Abstract
Background
Spinal cord injury (SCI) is an injury-triggered event that is associated with permanent neurologic deficit. The deficit instigated by SCI leads to medical co-morbidity, not only affecting sensory and motor capabilities, but also having an impact on the physiological and economic condition of the patient. Against this backdrop, the present study was carried out to investigate the effect of lycopsamine, a plant-derived alkaloid in SCI rats.
Material/Methods
The traumatic SCI injury in rats was created using a force-calibrated weight-drop device. The Basso-Beattie-Bresnahan (BBB) locomotor rating scale was used to investigate the functional consequences of SCI. DAPI (4′,6-diamidino-2-phenylindole) and Tunnel staining were used to detect apoptosis. Western blot and qRT-PCR was used to examine the protein and gene expressions, respectively.
Results
The results revealed that lycopsamine significantly (p<0.01) improved locomotory function in SCI rats. Lycopsamine also significantly (p<0.01) decreased the lesion area of the SCI rats. Investigation of the effect of lycopsamine on cell death following SCI revealed that lycopsamine reduces apoptotic cell death following SCI. The lycopsamine-induced reduction in apoptosis was allied with downregulation of calpain, cleaved caspase 3 and 9, and Bax. However, the expression of BCl-2 was significantly upregulated. Furthermore, lycopsamine significantly (p<0.01) upregulated the expression of interleukin-10 (IL-10) and decreased the expression of tumor necrosis factor-α (TNF-α).
Conclusions
Lycopsamine exerts protective effects in PCI rats by improving functional recovery and suppressing apoptosis.
MeSH Keywords: Apoptosis, Gene Expression, Spinal Cord Injuries
Background
Natural products from animals, plants, and microbes have bestowed mankind with a diversity of chemical scaffolds used in alleviating human ailments [1]. It has been reported that around 70% of the world population is dependent on use of plants for their primary health care [2], and many molecules of plant origin have been used in the treatment of devastating diseases such as cancer [3]. Plant metabolites such as campothecins are used in the treatment of cancer, and artemisinin has been used for the treatment of malaria [4,5]. It is belived that plants or molecules isolated from plants may prove beneficial in the treatment of spinal cord injury (SCI). SCI is a devastating condition, and in the USA alone, approximately 0.3 million cases of SCI were reported and around 11 000 new cases are reported annually. Billions of dollars in the USA alone are spent on this disease, making it one of the most economically devastating diseases [6]. Spinal cord injury may be associated with permanent neurological deficits [7]. Besides affecting sensory and motor capabilities, SCI may have physiological and economical effects [8]. The Edwin Smith papyrus was written by an Egyptian physician in 1700 BC, and is the earliest known document stating that SCI as an “ailment not to be treated” [9]. Since then, SCI has been regarded as a devastating condition in which most of the cases die before any patient care is given. Against this backdrop, plant-derived molecules can be screened to identify molecules that may prove beneficial in the management of SCI. Lycopsamine is a pyrrolizidine alkaloid that has been isolated from a number of plant species [10]. It has been reported to exhibit tremendous pharmacological potential [11]. Previous investigations have revealed that alkaloids such as gelsemine exert beneficial effects in spinal cord injury [12]. Herein, we for the first time, we examine the effects of lycopsamine in SCI rat models. Wes found that lycopsamine exhibits protective effects in SCI rats by reducing cell death and improving functional recovery. We propose that lycopsamine may prove beneficial in the management of SCI and warrants further investigation.
Material and Methods
Spinal cord injury
The traumatic injury in rats was triggered using a force-calibrated weight-drop device [13]. The male Sprague-Dawley rats (male, 235–245 g) were procured from the Animal Centre of Taizhou People’s Hospital, Taizhou, and housed in sterile stainless-steel cages with a 12-h light: dark cycle at 22˚C and with ~50% relative humidity. The rats were anesthetized with isoflurane and the whole procedure was carried out as described previously [14]. The international standards for animal care and ethics were followed [15] and the study was approved by the Animal Ethics Committee of Taizhou People’s Hospital, Taizhou under approval number ECSHT-AS345/2017.
