Abstract
An early sign of islet failure in type 2 diabetes (T2D) is the loss of normal patterns of pulsatile insulin release. Disruptions in pulsatility are associated with a left shift in glucose sensing that can cause excessive insulin release in low glucose (relative hyperinsulinemia, a hallmark of early T2D) and β-cell exhaustion, leading to inadequate insulin release during hyperglycemia. Our hypothesis was that reducing excessive glucokinase activity in diabetic islets would improve their function. Isolated mouse islets were exposed to glucose and varying concentrations of the glucokinase inhibitor d-mannoheptulose (MH) to examine changes in intracellular calcium ([Ca2+]i) and insulin secretion. Acutely exposing islets from control CD-1 mice to MH in high glucose (20 mM) dose dependently reduced the size of [Ca2+]i oscillations detected by fura-2 acetoxymethyl. Glucokinase activation in low glucose (3 mM) had the opposite effect. We then treated islets from male and female db/db mice (age, 4 to 8 weeks) and heterozygous controls overnight with 0 to 10 mM MH to determine that 1 mM MH produced optimal oscillations. We then used 1 mM MH overnight to measure [Ca2+]i and insulin simultaneously in db/db islets. MH restored oscillations and increased insulin secretion. Insulin secretion rates correlated with MH-induced increases in amplitude of [Ca2+]i oscillations (R2 = 0.57, P < 0.01, n = 10) but not with mean [Ca2+]i levels in islets (R2 = 0.05, not significant). Our findings show that correcting glucose sensing can restore proper pulsatility to diabetic islets and improved pulsatility correlates with enhanced insulin secretion.
Islets of Langerhans are micro-organs within the pancreas that are responsible for regulating blood glucose and body energy metabolism (1). In response to increased blood glucose after a meal, β-cells within the islet secrete insulin to stimulate the conversion of glucose to glycogen in the liver, suppress hepatic glucose output, and facilitate the uptake of glucose into target tissues, in particular, muscle and fat. The “consensus model” provides a detailed description of how β-cells respond to glucose stimulation (2–4). In brief, glucose is taken up through glucose transporters and metabolized through glycolysis and the tricarboxylic acid cycle to increase ATP production. The resulting increase in the ATP/ADP ratio closes ATP-sensitive potassium channels, which initiates a large influx of Ca2+ to trigger insulin release. Additional insulin release occurs independently of the consensus pathway via multiple interconnected mechanisms, collectively called the “metabolic amplifying pathway” (5). Under normal physiological conditions, glucose is tightly regulated in the body (~6 to ~9 mM glucose); thus, the extreme glucose excursions that produce biphasic insulin release are not observed. Instead, endogenous cycles in glucose metabolism produce pulses of insulin at ~5-minute intervals. The pulses are large after meals and small between meals and overnight.
Why is insulin pulsatility important? Delivering intermittent insulin to healthy individuals has been shown to produce a more effective insulin response than continuous insulin delivery (6–9). More importantly, patients with type 2 diabetes (T2D) respond more effectively to pulsatile insulin than to continuous delivery (10–12). The loss of pulsatile insulin secretion occurs during the early stages of T2D, with a reduction in the amplitude of the insulin pulses linked to subsequent diabetes (13–17). The close relatives of patients with diabetes have also demonstrated considerable degradation of pulsatile insulin secretion despite having clinically normal glucose tolerance and insulin sensitivity (18, 19). Studies have shown in dogs and rats that pulsatile insulin is more efficacious in suppressing hepatic glucose production than is continuous insulin because pulsatile delivery potentiated insulin receptor signal transduction in the liver (20–22). The loss of pulsatility might be a source of reduced hepatic insulin clearance and reduced glucose conversion to glycogen that might trigger excess insulin, leading to a vicious cycle. At the islet level, nonoscillatory islets are associated with reduced glucose responsiveness and greater signs of endoplasmic reticulum stress compared with oscillatory islets (23). Thus, considerable evidence has suggested that pulsatile insulin secretion is important to the regulation of blood glucose and that the loss of pulsatility is a potential early warning sign of emerging disease (24, 25).
Relative hyperinsulinemia, an elevated fasting insulin level relative to the glucose level, has been reported for several diabetes-prone populations (26, 27). Evidence suggests that the hypersecretion of insulin may play a primary role in the pathogenesis of T2D. Hyperinsulinemia is among some of the earliest detectable changes in metabolism in diabetes-prone people (28–30), in nonhuman primates (31), and in several rodent models of T2D (32–36). It is not known whether increased insulin levels are a cause or an effect of insulin resistance. Studies have shown that basal hyperinsulinemia in healthy normoglycemic adults can predict the likelihood of developing T2D in the next ≥20 years independently of the presence of insulin resistance (28, 29). Recent work has also suggested that normal islets develop a “left shift” in glucose sensitivity owing to chronic exposure to nutrient-derived factors, including glucose and free fatty acids (37–39). A left shift means that lower levels of glucose are sufficient to trigger a response (left being toward zero in a dose–response curve). This supports previous observations that a 90% pancreatectomy causes a left shift in glucose sensitivity owing to the massively increased workload on the remaining islets (40, 41). Moreover, another study showed that insulin secretion rates from perfused pancreas in Zucker diabetic fatty rats are maximal at 3 to 5 mM glucose and actually decline with an increased glucose load (34). This relationship between insulin and glucose was described as “paradoxical” (34). The effect was attributed to greater rates of glycolysis in low glucose conditions (34, 42) and suggested aberrant β-cell glucose sensing. Thus, a left shift in glucose sensitivity could be considered a normal compensatory response to nutrient overload (38). However, this same adaptation could be harmful to diabetes-prone islets if the push to secrete more insulin impairs the processes that couple nutrient stimulation to insulin secretion (43).
In the present study, we found that mildly reducing glucokinase activity in islets from newly diabetic db/db mice can “right shift” responses and restore normal glucose sensing. After overnight treatment with the glucokinase-specific inhibitor d-mannoheptulose (MH), the disruptions in pulsatility observed in these diabetic islets in postprandial glucose levels (11 mM) were ameliorated. Robust endogenous pulsatility was restored to the physiological range of glucose and insulin release also “paradoxically” increased. Rather than trying to stimulate more and more insulin release from β-cells, our work has provided proof of concept that slightly reducing glycolytic rates can enhance, rather than inhibit, insulin secretion by restoring endogenous pulsatility and normal glucose sensing. This represents a substantial shift in the perception of how to treat the β-cell in the early stages of T2D.
Materials and Methods
Mice
Male and female db/db mice at 4 to 8 weeks of age and age-matched heterozygous male and female mice were used for these studies. The mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in a pathogen-free facility at Ohio University or the University of Virginia. Most studies were conducted using male mice. Islets from two female db/db mice and one heterozygous control were used for the studies of the relative glucose sensitivity of normal islets vs islets from db/db mice. Islets from one female db/db and one heterozygous control were used for studies of the effect of different doses of overnight MH treatment on overall intracellular calcium ([Ca2+]i) oscillations in islets from db/db and heterozygous mice. The respective institutional animal care and use committees approved all protocols used in these studies. For a subset of studies, outbred male CD-1 mice aged 8 to 14 weeks (Envigo, Indianapolis, IN) were used as a control strain to test the effects of MH on glucose sensitivity in isolated islets.
Chemicals
MH was purchased from Carbosynth (San Diego, CA) or Millipore-Sigma (St. Louis, MO) with no observed differences in the effects from the different sources. Ro-28-1675 was purchased from Axon Medchem (Reston, VA) All other reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless indicated otherwise.
Islet isolation
Pancreatic islets were isolated and cultured as described previously (44). After isolation, the islets were placed overnight in RPMI 1640 media (Invitrogen, Carlsbad, CA) containing 11 mM glucose, 10% fetal bovine serum, and 1% penicillin/streptomycin. The experiments were performed 1 to 2 days after isolation.
[Ca2+]i
Islets were loaded with 1 µM fura-2 acetoxymethyl (AM) (Thermo Fisher Scientific, Waltham, MA) in a mixture of two modified Krebs-Ringer bicarbonate (KRB) solutions: a low glucose solution (3 mM glucose, 134.5 mM NaCl, 3 mM CaCl2, 5 mM KCl, 2 mM MgCl2, 10 mM HEPES; pH 7.4) and a high glucose solution (28 mM glucose, 122 mM NaCl, 3 mM CaCl2, 5 mM KCl, 2 mM MgCl2, 10 mM HEPES; pH 7.4). These solutions were mixed in appropriate proportional volumes to produce various glucose concentrations. A separate solution was made for the 0 mM glucose related to the data from the studies of the relative glucose sensitivity of normal islets vs islets from db/db mice and for glucose-stimulated insulin secretion studies. The islets were loaded for 30 minutes at 37°C and 5% CO2 and then transferred to the recording chamber for an additional 10 minutes. [Ca2+]i was measured using the ratiometric [Ca2+]i indicator fura-2AM, as described previously (45).
Simultaneous measurements of [Ca2+]i and insulin secretion
Islets were incubated in fura-2AM for 30 minutes and then transferred to the recording chamber as described in the preceding section. The total number of islets in the chamber was counted while 11 mM glucose solution was perifused through the chamber for 5 minutes into a waste container. At the 5-minute mark, the [Ca2+]i recording began and the perifusion system began recirculating a 500-µL volume of 11 mM glucose solution contained in a 1.5-mL microcentrifuge tube. At the end of the 30-minute recording, all supernatant in the chamber was drained into the 1.5-mL microcentrifuge tube, and the islets were collected in a second labeled tube. Supernatant and islets were spun down, 500 µL of the supernatant was drawn off for insulin measurements, and the volume of the remaining supernatant was measured to normalize the insulin to the fluid volume. The chamber was dried between experiments to remove any insulin-containing supernatant from the previous experiment. Insulin secretion was determined using an ELISA (Alpco, Salem, NH), as described previously (46). The inter- and intra-assay variability was <10%.
