fibroblast growth factor 21, through binding to its receptor, stimulated insulin release from isolated islets of diabetic rodent models under high-glucose conditions (37). RTKs have also been found to play a role in the negative feedback of insulin release, as observed with bidirectional signaling through EphA (21, 38). Although there are many RTKs in β-cells that contribute to their overall function, we will focus on the selected RTKs c-Kit and IR and their roles in insulin release.
c-Kit Activity in Islet Function and Insulin Secretion
c-Kit is the RTK for the ligand stem cell factor (SCF) and, similar to the islet RTKs discussed earlier, was observed in embryonic and newborn islets and become restricted to a small subpopulation of β-cells in the postnatal and adult rat pancreas (39–42). SCF treatment induced ERK phosphorylation in the INS-1 cell line and increased insulin+ cell expression in the PANC-1 cell line, highlighting its importance in β-cell development (41, 43). By using various mouse models, our research group has demonstrated that c-Kit activity is necessary in mature β-cells to maintain islet function and normoglycemia. Heterozygous mice (c-KitWv/+) with an intracellular point mutation that disrupted receptor activation displayed reduced insulin content and glucose-stimulated insulin secretion (44). In contrast to the c-KitWv/+ model, mice with a β-cell–specific overexpression of the human c-KIT receptor (c-KitβTg) had markedly greater islet mass, proliferation, and insulin release compared with wild-type controls (45). Increased vascularization was also noted in the islets of c-KitβTg mice through increased β-cell Vegf-a production, which promoted insulin secretion in mice on a normal diet but led to inflammation-induced β-cell apoptosis and islet dysfunction in mice on a long-term (22 weeks) high-fat diet (46). These in vivo studies have demonstrated that c-Kit signaling is necessary for β-cell development, survival, and function, yet the prolonged activation of the receptor may promote detrimental effects under metabolic stresses.
Although c-Kit activation on β-cells increased insulin production and release, the exact role of c-Kit signaling in the mechanism of insulin granule exocytosis is unknown. Secretory control through c-Kit activation has been extensively assessed in mast cells of the immune system, where histamine and IL-6 release were stimulated with SCF administration (47, 48). In mast cells, c-Kit activation via the phosphatidylinositol 3-kinase (PI3K) pathway and cytoskeletal remodeling were necessary for secretion (49, 50). Mice with a mutation that rendered c-Kit inactive exhibited reduced downstream PI3K/Akt signaling and decreased granule release response from mast cells (51). Although the direct role of c-Kit signaling on the regulation of β-cell insulin granule exocytosis has not been documented, we have found that c-KitβTg islets contained significantly elevated IR mRNA and protein expression (45). In light of these results, additional research is warranted to determine whether c-Kit directly regulates granule release in β-cells or requires interplay with other β-cell RTKs, including IR, for insulin exocytosis.
The Role of the β-Cell Autocrine Insulin/IR Axis in the Regulation of Insulin Secretion
In contrast to c-Kit, numerous studies have examined the capacity of IR activation to stimulate insulin release. There is little consensus on the role of insulin-stimulated insulin secretion because multiple studies have presented both positive and negative regulatory roles (summarized in Fig. 2). Findings from both in vitro cell models and in vivo rodent studies indicate that maintenance of the insulin/IR/insulin receptor substrate (IRS) signaling axis is important for biphasic insulin signaling and insulin granule exocytotic machinery. Transfection of βTC6-F7 cells with an overexpression of intracellular kinase mutated IR, which yielded reduced IR activity when compared with an overexpressing wild-type IR line, was shown to impair glucose-induced insulin secretion (52). The earliest study of mice with a β-cell–specific IR knockout demonstrated that reducing the presence of IR interfered with first phase insulin secretion (53). More recently, the adaptor protein APPL1, a regulator of Akt and an anchor between IR and IRS-1 (54), was identified as a potential upstream factor for the regulation of soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs) Syntaxin 1a, Snap25, and Vamp2 in murine β-cells (55). Isolated single murine β-cells also demonstrated increased exocytotic events when stimulated with high doses of insulin (100 nM) and impaired secretion when pretreated with IR neutralization antibody (56).
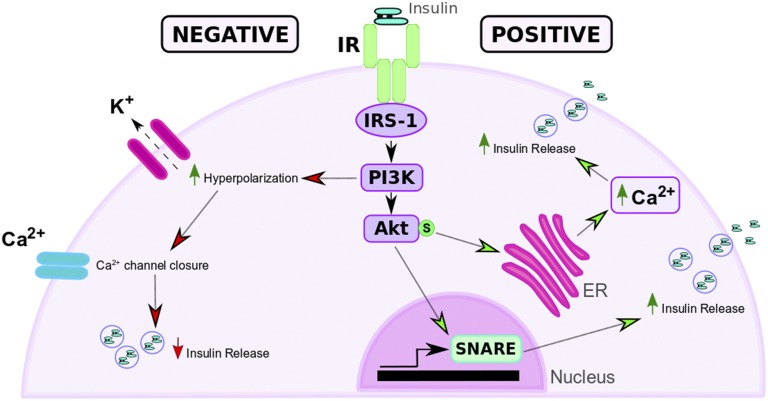
Figure 2.
Effects of IR activation on insulin secretion. Insulin autocrine stimulation of β-cells can induce positive or negative feedback on insulin release. Positive feedback (right, green arrowheads): activation of the IR/IRS-1 pathway induces Akt phosphorylation, which increases intracellular Ca2+ release from the ER and SNARE protein levels and leads to increased insulin release from β-cells. Negative feedback (left, red arrowheads): insulin stimulation activates the IR/IRS/PI3K pathway, resulting in hyperpolarization of membrane KATP channel and a reduction in Ca2+-stimulated insulin release.