Animal grouping and treatments
A total of 24 injured rats were randomly divided in 2 groups. Lycopsamine (98% pure, obtained from Sigma-Aldrich) was dissolved in 0.1% dimethyl sulfoxide (DMSO) and administrated intraperitoneally after injury in rats. The vehicle rats received normal saline and the treatment group received 30 mg/kg of lycopsamine once daily. At the end of the study, the animals were sacrificed by deep anesthesia with isoflurane and death was confirmed by monitoring the heart beat.
Behavioural testing
The Basso-Beattie-Bresnahan (BBB) locomotor rating scale [16] was used to investigate the functional consequences of SCI and to verify the effects of lycopsamine on recovery.
Analysis of lesion area
The lycopsamine-treated and the vehicle treated rats were anesthetized and intracardially perfused with PBS (0.1 M, pH 7.4) and then with paraformaldehyde in PBS (pH 7.4). The histological analysis was performed by cutting a 2-cm cord segment centred at the site of injury from the vertebral column. The segment was fixed and kept in sucrose (30%) in phosphate-buffered saline (PBS). The segment was then embedded in an optical cutting temperature (OCT) compound and the 8–10-μm sections were cut, stained with cresyl violet, and observed under a microscope, and the cavitation area was determined using the Metamorphor imaging program (Universal Imaging Corp., West Chester, PA).
Quantitative RT-PCR
RNA was isolated from tissues by Trizol reagent and then transcribed into cDNA using a RevertAid cDNA synthesis kit. The expression was examined by qRT-PCR as described previously [17]. For real-time PCR, the cDNA was diluted 10 times and qRT-PCR was performed in triplicates in ABI StepOne Real-time (Applied Biosystems) using SYBR Green Master Mix (Fermentas) and gene-specific primers. The cycling parameters were 95°C for 20 s, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The relative quantification method (ΔΔ-CT) was used to evaluate quantitative variation between the replicates examined. The amplification of actin was used as an endogenous control to normalize all data.
DAPI and TUNEL assay
The apoptosis-inducing effects of lycopsamine were determined by 4′, 6-diamidino-2-phenylindole (DAPI) staining. In brief, the spinal cord sections were subjected to incubation, with DAPI (0.2 μg/ml) for 20 min at room temperature. The cells were then fixed with 70% methanol at −20°C overnight. Finally, the nuclear morphology of the stained cells was examined by fluorescence microscopy. Tunnel assay was also performed as described previously [18].
Western blotting
The tissues were homogenized in buffer and their protein concentrations were determined with Bradford method. Protein expression was measured using Western blotting as described previously [19]. The primary antibodies against Calpain, Caspase 3 and 9, Bcl-2, Bax, and GAPDH were obtained from Santa Cruz Biotechnology, Inc.
Statistical analysis
Data are shown as mean ±SEM. Statistical analysis was done using the t test with GraphPad prism 7 software. Values of p<0.05 were considered to be indicative of significant difference.
Results
Lycopsamine improves functional recovery of SCI rats
The impact of Lycopsamine on locomotory function was examined to see if lycopsamine exhibits protective effects on the functional recovery following SCI. It was observed that following SCI, all of the rats had paralysed hind limbs. A BBB test was used to assess the motor function of the hind limbs. It was found that the BBB scores were significantly higher in the lycopsamine-administered rats in comparison to the vehicle rats at 24 to 38 days following SCI (Figure 1).
Figure 1.
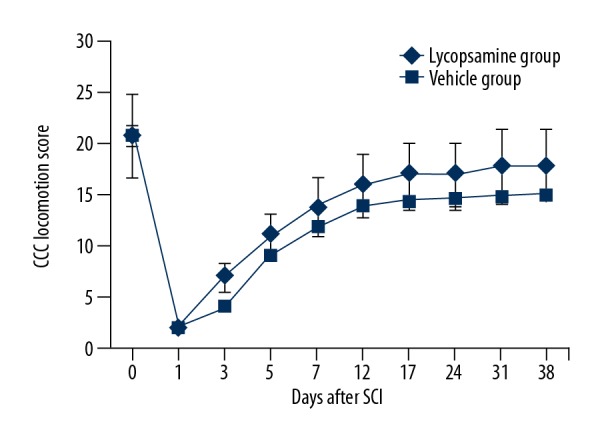
The BB locomotion scores for lycopsamine-treated and vehicle groups to the end of the study. Means of 3 replicates ±SD (* p<0.01).