Autofluorescence of nicotinamide adenine dinucleotide hydrate and reduced form of nicotinamide adenine dinucleotide phosphate
Islets were recorded for the autofluorescence produced by nicotinamide adenine dinucleotide hydrate and/or the reduced form of nicotinamide adenine dinucleotide phosphate [NAD(P)H] by imaging islets using 340 nm excitation and 520 nm emission light. Islets were recorded using a method similar to that described to measure [Ca2+]i but without using fura-2AM, as described previously (47).
ATP content
Sets of size-matched islets (15 islets for one trial and 20 islets for another trial) were incubated overnight with or without MH as described in the “Results” section. After treatment, the islets were incubated in KRB containing 0 mM glucose for 1 hour to reduce islet metabolism among all treatment groups and then transferred to a KRB solution containing 11 mM glucose for 1 hour. Islets were then collected and lysed. The ATPlite Luminescence Assay System (PerkinElmer, Waltham, MA) was used according to the manufacturer’s instructions to measure the ATP content in the islets.
Pulsatile analysis of [Ca2+]i patterns
Oscillatory islet activity was analyzed using CLUSTER8 pulse detection software (48), as previously described (43, 49–51). Within CLUSTER8, parameters were specified to appropriately identify pulses and filter out random pulse-like noise within the traces. The following CLUSTER8 parameters were used: two points were required for each peak or nadir, a t-score of two was required to detect an increase or decrease, and a t-score of four was required to detect outliers. For experimental data, three-point moving averages and an SD of 0.02 were used to reduce false-positive results due to noise in the data before analysis.
Statistical analysis
Standard statistical analysis tests were used. Paired and unpaired t tests were used for direct comparisons between two groups (db/db vs heterozygous, untreated vs MH-treated, and so forth), one-way ANOVA for multiple comparisons, and Fisher exact test for contingency tables.
Results
Oscillatory range of glycolytic activity in pancreatic islets
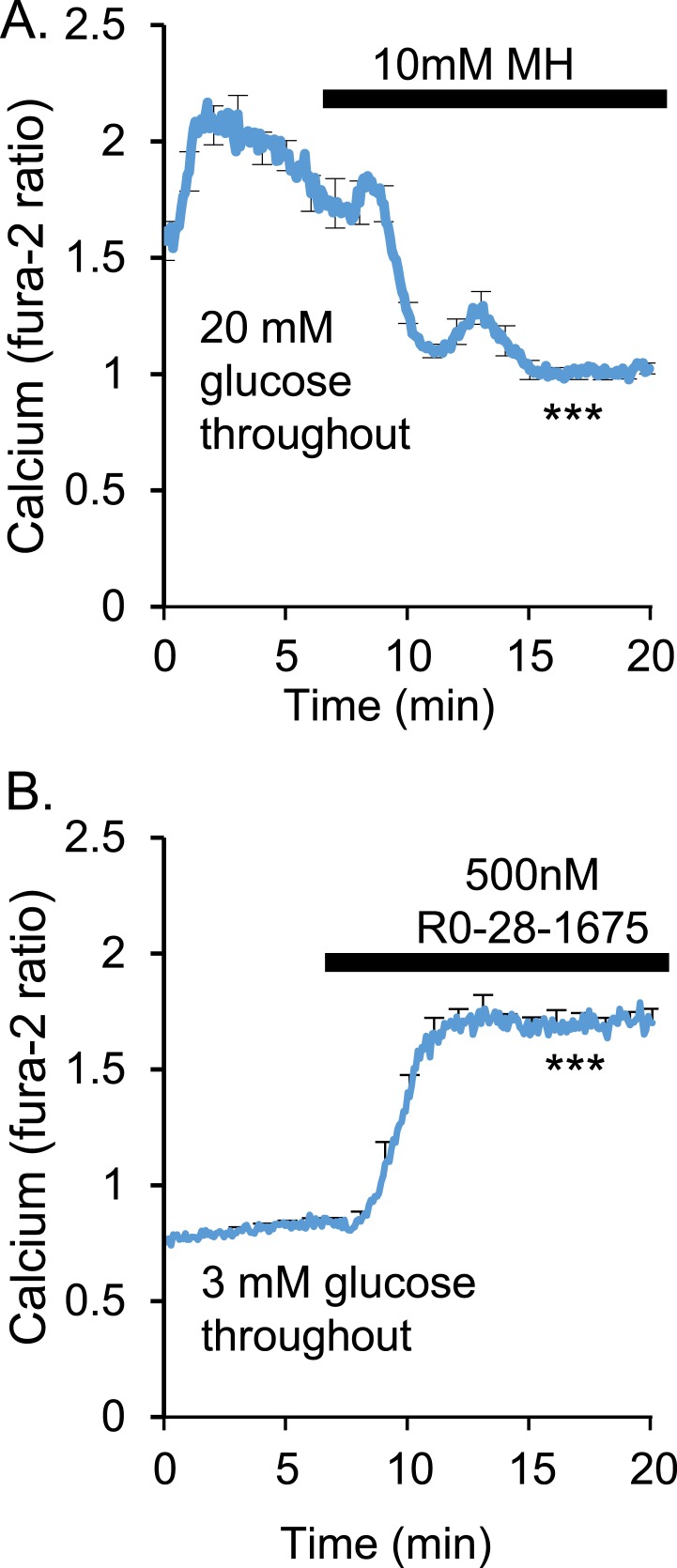
It is well-established that inhibiting glucokinase in the normal β-cell will block glycolysis and stop insulin release, causing hyperglycemia (52–56). To demonstrate this effect at the islet level, we exposed normal healthy islets isolated from adult outbred CD-1 mice to 10 mM MH in vitro to inhibit glucokinase activity. Acute MH exposure to islets in high 20-mM glucose at 5 minutes caused a consistent decrease in the ratiometric fura-2AM signal, indicating a reduction in [Ca2+]i, the proximal step to insulin secretion, which reaches a maximal effect by 15 minutes (Fig. 1A). This reduction in [Ca2+]i was observed among all islets tested (n = 17 islets; P < 0.001). The opposite effect can be produced by treating islets in low 3-mM glucose with the glucokinase activator Ro-28-1675. [Ca2+]i increased rapidly to a plateau after exposure to the glucokinase activator among all islets tested (n = 10 islets, P < 0.001; Fig. 1B).
Figure 1.
[Ca2+]i levels depend on glucokinase activity. (A) In high glucose (20 mM), acute exposure with a 10-mM concentration of the glucokinase inhibitor MH caused a rapid decrease in [Ca2+]i to levels typically observed in very low glucose (n = 17 islets, ***P < 0.001). (B) Stimulation of glucokinase activity with 500 nM Ro-28-1675 in low glucose (3 mM) led to constitutively high [Ca2+]i (n = 10 islets, ***P < 0.001).
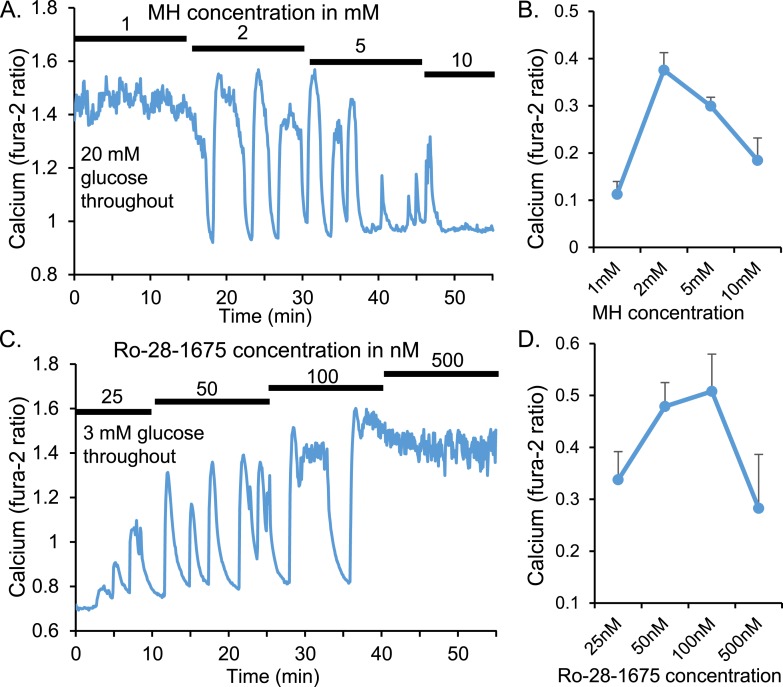
We have demonstrated how manipulating glycolytic activity to much smaller degrees can affect pulsatility as measured by changes in the patterns of [Ca2+]i in CD-1 mice (Fig. 2). MH exposure in high 20-mM glucose reduced the saturated rates of glycolysis to reveal large amplitude pulses (also called “oscillations”) in [Ca2+]i, which diminished dose dependently with increasing MH doses until the islets were fully inhibited at 10 mM MH (Fig. 2A).
Figure 2.