A review of the literature suggests that IR/IRS activity differentially affects intracellular Ca2+ influx from the cell membrane and endoplasmic reticulum (ER), which may explain the contrasting effects of autocrine insulin action during insulin release. It has been shown that IR signaling does not affect membrane depolarization–induced secretion but is necessary for mobilizing Ca2+ from intracellular stores (56). Insulin-stimulated isolated murine β-cells demonstrated hyperpolarization of the cell and mitochondrial membranes through activated KATP channels, which subsequently reduced the levels of intracellular Ca2+ necessary for insulin exocytosis (57). However, another report showed that treating murine β-cells with 100 nM of insulin heightened intracellular Ca2+ release from the ER, resulting in increased insulin secretion (58). This pathway relied on activation through the IR/IRS-1/Akt axis and is independent of KATP-induced depolarization. Irs-1 colocalized with the sarcoendoplasmic reticulum Ca2+ ATPase in βTC6-F7 cells, which inhibited Ca2+ reuptake into the ER and increased insulin secretion (59). Irs-1−/− mice displayed reduced insulin granule exocytosis because of a shortened transient period of high intracellular Ca2+ levels after glucose stimulation (60), indicating that the IR/IRS-1 signaling axis is important for ER-regulated Ca2+ release. It has also been reported that isolated rat islets increased insulin release when Irs-1 was inhibited in a high-glucose environment (61), which suggests that IRS-1–induced insulin secretion can also be affected by glycemic levels. The data available at this time indicate that additional research is needed to determine the contrasting effects of insulin stimulation on intracellular Ca2+ levels and the downstream signaling pathways that connect receptor activation to granule exocytosis.
Interplay Between c-Kit and IR Signaling on Insulin Exocytosis
The role of Akt signaling through c-Kit and IR
Signaling through the PI3K/Akt pathway is well established in c-Kit– and IR-activated β-cells (45, 62). Importantly, Akt activation through the conversion of phosphatidylinositols via PI3K has been demonstrated in multiple experimental studies to promote insulin secretion. Loss of class IA PI3Ks in β-cells reduced intracellular Ca2+ levels and SNARE proteins, which inhibited insulin granule exocytosis (63). The α isoform of class II PI3Ks has also been linked to downstream Akt1 activation and insulin release under glucose-induced conditions (64). Akt is necessary for the phosphorylation of the Rab GTPase-activating protein AS160, responsible for glucose-stimulated insulin release, through IR/IRS-2 signaling (65). Focal adhesion kinase, which controls cytoskeletal remodeling and vesicle trafficking through integrin-initiated signaling, also phosphorylated the Akt/AS160 complex, indicating that this pathway may serve a role in focal adhesion kinase–mediated insulin release (66). In vitro studies have also determined that β-cell autocrine insulin signaling, in a glucose-induced environment, is necessary for activation of the PI3K/Akt pathway (67), which highlights the necessity of this signaling axis for insulin secretion. Our own studies have determined that c-Kit activation in mouse islets resulted in phosphorylation of serines 473 and serine 9 of Akt and GSK3β, respectively, leading to increased insulin secretion (45, 68). Recent work by others has shown that β-cell mTOR inactivation reduced Akt phosphorylation and inhibited insulin secretion without affecting islet mass (69). We also found that mTOR signaling in c-KitβTg mice is necessary for β-cell Vegf-a production and the subsequent promotion of islet vascularization and insulin secretion (46).
Interestingly, a temporal effect for PI3K suppression on rodent islets has been identified, where short-term inhibition of PI3K promoted increased newcomer granule exocytosis, whereas prolonged inhibition compromised insulin release (70). Additional support for an inhibitory role of the PI3K/Akt pathway was provided through experiments with p85α−/− mice, which revealed that high-glucose treatments increased insulin release (71). It should also be noted that PI3K signaling through IR activation has been shown to inhibit insulin release in human islets (72). Although activation of the PI3K/Akt pathway has been reported to have differing effects on insulin secretion, it remains an important regulator of exocytosis in β-cells.
Proposed model of c-Kit and IR interplay for insulin release
c-KitβTg islets exhibited increased expression of Ir and Irs-1 (45), which establishes a link between the c-Kit and IR/IRS pathways that can contribute to the insulin release axis. Increased insulin release was initially observed in 8-week c-KitβTg mice and continued to 28 weeks of age (46). These results suggest that transient c-Kit signaling in β-cells can lead to IR/IRS upregulation through the following: (i) a direct interaction that affects the activity of the IR/IRS pathway [previously proposed in Feng et al. (73)]; and (ii) indirect IR activation through increased insulin secretion from c-Kit signaling (Fig. 3A). However, recent data from our group indicated that the prolonged expression of c-Kit on β-cells resulted in negative feedback through serine phosphorylation of Irs-1Ser612, a known mechanism that downregulates IR/IRS signaling (75). This led to reduced signaling through the Akt pathway and subsequent defective insulin release. Decreasing the expression of IR on the β-cells of aging c-KitβTg mice improved their glucose tolerance. Interestingly, a recent study identified that IGF-1R was increased in aged β-cells that have poor insulin release (76). Activation of PDGFR has also been shown to promote IRS serine phosphorylation in adipocyte and vascular smooth muscle cells through PI3K/Akt/mTOR signaling (77, 78). The effects of c-Kit overexpression on β-cells can therefore influence the serine phosphorylation of IRS and promote dysregulated signaling of this axis through direct feedback from c-Kit–activated Akt signaling or from prolonged c-Kit–initiated insulin release (Fig. 3B). This evidence suggests that the IR axis may have a detrimental effect on insulin secretion in aged β-cells and that regulation of c-Kit–IR interplay must be considered to maintain appropriate insulin levels that contribute to the alleviation of hyperglycemia.