Lycopsamine reduces cell death
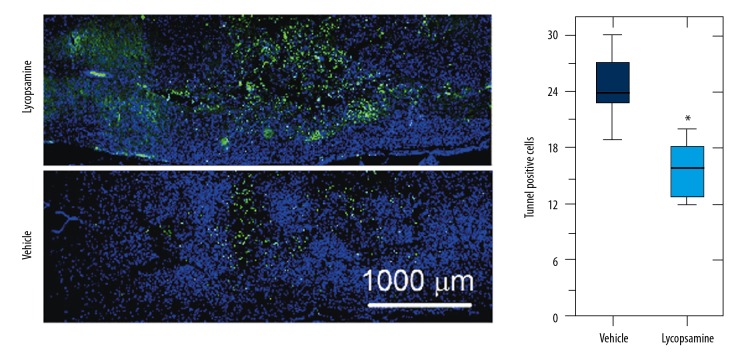
Spinal cord injury triggers apoptosis of the neurons and glial cells. Therefore, we sought to investigate if lycopsamine prevents the neurons and glial cells from undergoing cell death. Firstly, we carried out the DAPI, and TUNEL assay of the spinal cord sections were carried out. The results showed higher percentage of the apoptotic cells (TUNEL-positive) in the vehicle-treated rats in comparison to the lycopsamine-treated rats (Figure 2). These results suggest that lycopsamine reduces apoptotic cell death following SCI.
Figure 2.
DAPI and TUNEL assays showing apoptotic cells in lycopsamine-treated and vehicle groups. Means of 3 replicates ±SD (* p<0.01). The blue cells represent the DAPI-stained cells and the green cells represent the apoptotic cells as determined by TUNEL assay.
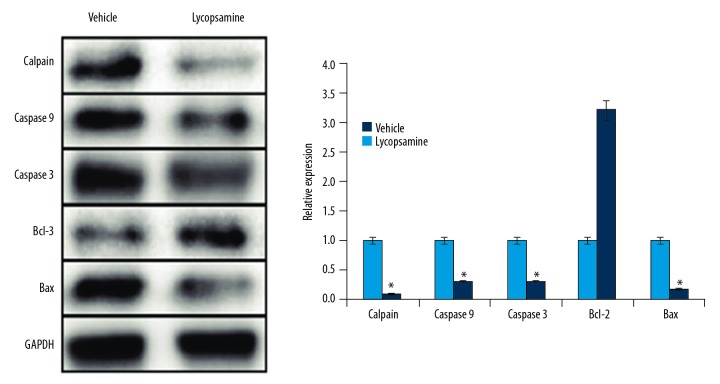
Lycopsamine modulates apoptosis-related protein expression
Since DAPI staining revealed that lycopsamine inhibits apoptosis following SCI, we sought to assess its effects on the expression of apoptosis-related proteins by use of Western blot analysis. The results showed that the expression of calpain, cleaved caspase 3 and 9, and Bax was significantly downregulated in the lycopsamine-treated rats in comparison to the vehicle group. However, the expression of BCl-2 was significantly upregulated (Figure 3). These results clearly indicate that lycopsamine inhibits apoptotic cell death of the neurons and glial cells by modulating the expression of apoptosis-related proteins.
Figure 3.
Western blot analysis showing the expression of Calpain, Caspase-3 and 9, Bax, and Bcl-2 in lycopsamine-treated and vehicle groups. The experiment was repeated 3 times.