Islet pulsatility depends on glucokinase activity. (A) Representative [Ca2+]i trace showing increasing doses of the glucokinase inhibitor MH in high (20 mM) glucose causes constitutively high [Ca2+]i to go through a range of pulsatile patterns to basal [Ca2+]i. (B) Mean amplitude ± SEM of [Ca2+]i oscillations for each MH concentration (n = 12 islets). (C) Stimulation of glucokinase activity by Ro-28-1675 led to a dose-dependent increase in [Ca2+]i pulsatility to constitutive calcium influx at the highest Ro-28-1675 concentration (n = 12 islets). (D) Mean amplitude ± SEM of [Ca2+]i oscillations for each Ro-28-1675 concentration.
The amplitude of [Ca2+]i oscillations detected by the CLUSTER8 pulse detection algorithm follows an “inverted U” dose response (Fig. 2B) (57). When glucokinase activity is high, [Ca2+]i will be maintained at a plateau with very little deviation, resulting in small amplitude pulses (or no detectable pulses at all). As the increasing MH levels decrease these saturating levels of glucokinase activity, oscillations in glycolysis and [Ca2+]i emerge. The amplitude of these oscillations diminishes as glucokinase activity is reduced further. This dose–response relationship is observed in reverse using the glucokinase activator Ro-28-1675 beginning in low glucose (Fig. 2C and 2D).
Together, these results show that a range of glycolytic activity supports pulsatility. This is consistent with previous work showing a range of glucose concentrations that support oscillatory activity in pancreatic islets (47, 58). Too little glucose will not trigger a sufficient shift in the ATP/ADP ratio to close ATP-sensitive potassium channels and activate voltage-gated calcium channels. Too much glucose will saturate glucokinase activity such that glycolytic cycling does not occur.
Acute exposure to MH generally does not restore normal pulsatility
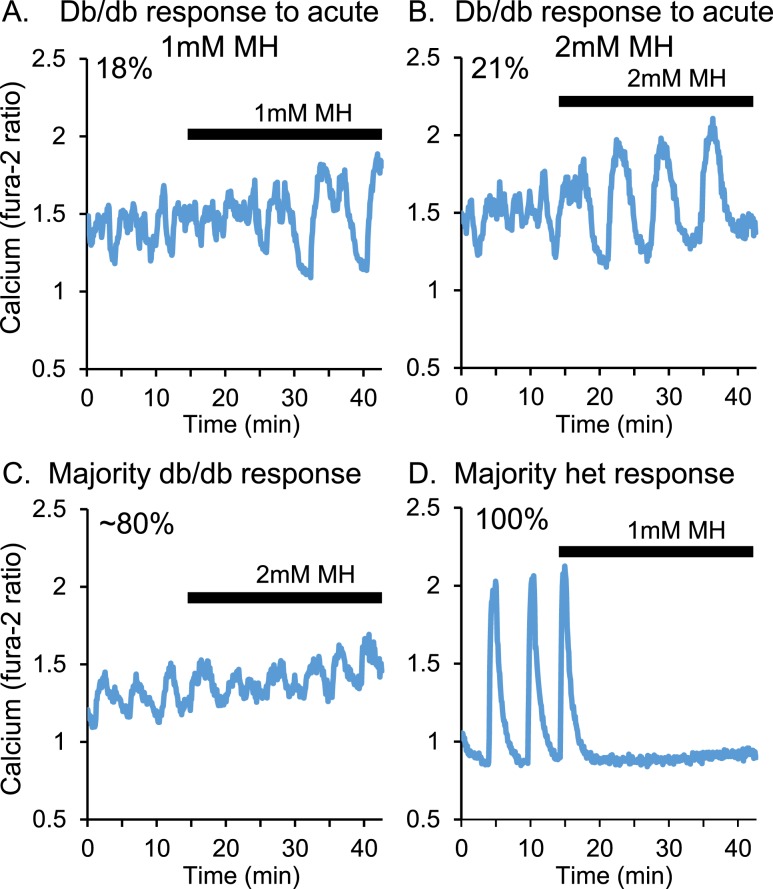
We previously reported that overnight MH exposure could substantially increase the amplitude and area of calcium oscillations in islets isolated from db/db mice and the percentage of islets capable of producing detectable oscillations [data shown in Fig. 7 of Corbin et al. (43)]. Acute exposure to MH, however, does not appear to affect the patterns of [Ca2+]i in islets from db/db mice to nearly the same degree. As shown in Fig. 3A, 1-mM MH exposure during a calcium recording only changed the calcium patterns in 18% of the islets tested (n = 4 of 22). Using 2 mM MH (Fig. 3B), 21% of islets (n = 3 of 14) from db/db mice showed an improvement in oscillatory activity. A large majority of islets showed no discernable change in the pattern after acute exposure to 2 mM MH (Fig. 3C). This lack of effect was observed in 82% of islets exposed to 1 mM MH and 79% of islets exposed to 2 mM MH. In contrast, 100% of islets from heterozygous controls (n = 39 of 39) showed an immediate decrease in [Ca2+]i (representative example provided in Fig. 3D). The effects of as little as 1 mM MH were similar to the effects of 5 or 10 mM MH on islets from outbred CD-1 mice. We could not determine the cause of this strain discrepancy. Overall, these data indicate that it is more difficult for MH to acutely affect calcium patterns in db/db islets compared with healthy islets, especially islets of the same background strain.
Figure 3.
Overnight MH exposure does not substantially alter [Ca2+]i patterns. Islets from db/db mice were acutely exposed to (A) 1 mM or (B) 2 mM MH and recorded for changes in [Ca2+]i. In a minority of cases [(A) 18%; (B) 21%], short-term MH exposure was sufficient to induce larger amplitude oscillations. (C) In ~80% of cases [(A) 82%; (B) 79%], no change in [Ca2+]i patterns was observed. (D) For islets from heterozygous (het) controls, acute 1 mM MH was sufficient to inhibit calcium influx in 100% of cases.
Islets from db/db mice have left-shifted glucose sensitivity
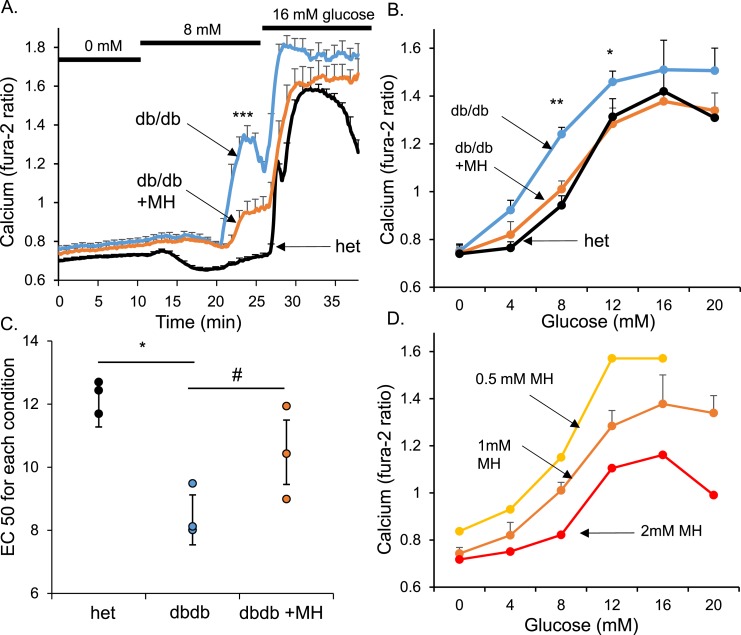
We next examined the relative glucose sensitivity of normal islets vs islets from db/db mice (age, 5 to 8 weeks) by measuring [Ca2+]i changes in response to different glucose concentrations after overnight incubation with or without various doses of MH. Islets from db/db mice and heterozygous controls were exposed to glucose steps of 0 to 8 to 16 mM or 4 to 12 to 20 mM during measurement of the changes in [Ca2+]i. An example of traces from one of three trials is shown in Fig. 4A for 0 to 8 to 16 mM glucose. The islets from heterozygous mice showed a distinct decrease in calcium (Fig. 4A; black trace) at the very end of the recording. This effect was caused by some of the islets entering into oscillatory activity toward the end of the recording. The mean [Ca2+]i levels during the last 10 minutes of each glucose step were recorded and plotted to produce glucose dose–response curves for [Ca2+]i. Islets from db/db mice (Fig. 4B; blue) were substantially left shifted compared with those from heterozygous controls (Fig. 4B; black). The mean EC50 calculated by curve fitting from three separate trials showed a clear left shift in glucose sensitivity of ~4 mM (Fig. 4C). Overnight treatment of 1 mM MH corrected this left shift to an EC50 closer to that of the heterozygous control and away from that of untreated db/db islets (P = 0.054). The maximal rate of reaction (Vmax) values did not differ significantly among the groups: 0.78 ± 0.07 for db/db, 0.68 ± 0.06 for heterozygous, and 0.66 ± 0.07 for db/db plus 1 mM MH. One trial was also conducted with heterozygous islets either treated with 1 mM MH or untreated. As expected, the islets exposed to MH had slightly reduced responses to glucose stimulation that reached statistical significance for 12, 16, and 20 mM glucose (P < 0.05). The sample size for each condition shown in Fig. 4B was 29 to 43 individually recorded islets for every glucose concentration from among three trials. The average response among the islets was combined to simplify the analysis and compare three trials using a paired t test. Despite this simplification, a clear left shift in glucose sensitivity was observed among the db/db islets, and overnight MH exposure partially corrected this left shift.
Figure 4.