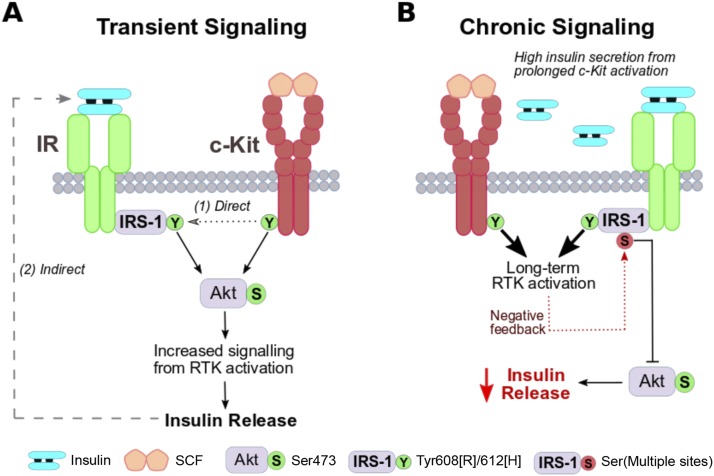
Figure 3.
Proposed c-Kit–IR interplay within the β-cell. (A) Transient receptor activation: c-Kit and IR both promote PI3K/Akt intracellular signaling, which leads to maintained insulin secretion. c-Kit receptor activation is proposed to promote upregulated IR/IRS signaling (via phosphorylation of tyrosine resides, green) through (i) direct activation and (ii) indirect activation through insulin secretion. Phosphorylation of IRS-1 tyrosine 608 in rodents and 612 in humans is linked to PI3K binding (74). (B) Chronic receptor activation: Sustained signaling through the PI3K/Akt pathway can lead to negative feedback through IRS-1 phosphorylation of serine residues (red) and impair insulin release. Multiple serine sites (e.g., Ser612) have been reported as phosphorylation targets from PI3K/Akt/mTOR/S6K1 signaling [for an extensive review, please see Copps and White (74)].
Potential Research Targets and Clinical RTK Therapeutic Applications for c-Kit–IR Pathway Activation
One of the major challenges for islet transplantation is preserving the insulin secretory efficacy of islets in patients. Inducing isolated islet proliferation and survival through ligand stimulation of RTKs has been examined as a potential therapy for improving islet transplantation. Increased HGF production from rodent islets via adenoviral transduction resulted in improved islet survival after transplantation and prevented the loss of intraportally transplanted islet function from immunosuppressive drug treatment (79, 80). Nonhuman primate islets transduced with murine HGF were also able to achieve euglycemic levels through increased islet survival when transplanted to streptozotocin (STZ)-induced diabetic mice (81). Similar improvements in islet survival and glucose tolerance in STZ-diabetic mice were noted when transplanted islets were pretreated with NGF (82). Improving the vascularization of rodent and human islets through their transfection with VEGF-A mRNA has been reported to increase β-cell volume (83) and restored glucose control in diabetic mouse models (83–85). The cotransplant of islet-containing scaffold grafts with a Vegf-a–releasing alginate sphere in STZ-diabetic mice also improved islet survival, vascularization, and the time interval for mice to reach restored normoglycemia (86). Additional studies have shown that delivering RTK ligands through transplanted islet-rich scaffolds or gels improved glucose homeostasis in diabetic mice (87, 88), which presents the effective role of RTK signaling as a promising strategy for extending the function of transplanted islets.
Our laboratory’s research proposed that RTK therapy targeting c-Kit and IR will increase the long-term function and survival of transplanted islets. Evidence from our previous work involving c-Kit signaling in murine islets has repeatedly shown that receptor activation increased insulin release under normal and short-term high-fat diet feeding (45) and promoted β-cell survival by downregulating the proapoptotic Fas receptor (89). c-Kit signaling also improved islet vascularization through increased β-cell Vegf-a secretion (46), which has been established as one strategy that can increase the survival and function of transplanted islets. The potential translation of c-Kit and SCF therapies in human islets has been demonstrated through our laboratory’s previous work on the human fetal pancreas. Phosphorylation of c-Kit in human islet-epithelial cell clusters in vitro, via either exogenous SCF treatment or antibody-targeted receptor activation, increased the proliferation and survival of insulin+ cells, increased the percentage of PDX-1+ and insulin+ cells found within the culture, and led to Akt phosphorylation (39, 90). Insulin treatment of isolated human and murine islets demonstrated a similar outcome, where islets had increased survival through Akt phosphorylation and Pdx1 nuclear translocation (62, 91). The activation of c-Kit and IR through exogenous SCF and insulin treatment, respectively, can therefore be used to improve long-term outcomes in recipients of transplanted islets.