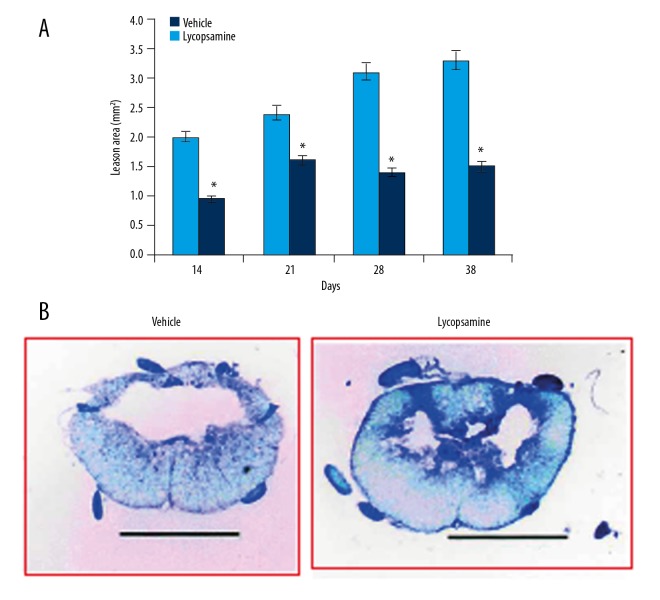
Lycopsamine reduces tissue necrosis after SCI
Although the lesion size increased continuously in lycopsamine- and vehicle-treated rats, a significant decrease was observed in lycopsamine-treated rats 14 days after SCI. The lesion size was considerably decreased at 28–38 days after SCI by lycopsamine in comparison to the vehicle-treated rats (Figure 4A). The lesion area was also visualised in the cresyl violet-stained sections of spinal cord, and larger lesions were observed in vehicle-treated group as compared to the lycopsamine-treated group (Figure 4B).
Figure 4.
(A) Lesion area (mm2) at indicated days (B) in cresyl violet-stained sections of spinal cord showing lesion area in lycopsamine-treated and vehicle groups. Mean of 3 replicates ±SD (* p<0.01).
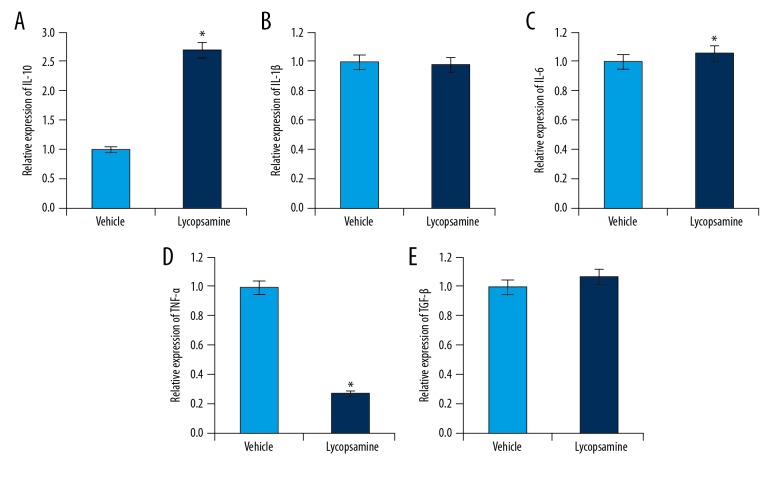
Lycopsamine enhanced the expression of IL-10 and decreased the expression of TNF-α
Given the antioxidant and anti-inflammatory effects of plant-derived natural products, we investigated the effect of lycopsamine on the expression of a few cytokines following SCI. The results of qRT-PCR revealed that lycopsamine treatment significantly increased the expression of IL-10, which was concomitant with downregulated expression of TNF-α. However, no apparent effect was observed on the expression of Il-1β, TGF-β, and IL-6 (Figure 5A–5E)
Figure 5.
Quantitative real-time expression analysis of (A) IL-10, (B) IL-1β (C) IL-6, (D) TNF-α, and (E) TGF-β. Mean of 3 replicates ±SD (* p<0.01).