The glucose-stimulated [Ca2+]i response was left shifted in islets from newly diabetic db/db mice, which was reversed by MH treatment. (A) Example of islet traces from untreated db/db islets (blue), MH-treated db/db islets (orange), and heterozygous (het) controls (black) that were stimulated from 0 to 8 to 16 mM glucose. Other islets were similarly stimulated from 4 to 12 to 20 mM glucose. (B) Mean [Ca2+]i response to glucose for each treatment group averaged from three trials. (C) EC50 for glucose stimulation calculated by sigmoidal curve fitting of each of three trials for all conditions. (D) Dose–response curves after overnight treatment with 0.5, 1, or 2 mM MH. MH showed a clear dose-dependent right shift and reduction of maximal [Ca2+]i response to glucose. #P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001.
We observed that MH has dose-dependent effects on both the EC50 and the Vmax. Overnight exposure to 2 mM MH appeared to right shift the EC50 beyond the heterozygous control and substantially reduced the Vmax (Fig. 4D). In contrast, 0.5 mM had less of an effect on these parameters compared with 1 mM MH exposure. Specifically, the Vmax values were 1.02 ± 0.04 for 0.5 mM MH, 0.72 ± 0.04 for 1 mM MH, and 0.49 ± 0.04 for 2 mM MH (all significantly different from each other; P < 0.01). These dose-dependent effects of MH have highlighted the strong sensitivity of islets to changes in glycolytic activity and the potential challenges in attempting to normalize glucose sensing as a potential therapeutic treatment of patients with T2D without also shutting down glucose-stimulated insulin secretion. Overall, our findings indicate that (i) islets from newly diabetic db/db mice have a left shift in glucose sensitivity and (ii) mildly reducing glycolytic activity with MH can reverse this left shift.
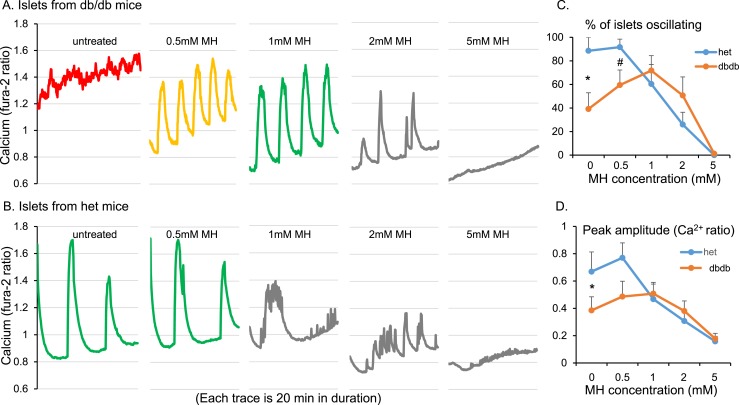
Optimal concentration of overnight MH to restore normal pulsatility
We next examined the effect of different doses of overnight MH treatment on overall [Ca2+]i oscillations in islets from db/db and heterozygous mice. Islets from db/db mice given no treatment had constitutively high levels of [Ca2+]i and no detectable oscillations with 11 mM glucose (Fig. 5A; red). After overnight exposure to 0.5 mM MH, the islets showed robust oscillations in [Ca2+]i (Fig. 5A; yellow). Even larger amplitude oscillations were observed after 1 mM MH (Fig. 5A; green). With 2 mM exposure, however, the amplitude and regularity of the oscillations declined, and oscillations in [Ca2+]i were nonexistent after 5 mM exposure (Fig. 5A; gray). Islets from heterozygous control mice were oscillatory in the untreated condition as expected, and oscillations were still robust with 0.5 mM MH treatment (Fig. 5B; green). However, oscillatory function declined dose dependently with 1, 2, and 5 mM MH (Fig. 5B; gray). The percentage of islets displaying detectable pulses, plotted against the MH dose for both strains, differed significantly between the db/db and heterozygous mouse islets (Fig. 5C). Maximal pulsatility was clearly observed with 1 mM MH in db/db islets in a classic inverted U-shaped curve. Heterozygous controls showed optimal pulsatility with no treatment (and similarly with 0.5 mM MH). As expected, the oscillatory capacity dose dependently declined with MH doses of 1, 2, and 5 mM (Fig. 5C). Similar trends were observed for the amplitude of [Ca2+]i oscillations (Fig. 5D).
Figure 5.
Overnight MH treatment produced a dose-dependent inverted U-shaped curve in islets from newly diabetic db/db mice. Islets from (A) db/db mice or (B) heterozygous (het) control mice were exposed to 0.5, 1, 2, or 5 mM MH overnight. (C) Percentage of islets displaying oscillations for each MH dose produced an inverted U-shaped curve for db/db and a dose-dependent decrease for het controls. (D) Mean amplitude of oscillations for each MH dose produced a pattern similar to that shown in (C). At least five islets were included for each condition for each of five trials. *P < 0.05, #P < 0.10 by paired t test.
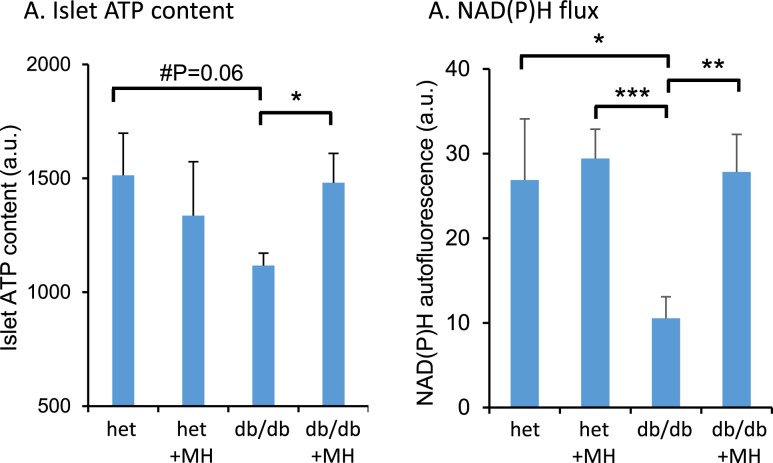
Increased ATP content and glucose-stimulated NAD(P)H flux in islets from db/db mice after overnight MH exposure
Having identified the optimal concentration of MH to improve oscillatory calcium flux, we next tested islets for effects of overnight exposure to 1 mM MH on downstream markers of glucose metabolism. The ATP content within islets after MH exposure and washout was significantly lower in the islets from db/db mice than that in the islets from heterozygous controls (Fig. 6A). However, treatment with MH restored ATP content to controls levels. Similarly, changes in NAD(P)H in response to stimulation from 3 to 20 mM glucose were reduced in db/db islets compared with heterozygous controls (Fig. 6B). Again, MH treatment restored glucose-stimulated NAD(P)H flux in islets from the db/db mice. These data suggest improved mitochondrial activity after overnight MH treatment.
Figure 6.
Overnight MH treatment enhanced ATP content and glucose-stimulated NAD(P)H flux in diabetic islets. (A) Islets from db/db mice and heterozygous (het) controls were incubated overnight in RPMI media with 11 mM glucose with or without 1 mM MH. After washout and additional incubation, islets were collected and lysed to detect ATP by luminescence assay; 15 to 20 islets per condition that had been size matched for islet mass were used for each of 8 replicates. (B) Islets were incubated with or without MH as described in (A). Islets were then imaged to detect changes in NAD(P)H from 3 to 20 mM glucose stimulation. The mean change in NAD(P)H is listed for each group of islets (n = five to nine islets for each treatment condition). *P < 0.05; **P < 0.01; ***P < 0.001.
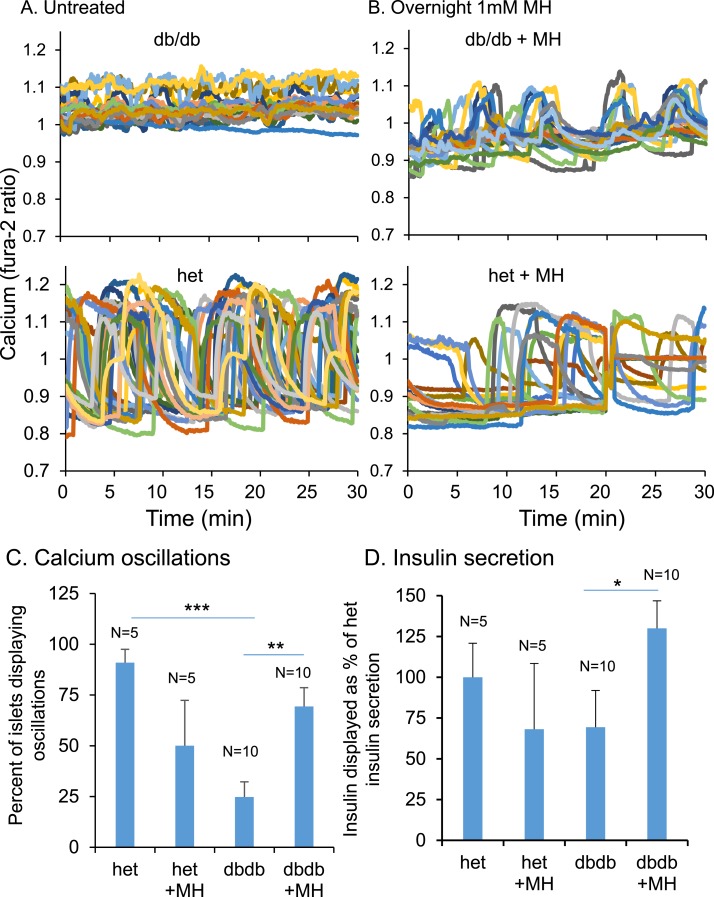
Simultaneous measurements of [Ca2+]i and insulin secretion showed that MH exposure restores pulsatility and enhances insulin release
We used the optimal dose of 1 mM MH in overnight treatment to measure the patterns of [Ca2+]i contemporaneously with insulin release into perifusate during a 30-minute recording. Islets isolated from db/db mice typically showed constitutively elevated [Ca2+]i (Fig. 7A; top). Islets from heterozygous control mice, in contrast, displayed robust high amplitude oscillations in [Ca2+]i (Fig. 7A; bottom). Islets from db/db mice exposed to 1 mM MH overnight showed a marked improvement in displaying [Ca2+]i oscillations and a reduction in mean [Ca2+]i (Fig. 7B; top). Heterozygous controls, which showed oscillations with 11 mM glucose optimally without treatment, responded to overnight MH exposure with a reduction in the frequency of oscillations and also a reduction in mean [Ca2+]i (Fig. 7B; bottom).