Although activation of c-Kit–IR signaling pathways increases the capacity for β-cells to proliferate, diminishes cellular apoptosis, and increases insulin release to improve glucose tolerance, there is the potential for negative effects to emerge from this RTK therapy. Because of its prosurvival and replicative effects, activation of c-Kit signaling through SCF stimulation has been identified in a variety of different tumor types, and SCF treatment in pancreatic cell lines positive for c-Kit expression has increased their proliferation and invasion (92, 93). High levels of circulating insulin have been shown to induce inflammation in mice fed a high-fat diet and reduce the lifespan and insulin sensitivity in aged mice, and subsequently induced a diabetic phenotype in these rodent models (94, 95). Inhibitors that target RTKs have been examined for their potential to alleviate diabetic complications that emerge from the negative effects of prolonged intracellular activation, such as the promotion of islet inflammation and eventual β-cell apoptosis (96). The development of RTK therapies for transplanted islets must identify an optimal window where transient receptor activation will alleviate hyperglycemia by improving insulin secretory function while minimizing the cellular dysfunction observed with prolonged activation.
Summary and Perspectives
The secretion of insulin from β-cells is a controlled process that can be stimulated through many different means. RTKs are essential for the maintenance of normal β-cell development and function, and they play an important role in the regulation of granule exocytosis. Activation and signaling through the receptor c-Kit can increase the release of insulin and improve glycemia. Although the role of insulin activation of IR is controversial, a number of studies that focused on the expression and activation of IR in β-cells have found that this receptor is positively associated with insulin exocytosis. Intracellular signaling through the PI3K/Akt pathway shared by both c-Kit and IR activation is necessary for regulating insulin granule release. Interplay between β-cell membrane receptors can modulate granule release, but sustained activation of c-Kit and IR in β-cells can blunt insulin secretion, leading to insufficient control of blood glucose. Understanding the mechanisms through which c-Kit and IR control the release of insulin is essential for the potential application of RTK-based therapies because determining appropriate temporal or dosage levels will be necessary to optimize β-cell function in diabetes treatment.
Acknowledgments
We thank Drs. Nica Borradaile and Savita Dhanvantari for their help in reviewing this manuscript. A review of current information was performed through a literature search using the PubMed and Google Scholar databases. Portions of the information regarding c-Kit’s role in islet development and function are based on multiple studies from our laboratory.
Financial Support: The work featured in this review was supported by funding from the Canadian Institute of Health Research (CIHR 89800 to R.W.).
Author Contributions: A.O. drafted the manuscript. R.W. generated the outline and edited the review.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- DDR1
discoidin domain receptor tyrosine kinase 1
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- EphA
erythropoietin-producing hepatoma A
- ER
endoplasmic reticulum
- FGFR1
fibroblast growth factor receptor 1
- GLP-1R
glucagon-like peptide-1 receptor
- GPCR
G protein–coupled receptor
- HGF
hepatocyte growth factor
- IGF-1R
IGF-1 receptor
- IR
insulin receptor
- IRS
insulin receptor substrate
- NGF
nerve growth factor
- PDGFR
platelet-derived growth factor receptor
- PI3K
phosphatidylinositol 3-kinase
- RTK
receptor tyrosine kinase
- SCF
stem cell factor
- SNARE
soluble N-ethylmaleimide–sensitive factor attachment protein receptor
- STZ
streptozotocin
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
References
- 1. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103(7):2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arrojo e Drigo R, Ali Y, Diez J, Srinivasan DK, Berggren PO, Boehm BO. New insights into the architecture of the islet of Langerhans: a focused cross-species assessment. Diabetologia. 2015;58(10):2218–2228. [DOI] [PubMed] [Google Scholar]
- 3. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19–39. [PMC free article] [PubMed] [Google Scholar]
- 4. MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(suppl 3):S434–S442. [DOI] [PubMed] [Google Scholar]
- 5. Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, Thorens B. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest. 2004;113(4):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest. 2012;122(1):388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miettinen PJ, Huotari M, Koivisto T, Ustinov J, Palgi J, Rasilainen S, Lehtonen E, Keski-Oja J, Otonkoski T. Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development. 2000;127(12):2617–2627. [DOI] [PubMed] [Google Scholar]
- 8. Miettinen PJ, Ustinov J, Ormio P, Gao R, Palgi J, Hakonen E, Juntti-Berggren L, Berggren PO, Otonkoski T. Downregulation of EGF receptor signaling in pancreatic islets causes diabetes due to impaired postnatal beta-cell growth. Diabetes. 2006;55(12):3299–3308. [DOI] [PubMed] [Google Scholar]