Discussion
SCI is a devastating condition and has a huge economic effect worldwide [20]. A significant number of patients across the globe are suffering from SCI and there is no complete treatment for this disease, which is why it is sometimes considered to an ‘aliment not to be treated’ disease. Although recent advances have improved management of SCI patients, complete treatment is still far from being a reality [21]. However, new treatment options are sometimes tried. Natural products, especially from plants, are considered as important sources of drugs. Herein, we examined the effects of a plant-derived alkaloid, lycopsamine, on apoptosis and functional recovery after SCI in a rat model. We found that BBB scores were significantly higher in lycopsamine-treated rats in comparison to the vehicle rats at 24 to 38 days. This result suggests that lycopsamine administration improves locomotory function in SCI rats. It has been reported that following SCI, the rate of apoptosis of neural and glial cells increases considerably [22]. However, in the present study, the DAPI and TUNEL assays revealed that apoptotic cells were significantly lower in the lycopsamine-treated SCI rats. This indicates that lycopsamine inhibits apoptosis in neural and glial cells. Proteins such as Caspase 3 and 9, Bax, and Bcl-2 are considered as important biomarkers of apoptosis [23]. In this study, we observed that lycopsamine caused significant downregulation in the expression of Bax, Calpain, and Caspase 3 and 9, which was associated with upregulation of Bcl-2 expression, further confirming that lycopsamine administration reduces apoptotic cell death following SCI. Additionally, the effects of lycopsamine were also investigated on the lesion area, and we found that after 14 days, the lesion area of the lycopsamine-treated SCI rats was significantly lower compared to the vehicle-treated rats. Inflammation plays an important role in development of several neurodegenerative diseases [24]. SCI also quickly triggers increases in proinflammatory cytokines such as IL-6, IL-1β, and TNF-α [25]. Moreover, injection of IL-10 during PCI reduces the levels of TNF-α [26]. Herein, we observed that lycopsamine administration decreased the expression of TNF-α, and that the expression of IL-10 was significantly increased, showing the protective effect of lycopsamine in PCI rats. Previous studies have also shown that naturally occurring compounds have protective effects on SCI. Ginsenosides are also reported to exert protective effects on SCI [27]. Similarly, flavonoids such as caffeic acid phenethyl ester improves spinal cord injury in rabbits [28].
Conclusions
The protective effect and functional improvement observed with lycopsamine treatment are probably mediated by the suppression of cell death and the expression of apoptosis-related proteins. It also upregulates the expression of IL-10 and inhibits the expression of TNF-α. Therefore, Lycopsamine may prove beneficial in the management of PCI, but further investigations are required to validate the present findings.
Footnotes
Source of support: Departmental sources
References
- 1.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–61. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 2.Greenwell M, Rahman PK. Medicinal plants: Their use in anticancer treatment. International J Pharm Sci Res. 2015;6(10):4103–9. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su Y, Wang Q, Wang C, et al. The treatment of Alzheimer’s disease using Chinese medicinal plants: From disease models to potential clinical applications. J Ethnopharmacol. 2014;152(3):403–23. doi: 10.1016/j.jep.2013.12.053. [DOI] [PubMed] [Google Scholar]
- 4.Nasri H. Herbal drugs and new concepts on its use. J Preven Epidemiol. 2016;1(1):1–7. [Google Scholar]
- 5.Patridge E, Gareiss P, Kinch MS, et al. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov Today. 2016;21(2):204–27. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 6.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359(9304):417–25. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz G, Fehlings MG. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: Improved behavioral and neuroanatomical recovery with riluzole. J Neurosur. 2001;94(2):245–56. doi: 10.3171/spi.2001.94.2.0245. [DOI] [PubMed] [Google Scholar]