Figure 7.
(A,B) Examples of a 30-min [Ca2+]i pattern from (top) db/db or (bottom) heterozygous (het) islets (A) untreated or (B) after overnight MH exposure. Sets of 30 islets per condition were used to collect perifusate during the [Ca2+]i recording. The perfusate was then assayed for insulin release. After MH exposure, both the percentage of islets displaying (C) oscillations and (D) insulin secretion decreased for islets from het mice (n = 5) but increased for islets from db/db mice (n = 10). *P < 0.05; **P < 0.01; ***P < 0.001.
We also examined insulin secretion during these conditions. The differences in the percentage of islets displaying oscillations due to MH treatment (Fig. 7C) were fairly similar to the rates of insulin secretion (Fig. 7D) among the groups. Regarding the correlations, for each of 10 trials, islets from the same mouse were used for untreated vs MH-treated conditions. We previously reported that oscillatory patterns can vary markedly from mouse to mouse but that islets isolated from any one mouse tended to have a very tight distribution of oscillatory activity, “an imprinted oscillation” (59, 60). For each pairing of untreated and MH-treated islets, we thus normalized the amplitude of [Ca2+]i oscillation in MH-treated islets to the amplitude of untreated islets as a percentage. The same normalization was computed for insulin secretion.
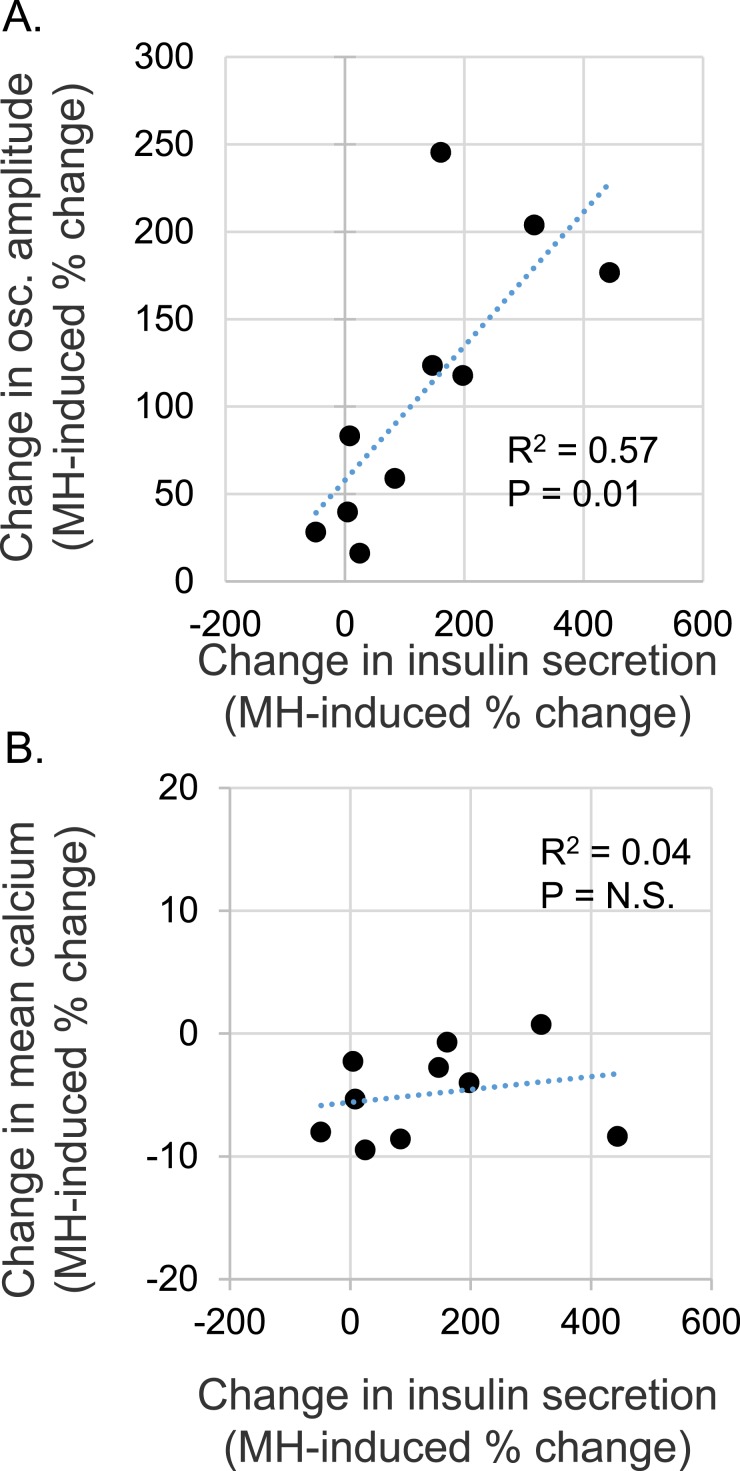
A scatter plot revealed a fairly tight correlation between the relative change in oscillatory amplitude and the relative change in insulin secretion (Fig. 8A). Thus, the greater the improvement in pulse amplitude, the greater the increase in insulin secretion. The area of pulses was also detected; however, these data were not accurate because pulses could sometimes be detected in the relatively flat and uneven patterns in untreated db/db islets that were low in amplitude but long in duration and, thus, large in area. Amplitude provided a much more accurate reading of the MH effects. The changes in mean [Ca2+]i levels were also plotted against insulin secretion but showed a very poor correlation (R2 = 0.04; Fig. 8B). We also observed slight correlations between the maximum (peak) [Ca2+]i (R2 = 0.18) and minimum (nadir) [Ca2+]i (R2 = 0.24). Although not very strong, these correlations further support the notion that restoring pulse amplitude (peaks and nadirs) would be a much greater indicator of proficient insulin secretion than the [Ca2+]i level. Thus, restoration of both stronger nadirs and peaks (increased amplitude) had by far the most striking correlation to insulin secretion. Islets from heterozygous mice showed reduced oscillations and reduced insulin secretion when treated with MH in four of five trials, consistent with the expected effects of glycolytic inhibition in normal healthy islets.
Figure 8.
Insulin secretion correlated with pulsatility but not [Ca2+]i levels. (A,B) Scatter plots of MH-induced differences in insulin secretion (x-axis) plotted against MH-induced differences in the (A) amplitude of oscillations or (B) mean [Ca2+]i level (y-axis) for islets from db/db mice. Each data point represents the secretion of a set of 30 islets paired with [Ca2+]i imaging data recorded simultaneously for each individual islet in 11 mM glucose for 30 min. These findings support the hypothesis that reducing excessive glycolytic activity restores glucose sensing and normal islet function.
Discussion
Glycolysis is a driving force in pulsatility in organisms as primitive as yeast (61, 62) and islets of every mammalian species studied. Glucokinase is an excellent glucose sensor for the β-cell owing to several unique biochemical characteristics: (i) non-Michaelis–Menten kinetics; (ii) affinity for glucose as a substrate within the physiological range for normal blood glucose levels (K0.5 of ~7 mM glucose); (iii) not shut off by its end product glucose-6-phosphate; (iv) shows a sigmoidal saturation curve for glucose with a Hill slope of ~1.7 and inflection point on the sigmoid of 4 to 5 mM glucose approximates the physiological threshold for glucose-stimulated insulin secretion in human islets; and (v) used by very few cells, primarily the endocrine pancreas and liver (63, 64). These characteristics uniquely permit glucokinase to closely monitor and respond to changes in blood glucose to secrete pulses of insulin accordingly.
Previous work has established that a range of glucose concentrations of ~8 to ~20 mM supports oscillatory activity in mouse pancreatic islets (47, 58). Islets secrete insulin in pulses at ~5-minute intervals. Pulses are large after meals and small between meals or during sleep. Below a certain glucose threshold, the ATP generated by glucose metabolism is insufficient to trigger pulsatile insulin release. Above a certain glucose level, pulses are not possible because glucose metabolism is so rapid that insulin secretion is constitutive. We found that this range of [Ca2+]i oscillations can be reproduced by increasing glucokinase activity in low glucose or reducing it in high glucose (Fig. 1).