- 9. Kritzik MR, Krahl T, Good A, Gu D, Lai C, Fox H, Sarvetnick N. Expression of ErbB receptors during pancreatic islet development and regrowth. J Endocrinol. 2000;165(1):67–77. [DOI] [PubMed] [Google Scholar]
- 10. Miralles F, Philippe P, Czernichow P, Scharfmann R. Expression of nerve growth factor and its high-affinity receptor Trk-A in the rat pancreas during embryonic and fetal life. J Endocrinol. 1998;156(3):431–439. [DOI] [PubMed] [Google Scholar]
- 11. Hald J, Galbo T, Rescan C, Radzikowski L, Sprinkel AE, Heimberg H, Ahnfelt-Rønne J, Jensen J, Scharfmann R, Gradwohl G, Kaestner KH, Stoeckert C Jr, Jensen JN, Madsen OD. Pancreatic islet and progenitor cell surface markers with cell sorting potential. Diabetologia. 2012;55(1):154–165. [DOI] [PubMed] [Google Scholar]
- 12. Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature. 2011;478(7369):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hakonen E, Ustinov J, Palgi J, Miettinen PJ, Otonkoski T. EGFR signaling promotes β-cell proliferation and survivin expression during pregnancy. PLoS One. 2014;9(4):e93651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demirci C, Ernst S, Alvarez-Perez JC, Rosa T, Valle S, Shridhar V, Casinelli GP, Alonso LC, Vasavada RC, García-Ocana A. Loss of HGF/c-Met signaling in pancreatic β-cells leads to incomplete maternal β-cell adaptation and gestational diabetes mellitus. Diabetes. 2012;61(5):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winzell MS, Ahrén B. G-protein-coupled receptors and islet function: implications for treatment of type 2 diabetes. Pharmacol Ther. 2007;116(3):437–448. [DOI] [PubMed] [Google Scholar]
- 16. Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther. 2013;139(3):359–391. [DOI] [PubMed] [Google Scholar]
- 17. Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni RN. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9(4):350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diao J, Asghar Z, Chan CB, Wheeler MB. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem. 2005;280(39):33487–33496. [DOI] [PubMed] [Google Scholar]
- 19. Hauge-Evans AC, Anderson RL, Persaud SJ, Jones PM. Delta cell secretory responses to insulin secretagogues are not mediated indirectly by insulin. Diabetologia. 2012;55(7):1995–2004. [DOI] [PubMed] [Google Scholar]
- 20. Muller D, Huang GC, Amiel S, Jones PM, Persaud SJ. Gene expression heterogeneity in human islet endocrine cells in vitro: the insulin signalling cascade. Diabetologia. 2007;50(6):1239–1242. [DOI] [PubMed] [Google Scholar]
- 21. Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, Wurst W, Nagamatsu S, Lammert E. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 2007;129(2):359–370. [DOI] [PubMed] [Google Scholar]
- 22. Hutchens T, Piston DW. EphA4 receptor forward signaling inhibits glucagon secretion from α-cells. Diabetes. 2015;64(11):3839–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kilkenny DM, Rocheleau JV. Fibroblast growth factor receptor-1 signaling in pancreatic islet beta-cells is modulated by the extracellular matrix. Mol Endocrinol. 2008;22(1):196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and beta cell proliferation. Dev Cell. 2006;10(3):397–405. [DOI] [PubMed] [Google Scholar]
- 25. Iwashita N, Uchida T, Choi JB, Azuma K, Ogihara T, Ferrara N, Gerber H, Kawamori R, Inoue M, Watada H. Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic beta cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia. 2007;50(2):380–389. [DOI] [PubMed] [Google Scholar]
- 26. Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, Hundemer GL, Kantz J, Thompson CS, Dai C, McGuinness OP, Powers AC. Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes. 2013;62(12):4154–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. Pancreatic islet production of vascular endothelial growth factor-a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–2985. [DOI] [PubMed] [Google Scholar]
- 28. Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, Shostak A, Radhika A, Poffenberger G, Quaggin SE, Jerome WG, Dumont DJ, Powers AC. Enhanced expression of VEGF-A in β cells increases endothelial cell number but impairs islet morphogenesis and β cell proliferation. Dev Biol. 2012;367(1):40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brissova M, Aamodt K, Brahmachary P, Prasad N, Hong JY, Dai C, Mellati M, Shostak A, Poffenberger G, Aramandla R, Levy SE, Powers AC. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration [published correction appears in Cell Metab. 2015;22(4):750]. Cell Metab. 2014;19(3):498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villalta SA, Lang J, Kubeck S, Kabre B, Szot GL, Calderon B, Wasserfall C, Atkinson MA, Brekken RA, Pullen N, Arch RH, Bluestone JA. Inhibition of VEGFR-2 reverses type 1 diabetes in NOD mice by abrogating insulitis and restoring islet function. Diabetes. 2013;62(8):2870–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Houtz J, Borden P, Ceasrine A, Minichiello L, Kuruvilla R. Neurotrophin signaling is required for glucose-induced insulin secretion. Dev Cell. 2016;39(3):329–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenbaum T, Sánchez-Soto MC, Hiriart M. Nerve growth factor increases insulin secretion and barium current in pancreatic beta-cells. Diabetes. 2001;50(8):1755–1762. [DOI] [PubMed] [Google Scholar]
- 33. Pingitore A, Caroleo MC, Cione E, Castañera Gonzalez R, Huang GC, Persaud SJ. Fine tuning of insulin secretion by release of nerve growth factor from mouse and human islet β-cells. Mol Cell Endocrinol. 2016;436:23–32. [DOI] [PubMed] [Google Scholar]
- 34. Roccisana J, Reddy V, Vasavada RC, Gonzalez-Pertusa JA, Magnuson MA, Garcia-Ocaña A. Targeted inactivation of hepatocyte growth factor receptor c-met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass. Diabetes. 2005;54(7):2090–2102. [DOI] [PubMed] [Google Scholar]
- 35. Xuan S, Kitamura T, Nakae J, Politi K, Kido Y, Fisher PE, Morroni M, Cinti S, White MF, Herrera PL, Accili D, Efstratiadis A. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J Clin Invest. 2002;110(7):1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR. Beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet. 2002;31(1):111–115. [DOI] [PubMed] [Google Scholar]
- 37. Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55(9):2470–2478. [DOI] [PubMed] [Google Scholar]
- 38. Jain R, Jain D, Liu Q, Bartosinska B, Wang J, Schumann D, Kauschke SG, Eickelmann P, Piemonti L, Gray NS, Lammert E. Pharmacological inhibition of Eph receptors enhances glucose-stimulated insulin secretion from mouse and human pancreatic islets. Diabetologia. 2013;56(6):1350–1355. [DOI] [PubMed] [Google Scholar]
- 39. Li J, Quirt J, Do HQ, Lyte K, Fellows F, Goodyer CG, Wang R. Expression of c-Kit receptor tyrosine kinase and effect on beta-cell development in the human fetal pancreas. Am J Physiol Endocrinol Metab. 2007;293(2):E475–E483. [DOI] [PubMed] [Google Scholar]
- 40. Yashpal NK, Li J, Wang R. Characterization of c-Kit and nestin expression during islet cell development in the prenatal and postnatal rat pancreas. Dev Dyn. 2004;229(4):813–825. [DOI] [PubMed] [Google Scholar]
- 41. Rachdi L, El Ghazi L, Bernex F, Panthier JJ, Czernichow P, Scharfmann R. Expression of the receptor tyrosine kinase KIT in mature beta-cells and in the pancreas in development. Diabetes. 2001;50(9):2021–2028. [DOI] [PubMed] [Google Scholar]
- 42. Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92(4):1619–1649. [DOI] [PubMed] [Google Scholar]
- 43. Wu Y, Li J, Saleem S, Yee SP, Hardikar AA, Wang R. c-Kit and stem cell factor regulate PANC-1 cell differentiation into insulin- and glucagon-producing cells. Lab Invest. 2010;90(9):1373–1384. [DOI] [PubMed] [Google Scholar]
- 44. Krishnamurthy M, Ayazi F, Li J, Lyttle AW, Woods M, Wu Y, Yee SP, Wang R. c-Kit in early onset of diabetes: a morphological and functional analysis of pancreatic beta-cells in c-KitW-v mutant mice. Endocrinology. 2007;148(11):5520–5530. [DOI] [PubMed] [Google Scholar]
- 45. Feng ZC, Li J, Turco BA, Riopel M, Yee SP, Wang R. Critical role of c-Kit in beta cell function: increased insulin secretion and protection against diabetes in a mouse model. Diabetologia. 2012;55(8):2214–2225. [DOI] [PubMed] [Google Scholar]
- 46. Feng ZC, Popell A, Li J, Silverstein J, Oakie A, Yee SP, Wang R. c-Kit receptor signaling regulates islet vasculature, β-cell survival, and function in vivo. Diabetes. 2015;64(11):3852–3866. [DOI] [PubMed] [Google Scholar]
- 47. Columbo M, Horowitz EM, Botana LM, MacGlashan DW Jr, Bochner BS, Gillis S, Zsebo KM, Galli SJ, Lichtenstein LM. The human recombinant c-kit receptor ligand, rhSCF, induces mediator release from human cutaneous mast cells and enhances IgE-dependent mediator release from both skin mast cells and peripheral blood basophils. J Immunol. 1992;149(2):599–608. [PubMed] [Google Scholar]
- 48. Gagari E, Tsai M, Lantz CS, Fox LG, Galli SJ. Differential release of mast cell interleukin-6 via c-kit. Blood. 1997;89(8):2654–2663. [PubMed] [Google Scholar]
- 49. Vosseller K, Stella G, Yee NS, Besmer P. c-kit receptor signaling through its phosphatidylinositide-3′-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Mol Biol Cell. 1997;8(5):909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ito T, Smrž D, Jung MY, Bandara G, Desai A, Smržová Š, Kuehn HS, Beaven MA, Metcalfe DD, Gilfillan AM. Stem cell factor programs the mast cell activation phenotype. J Immunol. 2012;188(11):5428–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen S, Burgin S, McDaniel A, Li X, Yuan J, Chen M, Khalaf W, Clapp DW, Yang FC. Nf1−/− Schwann cell-conditioned medium modulates mast cell degranulation by c-Kit-mediated hyperactivation of phosphatidylinositol 3-kinase. Am J Pathol. 2010;177(6):3125–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu GG, Rothenberg PL. Insulin receptor signaling in the beta-cell influences insulin gene expression and insulin content: evidence for autocrine beta-cell regulation. Diabetes. 1998;47(8):1243–1252. [DOI] [PubMed] [Google Scholar]
- 53. Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96(3):329–339. [DOI] [PubMed] [Google Scholar]
- 54. Ryu J, Galan AK, Xin X, Dong F, Abdul-Ghani MA, Zhou L, Wang C, Li C, Holmes BM, Sloane LB, Austad SN, Guo S, Musi N, DeFronzo RA, Deng C, White MF, Liu F, Dong LQ. APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Reports. 2014;7(4):1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheng KK, Lam KS, Wu D, Wang Y, Sweeney G, Hoo RL, Zhang J, Xu A. APPL1 potentiates insulin secretion in pancreatic β cells by enhancing protein kinase Akt-dependent expression of SNARE proteins in mice. Proc Natl Acad Sci USA. 2012;109(23):8919–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aspinwall CA, Lakey JR, Kennedy RT. Insulin-stimulated insulin secretion in single pancreatic beta cells. J Biol Chem. 1999;274(10):6360–6365. [DOI] [PubMed] [Google Scholar]