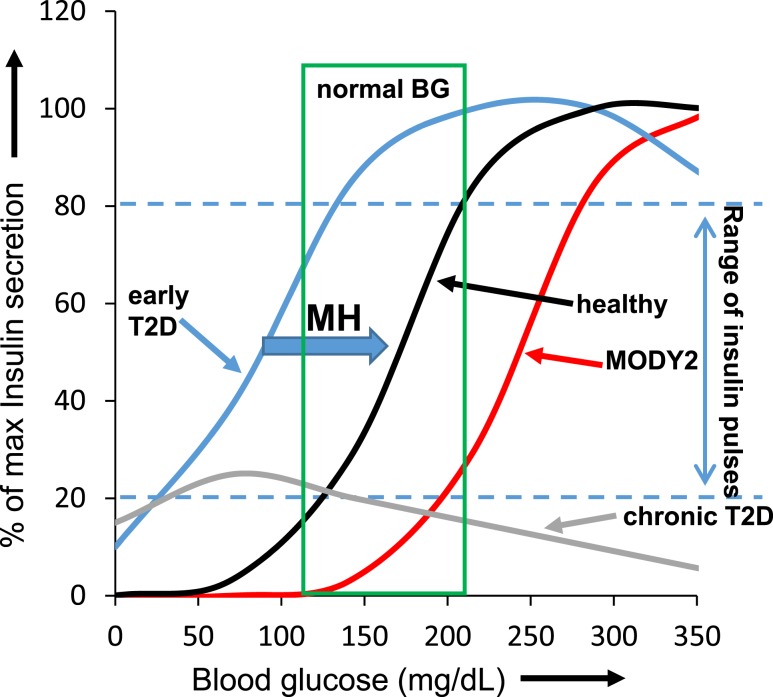
It is well established that fully inhibiting glucokinase in the normal β-cell will block glycolysis and shut off insulin release (52–54). Mutations in the glucokinase gene can cause hyperglycemia, such as observed in the more severe cases of mature-onset diabetes of the young 2 (MODY2) (55, 56). In the early stages of T2D, however, the increase in glucose sensitivity (left shift) is thought to make islets hyperinsulinemic in low glucose conditions (38, 43) but potentially overstimulated to the point of dysfunction in high glucose conditions (34, 39, 42). This effect has been modeled in Fig. 9 for mouse islets. The model also applies to human islets, albeit with different glucose ranges than shown in Fig. 9. Our model of shifting glucose sensitivities describes the relative relationship between the range of blood glucose values that support insulin pulses (“range of insulin pulses” between blue dashed blue lines) and the normal range of blood glucose values for healthy individuals (“normal BG”; green box). The range of glucose concentrations in which insulin pulses occur for normal healthy islets is shown inside the green box, indicating the normal physiological range of blood glucose, with larger insulin pulses for high glucose and smaller pulses for low glucose.
Figure 9.
Model of the range of blood glucose concentrations promoting insulin pulsatility. Normal nonfasted blood glucose range (for mice) boxed in green. Dashed blue lines indicate the range of pulsatile insulin release. Normal insulin release (black curve within the green box) is largely within the pulsatile range (between blue dashed lines). In early T2D, the curve (blue) is shifted: pulsatile insulin occurs at lower than normal glucose levels to the left of the green box, but maximum secretory capacity is still intact. However, this shift in glucose sensing results in excess insulin release with low glucose and a loss of pulsatility in normal postprandial glucose levels, which might further contribute to hepatic insulin resistance. Loss-of-function glucokinase mutations such as MODY2 in the most severe cases result in the opposite effect. Insulin pulsatility begins for MODY2 (red curve) in hyperglycemic conditions to the right of the green box. Chronic T2D (gray curve) results in substantial deterioration of insulin secretory capacity across all glucose levels. Note that the blood glucose concentrations are more closely related to the mouse; however, the curves also apply conceptually to humans.
In early T2D, however, this range shifts to the left of the green box, resulting in large pulses of insulin in hypoglycemic conditions (producing relative hyperinsulinemia) and continuous insulin release at all greater glucose levels (producing chronic overstimulation). Overnight MH treatment can reverse this effect, as indicated by the blue block arrow (Fig. 9), thus reducing excessive insulin release in low glucose conditions and restoring pulsatility to postprandial glucose levels. Loss-of-function mutations in glucokinase, the cause of MODY2, have the opposite effect. In a severe case of glucokinase loss, glucose sensing will be severely right shifted, such that islets are not stimulated in the normal range of blood glucose. Instead, stimulation of insulin pulses occurs in hyperglycemic conditions to the right of the normal range. In most clinical cases, MODY2 will be fairly mild, which would be modeled in Fig. 9 as a curve just a bit to the right of “healthy.” Finally, islet function in chronic T2D is substantially impaired, such that the maximum insulin secretion in response to glucose stimulation is inadequate. We observed that a small subset of islets is still capable of generating some degree of oscillatory activity in hypoglycemic conditions, although at substantially reduced amplitude (43).
MH was first identified in 1917 (65) and first shown to cause hyperglycemia in rats by inhibiting insulin release in 1957 (66). MH is a well-known seven-carbon sugar that binds selectively to glucokinase (EC 2.7.1.2), as opposed to hexokinase (EC 2.7.1.1), and acts as a competitive antagonist to prevent glucose phosphorylation, thus preventing glucose metabolism and blocking glucose-stimulated insulin secretion (54, 67). MH-induced inhibition of glycolysis would thus decrease ATP production by reducing the available substrate for generating ATP and by activating AMP-activated protein kinase (68). However, MH can also increase glucagon and glucagonlike peptide 1 (GLP-1) levels in islets, which might increase cAMP levels in β-cells by activating adenylyl cyclase (69). The GLP-1/cAMP pathway plays an important role in insulin secretion by regulating exocytosis of insulin granules (70) (71) and can ameliorate endoplasmic reticulum-related stress (72) and reduce reactive oxygen species production in diabetic rodent islets to improve mitochondrial function (73, 74). Thus, in addition to restoring pulsatility and normal glucose sensing, MH might also improve mitochondrial health by increasing GLP-1/cAMP signaling. This could explain, at least in part, our observed increases in ATP content and NAD(P)H flux, which suggest enhanced mitochondrial activity.
Many pharmaceutical endeavors have focused on developing glucokinase activators to enhance β-cell function as a therapeutic approach for treating T2D at a stage in the disease process when insulin levels are decreasing (63, 75–77), with disappointing clinical results (78). Our approach has been to restore normal glucose sensing and endogenous pulsatility in islets at an earlier stage of the disease when insulin secretory capacity is still intact by slightly reducing glycolytic rates with the glucokinase inhibitor MH. This approach should both reduce the risk of hyperinsulinemia by reducing insulin release in the fasted state when blood glucose is lower and prevent hyperglycemia by augmenting insulin release to appropriate levels during periods of high blood glucose conditions. MH is generally regarded as safe, and MH effects are limited to a small fraction of organs that preferentially use glucokinase over hexokinase, primarily the liver and endocrine pancreas (63, 64). MH thus has therapeutic potential for restoring normal islet function before the development of hyperglycemia and/or T2D. The restoration of pulsatility might even help to reduce insulin resistance by improving the hepatic response to insulin (21, 22).
The caveats to this potential approach that should also be acknowledged are numerous. First, our data suggest that “the sweet spot” for MH treatment would need to be found between having little to no effect on glucose sensing at low MH doses to substantially inhibiting insulin secretion by right shifting too far at higher MH doses, thus also reducing the maximum insulin response to glucose stimulation (Fig. 4D). If a drug could be developed with similar specificity for glucokinase but a much wider range of therapeutic concentrations, this concern could be minimized. Second, although a vast majority of cells in the body express hexokinase, in contrast to MH’s target glucokinase, MH treatment in vivo could affect a few other systems in the body, including the liver, brain, and kidney. Direct effects of MH on these other tissues could affect the efficacy we observed in these in vitro studies, although MH-induced improvements to insulin pulses should potentially enhance hepatic insulin signaling (21, 22), rather than inhibit it. Third, MH therapy might or might not stop or even slow the progression of T2D. Left shifting occurs in glucose sensitivity under a variety of metabolic stress conditions, including chronic exposure to nutrient-derived factors such as glucose and free fatty acids (37–39) and partial pancreatectomy (40, 41). It is not clear whether elevated glycolytic activity is a cause or merely a symptom of metabolic dysfunction in the islet. If not the cause, it is quite possible that β-cell decline could continue even with MH treatment. Despite these concerns, our findings have provided the proof of concept that reducing, rather than stimulating, glycolytic activity might improve β-cell function in the very early stages of T2D.
Conclusions
We have demonstrated that (i) islets from db/db mice in the early stage of T2D progression have a left shift in glucose, leading to disruptions in the normal range of pulsatility; (ii) reducing glycolytic activity paradoxically increases ATP content and NAD(P)H flux in db/db islets; (iii) reducing glycolytic activity can restore pulsatility to the normal range; and (iv) restored pulsatility correlates closely with improved insulin secretion. We hope to address in the future whether reducing glycolytic activity improves insulin secretion because insulin pulses are restored or simply result from some generalized effect of “β-cell rest” (79–82). Another goal is to determine whether pulsatility is requisite to normal islet health and function. We recently developed a perifusion system that can be used to “force oscillations” and monitor islet activity at different parts of the stimulus-secretion coupling pathway (83) to test this hypothesis. Thus, future studies are needed to address the exact relationship between glucose sensing and insulin secretion in conditions of metabolic distress and the role that pulsatility plays in these processes.