- 57. Khan FA, Goforth PB, Zhang M, Satin LS. Insulin activates ATP-sensitive K(+) channels in pancreatic beta-cells through a phosphatidylinositol 3-kinase-dependent pathway. Diabetes. 2001;50(10):2192–2198. [DOI] [PubMed] [Google Scholar]
- 58. Aspinwall CA, Qian WJ, Roper MG, Kulkarni RN, Kahn CR, Kennedy RT. Roles of insulin receptor substrate-1, phosphatidylinositol 3-kinase, and release of intracellular Ca2+ stores in insulin-stimulated insulin secretion in beta-cells. J Biol Chem. 2000;275(29):22331–22338. [DOI] [PubMed] [Google Scholar]
- 59. Borge PD Jr, Wolf BA. Insulin receptor substrate 1 regulation of sarco-endoplasmic reticulum calcium ATPase 3 in insulin-secreting beta-cells. J Biol Chem. 2003;278(13):11359–11368. [DOI] [PubMed] [Google Scholar]
- 60. Kulkarni RN, Roper MG, Dahlgren G, Shih DQ, Kauri LM, Peters JL, Stoffel M, Kennedy RT. Islet secretory defect in insulin receptor substrate 1 null mice is linked with reduced calcium signaling and expression of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)-2b and -3. Diabetes. 2004;53(6):1517–1525. [DOI] [PubMed] [Google Scholar]
- 61. Araujo EP, Amaral ME, Souza CT, Bordin S, Ferreira F, Saad MJ, Boschero AC, Magalhães EC, Velloso LA. Blockade of IRS1 in isolated rat pancreatic islets improves glucose-induced insulin secretion. FEBS Lett. 2002;531(3):437–442. [DOI] [PubMed] [Google Scholar]
- 62. Aikin R, Hanley S, Maysinger D, Lipsett M, Castellarin M, Paraskevas S, Rosenberg L. Autocrine insulin action activates Akt and increases survival of isolated human islets. Diabetologia. 2006;49(12):2900–2909. [DOI] [PubMed] [Google Scholar]
- 63. Kaneko K, Ueki K, Takahashi N, Hashimoto S, Okamoto M, Awazawa M, Okazaki Y, Ohsugi M, Inabe K, Umehara T, Yoshida M, Kakei M, Kitamura T, Luo J, Kulkarni RN, Kahn CR, Kasai H, Cantley LC, Kadowaki T. Class IA phosphatidylinositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell Metab. 2010;12(6):619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leibiger B, Moede T, Uhles S, Barker CJ, Creveaux M, Domin J, Berggren PO, Leibiger IB. Insulin-feedback via PI3K-C2alpha activated PKBalpha/Akt1 is required for glucose-stimulated insulin secretion. FASEB J. 2010;24(6):1824–1837. [DOI] [PubMed] [Google Scholar]
- 65. Bouzakri K, Ribaux P, Tomas A, Parnaud G, Rickenbach K, Halban PA. Rab GTPase-activating protein AS160 is a major downstream effector of protein kinase B/Akt signaling in pancreatic beta-cells. Diabetes. 2008;57(5):1195–1204. [DOI] [PubMed] [Google Scholar]
- 66. Rondas D, Tomas A, Soto-Ribeiro M, Wehrle-Haller B, Halban PA. Novel mechanistic link between focal adhesion remodeling and glucose-stimulated insulin secretion. J Biol Chem. 2012;287(4):2423–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hagren OI, Tengholm A. Glucose and insulin synergistically activate phosphatidylinositol 3-kinase to trigger oscillations of phosphatidylinositol 3,4,5-trisphosphate in beta-cells. J Biol Chem. 2006;281(51):39121–39127. [DOI] [PubMed] [Google Scholar]
- 68. Feng ZC, Donnelly L, Li J, Krishnamurthy M, Riopel M, Wang R. Inhibition of Gsk3β activity improves β-cell function in c-KitWv/+ male mice. Lab Invest. 2012;92(4):543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alejandro EU, Bozadjieva N, Blandino-Rosano M, Wasan MA, Elghazi L, Vadrevu S, Satin L, Bernal-Mizrachi E. Overexpression of kinase-dead mTOR impairs glucose homeostasis by regulating insulin secretion and not β-cell mass. Diabetes. 2017;66(8):2150–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Aoyagi K, Ohara-Imaizumi M, Nishiwaki C, Nakamichi Y, Ueki K, Kadowaki T, Nagamatsu S. Acute inhibition of PI3K-PDK1-Akt pathway potentiates insulin secretion through upregulation of newcomer granule fusions in pancreatic β-cells. PLoS One. 2012;7(10):e47381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eto K, Yamashita T, Tsubamoto Y, Terauchi Y, Hirose K, Kubota N, Yamashita S, Taka J, Satoh S, Sekihara H, Tobe K, Iino M, Noda M, Kimura S, Kadowaki T. Phosphatidylinositol 3-kinase suppresses glucose-stimulated insulin secretion by affecting post-cytosolic [Ca(2+)] elevation signals. Diabetes. 2002;51(1):87–97. [DOI] [PubMed] [Google Scholar]
- 72. Persaud SJ, Asare-Anane H, Jones PM. Insulin receptor activation inhibits insulin secretion from human islets of Langerhans. FEBS Lett. 2002;510(3):225–228. [DOI] [PubMed] [Google Scholar]
- 73. Feng ZC, Riopel M, Popell A, Wang R. A survival kit for pancreatic beta cells: stem cell factor and c-Kit receptor tyrosine kinase. Diabetologia. 2015;58(4):654–665. [DOI] [PubMed] [Google Scholar]
- 74. Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55(10):2565–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oakie A, Feng Z-C, Li J, Silverstein J, Wang R. Overexpression of c-Kit in aged mice stimulates beta cell proliferation, but leads to impaired beta cell function. Program of the 53rd Annual Meeting of the European Association for the Study of Diabetes. Lisbon, Portugal [abstract]. Diabetologia. 2017;60(suppl 1):S102. Abstract 220. [Google Scholar]
- 76. Aguayo-Mazzucato C, van Haaren M, Mruk M, Lee TB Jr, Crawford C, Hollister-Lock J, Sullivan BA, Johnson JW, Ebrahimi A, Dreyfuss JM, Van Deursen J, Weir GC, Bonner-Weir S. β Cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab. 2017;25(4):898–910.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Staubs PA, Nelson JG, Reichart DR, Olefsky JM. Platelet-derived growth factor inhibits insulin stimulation of insulin receptor substrate-1-associated phosphatidylinositol 3-kinase in 3T3-L1 adipocytes without affecting glucose transport. J Biol Chem. 1998;273(39):25139–25147. [DOI] [PubMed] [Google Scholar]
- 78. Zhao Y, Biswas SK, McNulty PH, Kozak M, Jun JY, Segar L. PDGF-induced vascular smooth muscle cell proliferation is associated with dysregulation of insulin receptor substrates. Am J Physiol Cell Physiol. 2011;300(6):C1375–C1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Garcia-Ocana A, Takane KK, Reddy VT, Lopez-Talavera JC, Vasavada RC, Stewart AF. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces beta cell death. J Biol Chem. 2003;278(1):343–351. [DOI] [PubMed] [Google Scholar]
- 80. Lopez-Talavera JC, Garcia-Ocaña A, Sipula I, Takane KK, Cozar-Castellano I, Stewart AF. Hepatocyte growth factor gene therapy for pancreatic islets in diabetes: reducing the minimal islet transplant mass required in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology. 2004;145(2):467–474. [DOI] [PubMed] [Google Scholar]
- 81. Fiaschi-Taesch NM, Berman DM, Sicari BM, Takane KK, Garcia-Ocaña A, Ricordi C, Kenyon NS, Stewart AF. Hepatocyte growth factor enhances engraftment and function of nonhuman primate islets. Diabetes. 2008;57(10):2745–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Miao G, Mace J, Kirby M, Hopper A, Peverini R, Chinnock R, Shapiro J, Hathout E. In vitro and in vivo improvement of islet survival following treatment with nerve growth factor. Transplantation. 2006;81(4):519–524. [DOI] [PubMed] [Google Scholar]
- 83. Staels W, Verdonck Y, Heremans Y, Leuckx G, De Groef S, Heirman C, de Koning E, Gysemans C, Thielemans K, Baeyens L, Heimberg H, De Leu N. Vegf-A mRNA transfection as a novel approach to improve mouse and human islet graft revascularisation. Diabetologia. 2018;61(8):1804–1810. [DOI] [PubMed] [Google Scholar]
- 84. Shimoda M, Chen S, Noguchi H, Matsumoto S, Grayburn PA. In vivo non-viral gene delivery of human vascular endothelial growth factor improves revascularisation and restoration of euglycaemia after human islet transplantation into mouse liver. Diabetologia. 2010;53(8):1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cross SE, Richards SK, Clark A, Benest AV, Bates DO, Mathieson PW, Johnson PR, Harper SJ, Smith RM. Vascular endothelial growth factor as a survival factor for human islets: effect of immunosuppressive drugs. Diabetologia. 2007;50(7):1423–1432. [DOI] [PubMed] [Google Scholar]
- 86. Gebe JA, Preisinger A, Gooden MD, D’Amico LA, Vernon RB. Local, controlled release in vivo of vascular endothelial growth factor within a subcutaneous scaffolded islet implant reduces early islet necrosis and improves performance of the graft. Cell Transplant. 2018;27(3):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stendahl JC, Wang LJ, Chow LW, Kaufman DB, Stupp SI. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86(3):478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Najjar M, Manzoli V, Abreu M, Villa C, Martino MM, Molano RD, Torrente Y, Pileggi A, Inverardi L, Ricordi C, Hubbell JA, Tomei AA. Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol Bioeng. 2015;112(9):1916–1926. [DOI] [PubMed] [Google Scholar]
- 89. Feng ZC, Riopel M, Li J, Donnelly L, Wang R. Downregulation of Fas activity rescues early onset of diabetes in c-Kit(Wv/+) mice. Am J Physiol Endocrinol Metab. 2013;304(6):E557–E565. [DOI] [PubMed] [Google Scholar]
- 90. Li J, Goodyer CG, Fellows F, Wang R. Stem cell factor/c-Kit interactions regulate human islet-epithelial cluster proliferation and differentiation. Int J Biochem Cell Biol. 2006;38(5-6):961–972. [DOI] [PubMed] [Google Scholar]
- 91. Johnson JD, Bernal-Mizrachi E, Alejandro EU, Han Z, Kalynyak TB, Li H, Beith JL, Gross J, Warnock GL, Townsend RR, Permutt MA, Polonsky KS. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci USA. 2006;103(51):19575–19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, Rosenblum M, Mikkelsen T, Fine HA. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9(4):287–300. [DOI] [PubMed] [Google Scholar]
- 93. Yasuda A, Sawai H, Takahashi H, Ochi N, Matsuo Y, Funahashi H, Sato M, Okada Y, Takeyama H, Manabe T. The stem cell factor/c-kit receptor pathway enhances proliferation and invasion of pancreatic cancer cells. Mol Cancer. 2006;5(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, Bamji SX, Clee SM, Johnson JD. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16(6):723–737. [DOI] [PubMed] [Google Scholar]
- 95. Templeman NM, Flibotte S, Chik JHL, Sinha S, Lim GE, Foster LJ, Nislow C, Johnson JD. Reduced circulating insulin enhances insulin sensitivity in old mice and extends lifespan. Cell Reports. 2017;20(2):451–463. [DOI] [PubMed] [Google Scholar]
- 96. Fountas A, Diamantopoulos LN, Tsatsoulis A. Tyrosine kinase inhibitors and diabetes: a novel treatment paradigm? [published correction appears in Trends Endocrinol Metab. 2016;27(1):65]. Trends Endocrinol Metab. 2015;26(11):643–656. [DOI] [PubMed] [Google Scholar]