Acknowledgments
Financial Support : This work was funded by the Osteopathic Heritage Foundation, Ohio University Heritage College of Osteopathic Medicine, and Mouse Metabolic Phenotyping Centers MICROMouse program (Grant DK076169).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AM
acetoxymethyl
- [Ca2+]i
intracellular calcium
- GLP-1
glucagonlike peptide 1
- KRB
Krebs-Ringer bicarbonate
- MH
d-mannoheptulose
- MODY2
mature-onset diabetes of the young 2
- NAD(P)H
nicotinamide adenine dinucleotide hydrate and/or the reduced form of nicotinamide adenine dinucleotide phosphate
- T2D
type 2 diabetes
- Vmax
maximal rate of reaction
References
- 1. Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. [DOI] [PubMed] [Google Scholar]
- 2. Ashcroft FM, Rorsman P. ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion. Biochem Soc Trans. 1990;18(1):109–111. [DOI] [PubMed] [Google Scholar]
- 3. Maechler P, Wollheim CB. Mitochondrial signals in glucose-stimulated insulin secretion in the beta cell. J Physiol. 2000;529(Pt 1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52(5):739–751. [DOI] [PubMed] [Google Scholar]
- 5. Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751–1760. [DOI] [PubMed] [Google Scholar]
- 6. Matthews DR, Lang DA, Burnett MA, Turner RC. Control of pulsatile insulin secretion in man. Diabetologia. 1983;24(4):231–237. [DOI] [PubMed] [Google Scholar]
- 7. Schmitz O, Arnfred J, Nielsen OH, Beck-Nielsen H, Orskov H. Glucose uptake and pulsatile insulin infusion: euglycaemic clamp and [3-3H]glucose studies in healthy subjects. Acta Endocrinol (Copenh). 1986;113(4):559–563. [DOI] [PubMed] [Google Scholar]
- 8. Paolisso G, Sgambato S, Torella R, Varricchio M, Scheen A, D’Onofrio F, Lefèbvre PJ. Pulsatile insulin delivery is more efficient than continuous infusion in modulating islet cell function in normal subjects and patients with type 1 diabetes. J Clin Endocrinol Metab. 1988;66(6):1220–1226. [DOI] [PubMed] [Google Scholar]
- 9. Paolisso G, Sgambato S, Gentile S, Memoli P, Giugliano D, Varricchio M, D’Onofrio F. Advantageous metabolic effects of pulsatile insulin delivery in noninsulin-dependent diabetic patients. J Clin Endocrinol Metab. 1988;67(5):1005–1010. [DOI] [PubMed] [Google Scholar]
- 10. Bratusch-Marrain PR, Komjati M, Waldhäusl WK. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes. 1986;35(8):922–926. [DOI] [PubMed] [Google Scholar]
- 11. Komjati M, Bratusch-Marrain P, Waldhäusl W. Superior efficacy of pulsatile versus continuous hormone exposure on hepatic glucose production in vitro. Endocrinology. 1986;118(1):312–319. [DOI] [PubMed] [Google Scholar]
- 12. Koopmans SJ, Sips HC, Krans HM, Radder JK. Pulsatile intravenous insulin replacement in streptozotocin diabetic rats is more efficient than continuous delivery: effects on glycaemic control, insulin-mediated glucose metabolism and lipolysis. Diabetologia. 1996;39(4):391–400. [DOI] [PubMed] [Google Scholar]
- 13. Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30(5):435–439. [DOI] [PubMed] [Google Scholar]
- 14. Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, Frank BH, Galloway JA, Van Cauter E. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1988;318(19):1231–1239. [DOI] [PubMed] [Google Scholar]
- 15. Ristow M, Carlqvist H, Hebinck J, Vorgerd M, Krone W, Pfeiffer A, Müller-Wieland D, Ostenson CG. Deficiency of phosphofructo-1-kinase/muscle subtype in humans is associated with impairment of insulin secretory oscillations. Diabetes. 1999;48(8):1557–1561. [DOI] [PubMed] [Google Scholar]
- 16. Hollingdal M, Juhl CB, Pincus SM, Sturis J, Veldhuis JD, Polonsky KS, Pørksen N, Schmitz O. Failure of physiological plasma glucose excursions to entrain high-frequency pulsatile insulin secretion in type 2 diabetes. Diabetes. 2000;49(8):1334–1340. [DOI] [PubMed] [Google Scholar]
- 17. Song SH, Rhodes CJ, Veldhuis JD, Butler PC. Diazoxide attenuates glucose-induced defects in first-phase insulin release and pulsatile insulin secretion in human islets. Endocrinology. 2003;144(8):3399–3405. [DOI] [PubMed] [Google Scholar]
- 18. O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med. 1988;318(19):1225–1230. [DOI] [PubMed] [Google Scholar]
- 19. Nyholm B, Pørksen N, Juhl CB, Gravholt CH, Butler PC, Weeke J, Veldhuis JD, Pincus S, Schmitz O. Assessment of insulin secretion in relatives of patients with type 2 (non-insulin-dependent) diabetes mellitus: evidence of early beta-cell dysfunction. Metabolism. 2000;49(7):896–905. [DOI] [PubMed] [Google Scholar]
- 20. Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54(6):1649–1656. [DOI] [PubMed] [Google Scholar]
- 21. Matveyenko AV, Veldhuis JD, Butler PC. Adaptations in pulsatile insulin secretion, hepatic insulin clearance, and beta-cell mass to age-related insulin resistance in rats. Am J Physiol Endocrinol Metab. 2008;295(4):E832–E841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matveyenko AV, Liuwantara D, Gurlo T, Kirakossian D, Dalla Man C, Cobelli C, White MF, Copps KD, Volpi E, Fujita S, Butler PC. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes. 2012;61(9):2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jahanshahi P, Wu R, Carter JD, Nunemaker CS. Evidence of diminished glucose stimulation and endoplasmic reticulum function in nonoscillatory pancreatic islets. Endocrinology. 2009;150(2):607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pørksen N, Hollingdal M, Juhl C, Butler P, Veldhuis JD, Schmitz O. Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes. 2002;51(Suppl 1):S245–S254. [DOI] [PubMed] [Google Scholar]
- 25. Satin LS, Butler PC, Ha J, Sherman AS. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol Aspects Med. 2015;42:61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aronoff SL, Bennett PH, Gorden P, Rushforth N, Miller M. Unexplained hyperinsulinemia in normal and “prediabetic” Pima Indians compared with normal Caucasians. An example of racial differences in insulin secretion. Diabetes. 1977;26(9):827–840. [DOI] [PubMed] [Google Scholar]
- 27. Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med. 1993;329(27):1988–1992. [DOI] [PubMed] [Google Scholar]
- 28. Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49(12):2094–2101. [DOI] [PubMed] [Google Scholar]
- 29. Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up: a preliminary report. Diabetes Care. 2009;32(8):1464–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leahy JL, Hirsch IB, Peterson KA, Schneider D. Targeting beta-cell function early in the course of therapy for type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(9):4206–4216. [DOI] [PubMed] [Google Scholar]
- 31. Hansen BC, Bodkin NL. Beta-cell hyperresponsiveness: earliest event in development of diabetes in monkeys. Am J Physiol. 1990;259(3 Pt 2):R612–R617. [DOI] [PubMed] [Google Scholar]
- 32. Chen NG, Tassava TM, Romsos DR. Threshold for glucose-stimulated insulin secretion in pancreatic islets of genetically obese (ob/ob) mice is abnormally low. J Nutr. 1993;123(9):1567–1574. [DOI] [PubMed] [Google Scholar]
- 33. Koyama K, Chen G, Lee Y, Unger RH. Tissue triglycerides, insulin resistance, and insulin production: implications for hyperinsulinemia of obesity. Am J Physiol. 1997;273(4 Pt 1):E708–E713. [DOI] [PubMed] [Google Scholar]
- 34. Zhou YP, Cockburn BN, Pugh W, Polonsky KS. Basal insulin hypersecretion in insulin-resistant Zucker diabetic and Zucker fatty rats: role of enhanced fuel metabolism. Metabolism. 1999;48(7):857–864. [DOI] [PubMed] [Google Scholar]
- 35. Gray SL, Donald C, Jetha A, Covey SD, Kieffer TJ. Hyperinsulinemia precedes insulin resistance in mice lacking pancreatic beta-cell leptin signaling. Endocrinology. 2010;151(9):4178–4186. [DOI] [PubMed] [Google Scholar]
- 36. Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu K-Y, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, Bamji SX, Clee SM, Johnson JD. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16(6):723–737. [DOI] [PubMed] [Google Scholar]
- 37. Erion KA, Berdan CA, Burritt NE, Corkey BE, Deeney JT. Chronic exposure to excess nutrients left-shifts the concentration dependence of glucose-stimulated insulin secretion in pancreatic β-cells. J Biol Chem. 2015;290(26):16191–16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glynn E, Thompson B, Vadrevu S, Lu S, Kennedy RT, Ha J, Sherman A, Satin LS. Chronic glucose exposure systematically shifts the oscillatory threshold of mouse islets: Experimental evidence for an early intrinsic mechanism of compensation for hyperglycemia. Endocrinology. 2016;157(2):611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ha J, Satin LS, Sherman AS. A mathematical model of the pathogenesis, prevention and reversal of type 2 diabetes. Endocrinology. 2016;157(2):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leahy JL, Bumbalo LM, Chen C. Beta-cell hypersensitivity for glucose precedes loss of glucose-induced insulin secretion in 90% pancreatectomized rats. Diabetologia. 1993;36(12):1238–1244. [DOI] [PubMed] [Google Scholar]
- 41. Liu YQ, Tornheim K, Leahy JL. Fatty acid-induced beta cell hypersensitivity to glucose: increased phosphofructokinase activity and lowered glucose-6-phosphate content. J Clin Invest. 1998;101(9):1870–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cockburn BN, Ostrega DM, Sturis J, Kubstrup C, Polonsky KS, Bell GI. Changes in pancreatic islet glucokinase and hexokinase activities with increasing age, obesity, and the onset of diabetes. Diabetes. 1997;46(9):1434–1439. [DOI] [PubMed] [Google Scholar]
- 43. Corbin KL, Waters CD, Shaffer BK, Verrilli GM, Nunemaker CS. Islet hypersensitivity to glucose is associated with disrupted oscillations and increased impact of proinflammatory cytokines in islets from diabetes-prone male mice. Endocrinology. 2016;157(5):1826–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009;11(1):3–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Corbin KL, Hall TE, Haile R, Nunemaker CS. A novel fluorescence imaging approach for comparative measurements of pancreatic islet function in vitro. Islets. 2011;3(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crim WS, Wu R, Carter JD, Cole BK, Trace AP, Mirmira RG, Kunsch C, Nadler JL, Nunemaker CS. AGI-1067, a novel antioxidant and anti-inflammatory agent, enhances insulin release and protects mouse islets. Mol Cell Endocrinol. 2010;323(2):246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nunemaker CS, Bertram R, Sherman A, Tsaneva-Atanasova K, Daniel CR, Satin LS. Glucose modulates [Ca2+]i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophys J. 2006;91(6):2082–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250(4 Pt 1):E486–E493. [DOI] [PubMed] [Google Scholar]
- 49. Nunemaker CS, Wasserman DH, McGuinness OP, Sweet IR, Teague JC, Satin LS. Insulin secretion in the conscious mouse is biphasic and pulsatile. Am J Physiol Endocrinol Metab. 2006;290(3):E523–E529. [DOI] [PubMed] [Google Scholar]
- 50. Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RK. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes. 2012;61(7):1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ren J, Sherman A, Bertram R, Goforth PB, Nunemaker CS, Waters CD, Satin LS. Slow oscillations of KATP conductance in mouse pancreatic islets provide support for electrical bursting driven by metabolic oscillations. Am J Physiol Endocrinol Metab. 2013;305(7):E805–E817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. German MS. Glucose sensing in pancreatic islet beta cells: the key role of glucokinase and the glycolytic intermediates. Proc Natl Acad Sci USA. 1993;90(5):1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balkan B, Dunning BE. Glucosamine inhibits glucokinase in vitro and produces a glucose-specific impairment of in vivo insulin secretion in rats. Diabetes. 1994;43(10):1173–1179. [DOI] [PubMed] [Google Scholar]
- 54. Sweet IR, Li G, Najafi H, Berner D, Matschinsky FM. Effect of a glucokinase inhibitor on energy production and insulin release in pancreatic islets. Am J Physiol. 1996;271(3 Pt 1):E606–E625. [DOI] [PubMed] [Google Scholar]
- 55. Byrne MM, Sturis J, Clément K, Vionnet N, Pueyo ME, Stoffel M, Takeda J, Passa P, Cohen D, Bell GI, Velho G, Froguel P, Polonsky KS. Insulin secretory abnormalities in subjects with hyperglycemia due to glucokinase mutations. J Clin Invest. 1994;93(3):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grupe A, Hultgren B, Ryan A, Ma YH, Bauer M, Stewart TA. Transgenic knockouts reveal a critical requirement for pancreatic beta cell glucokinase in maintaining glucose homeostasis. Cell. 1995;83(1):69–78. [DOI] [PubMed] [Google Scholar]
- 57. Sapolsky RM. Stress and the brain: individual variability and the inverted-U. Nat Neurosci. 2015;18(10):1344–1346. [DOI] [PubMed] [Google Scholar]
- 58. Luciani DS, Misler S, Polonsky KS. Ca2+ controls slow NAD(P)H oscillations in glucose-stimulated mouse pancreatic islets. J Physiol. 2006;572(Pt 2):379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nunemaker CS, Zhang M, Wasserman DH, McGuinness OP, Powers AC, Bertram R, Sherman A, Satin LS. Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo? Diabetes. 2005;54(12):3517–3522. [DOI] [PubMed] [Google Scholar]
- 60. Nunemaker CS, Dishinger JF, Dula SB, Wu R, Merrins MJ, Reid KR, Sherman A, Kennedy RT, Satin LS. Glucose metabolism, islet architecture, and genetic homogeneity in imprinting of [Ca2+](i) and insulin rhythms in mouse islets. PLoS One. 2009;4(12):e8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chance B, Estabrook RW, Ghosh A. Damped sinusoidal oscillations of cytoplasmic reduced pyridine nucleotide in yeast cells. Proc Natl Acad Sci USA. 1964;51(6):1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Laxman S, Tu BP. Systems approaches for the study of metabolic cycles in yeast. Curr Opin Genet Dev. 2010;20(6):599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Matschinsky FM, Zelent B, Doliba N, Li C, Vanderkooi JM, Naji A, Sarabu R, Grimsby J. Glucokinase activators for diabetes therapy: May 2010 status report. Diabetes Care. 2011;34(Suppl 2):S236–S243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nakamura A, Terauchi Y. Present status of clinical deployment of glucokinase activators. J Diabetes Investig. 2015;6(2):124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. La Forge FB. d-Mannoketoheptose, a new sugar from the avocado. J Biol Chem. 1917;28:511–522. [Google Scholar]
- 66. Simon E, Kraicer PF. Metabolism of mannoheptulose in the rat. I. Diabetogenic action. Arch Biochem Biophys. 1957;69:592–601. [DOI] [PubMed] [Google Scholar]
- 67. Ashcroft SJ, Randle PJ. Enzymes of glucose metabolism in normal mouse pancreatic islets. Biochem J. 1970;119(1):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93(4):891S–896S. [DOI] [PubMed] [Google Scholar]
- 69. Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose sensing in pancreatic β-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes. 2001;50(1):1–11. [DOI] [PubMed] [Google Scholar]
- 70. Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic β-cells. Nature. 2001;414(6865):808–812. [DOI] [PubMed] [Google Scholar]
- 71. Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser H-G, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem. 2003;278(10):8279–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tsunekawa S, Yamamoto N, Tsukamoto K, Itoh Y, Kaneko Y, Kimura T, Ariyoshi Y, Miura Y, Oiso Y, Niki I. Protection of pancreatic beta-cells by exendin-4 may involve the reduction of endoplasmic reticulum stress; in vivo and in vitro studies. J Endocrinol. 2007;193(1):65–74. [DOI] [PubMed] [Google Scholar]
- 73. Mukai E, Fujimoto S, Sato H, Oneyama C, Kominato R, Sato Y, Sasaki M, Nishi Y, Okada M, Inagaki N. Exendin-4 suppresses SRC activation and reactive oxygen species production in diabetic Goto-Kakizaki rat islets in an Epac-dependent manner. Diabetes. 2011;60(1):218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cheng Q, Law PK, de Gasparo M, Leung PS. Combination of the dipeptidyl peptidase IV inhibitor LAF237 [(S)-1-[(3-hydroxy-1-adamantyl)ammo]acetyl-2-cyanopyrrolidine] with the angiotensin II type 1 receptor antagonist valsartan [N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl)-[1,1′-biphenyl]-4-yl]methyl]-L-valine] enhances pancreatic islet morphology and function in a mouse model of type 2 diabetes. J Pharmacol Exp Ther. 2008;327(3):683–691. [DOI] [PubMed] [Google Scholar]
- 75. Matschinsky FM, Zelent B, Doliba NM, Kaestner KH, Vanderkooi JM, Grimsby J, Berthel SJ, Sarabu R. Research and development of glucokinase activators for diabetes therapy: theoretical and practical aspects. Handb Exp Pharmacol. 2011;203:357–401. [DOI] [PubMed] [Google Scholar]
- 76. Futamura M, Yao J, Li X, Bergeron R, Tran JL, Zycband E, Woods J, Zhu Y, Shao Q, Maruki-Uchida H, Goto-Shimazaki H, Langdon RB, Erion MD, Eiki J, Zhou YP. Chronic treatment with a glucokinase activator delays the onset of hyperglycaemia and preserves beta cell mass in the Zucker diabetic fatty rat. Diabetologia. 2012;55(4):1071–1080. [DOI] [PubMed] [Google Scholar]
- 77. Grewal AS, Sekhon BS, Lather V. Recent updates on glucokinase activators for the treatment of type 2 diabetes mellitus. Mini Rev Med Chem. 2014;14(7):585–602. [DOI] [PubMed] [Google Scholar]
- 78. Matschinsky FM. GKAs for diabetes therapy: why no clinically useful drug after two decades of trying? Trends Pharmacol Sci. 2013;34(2):90–99. [DOI] [PubMed] [Google Scholar]
- 79. Glaser B, Leibovich G, Nesher R, Hartling S, Binder C, Cerasi E. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh). 1988;118(3):365–373. [DOI] [PubMed] [Google Scholar]
- 80. Grill V, Björklund A. Overstimulation and beta-cell function. Diabetes. 2001;50(Suppl 1):S122–S124. [DOI] [PubMed] [Google Scholar]
- 81. Li Y, Xu W, Liao Z, Yao B, Chen X, Huang Z, Hu G, Weng J. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care. 2004;27(11):2597–2602. [DOI] [PubMed] [Google Scholar]
- 82. Brown RJ, Rother KI. Effects of beta-cell rest on beta-cell function: a review of clinical and preclinical data. Pediatr Diabetes. 2008;9(3 Pt 2):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Whitticar NB, Strahler EW, Rajan P, Kaya S, Nunemaker CS. An automated perifusion system for modifying cell culture conditions over time. Biol Proced Online. 2016;18(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]