- 8.Dobkin BH, Havton LA. Basic advances and new avenues in therapy of spinal cord injury. Ann Rev Med. 2004;55:255–61. doi: 10.1146/annurev.med.55.091902.104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26(24S):S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 10.Brown AW, Stegelmeier BL, Colegate SM, et al. The comparative toxicity of a reduced, crude comfrey (Symphytum officinale) alkaloid extract and the pure, comfrey-derived pyrrolizidine alkaloids, lycopsamine and intermedine in chicks (Gallus gallus domesticus) J App Toxicol. 2016;36(5):716–25. doi: 10.1002/jat.3205. [DOI] [PubMed] [Google Scholar]
- 11.Jedlinszki N, Balázs B, Csányi E, et al. Penetration of lycopsamine from a comfrey ointment through human epidermis. Reg Toxicol Pharmacol. 2017;83:1–4. doi: 10.1016/j.yrtph.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Venard C, Boujedaini N, Belon P, et al. Regulation of neurosteroid allopregnanolone biosynthesis in the rat spinal cord by glycine and the alkaloidal analogs strychnine and gelsemine. Neuroscience. 2008;153(1):154–61. doi: 10.1016/j.neuroscience.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Stokes BT. Experimental spinal cord injury: A dynamic and verifiable injury device. J Neurotrauma. 1992;9(2):129–34. doi: 10.1089/neu.1992.9.129. [DOI] [PubMed] [Google Scholar]
- 14.Page ST, Marck BT, Tolliver JM, et al. Selectivity of the anabolic steroid, 19-nor-4-androstenediol-3β, 17β-diol in male Sprague Dawley rats: Selective stimulation of muscle mass and bone mineral density relative to prostate mass. Endocrinol. 2007;149(4):1987–93. doi: 10.1210/en.2007-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Touitou Y, Smolensky MH, Portaluppi F. Ethics, standards, and procedures of animal and human chronobiology research. Chronobiol Int. 2006;23(6):1083–96. doi: 10.1080/07420520601055308. [DOI] [PubMed] [Google Scholar]
- 16.Barros Filho TE, Molina AE. Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats. Clinics. 2008;63(1):103–8. doi: 10.1590/s1807-59322008000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27(2–3):126–39. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Darzynkiewicz Z, Galkowski D, Zhao H. Analysis of apoptosis by cytometry using TUNEL assay. Methods. 2008;44(3):250–54. doi: 10.1016/j.ymeth.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peel M, Donachie W, Shaw A. Temperature-dependent expression of flagella of Listeria manocytogenes studied by electron microscopy, SDS-PAGE and Western blotting. Microbiol. 1988;134(8):2171–78. doi: 10.1099/00221287-134-8-2171. [DOI] [PubMed] [Google Scholar]
- 20.Ruschel J, Hellal F, Flynn KC, et al. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348:347–52. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brock JH, Graham L, Staufenberg E, et al. Bone marrow stromal cell intraspinal transplants fail to improve motor outcomes in a severe model of spinal cord injury. J Neurotrauma. 2016;33(12):1103–14. doi: 10.1089/neu.2015.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang P, Hou H, Zhang L, et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49(1):276–87. doi: 10.1007/s12035-013-8518-3. [DOI] [PubMed] [Google Scholar]
- 23.Baharara J, Namvar F, Ramezani T, et al. Silver nanoparticles biosynthesized using Achillea biebersteinii flower extract: Apoptosis induction in MCF-7 cells via caspase activation and regulation of Bax and Bcl-2 gene expression. Molecules. 2015;20(2):2693–706. doi: 10.3390/molecules20022693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amor S, Peferoen LA, Vogel DY, et al. Inflammation in neurodegenerative diseases – an update. Immunol. 2014;142(2):151–66. doi: 10.1111/imm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, López-Vales R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. 2016;64(12):2079–92. doi: 10.1002/glia.23041. [DOI] [PubMed] [Google Scholar]
- 26.Ko WK, Kim SJ, Jo MJ, et al. Ursodeoxycholic acid inhibits inflammatory responses and promotes functional recovery after spinal cord injury in rats. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-0994-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Liao B, Newmark H, Zhou R. Neuroprotective effects of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in vitro. Exp Neurol. 2002;173(2):224–34. doi: 10.1006/exnr.2001.7841. [DOI] [PubMed] [Google Scholar]
- 28.Ilhan A, Koltuksuz U, Ozen S, et al. The effects of caffeic acid phenethyl ester (CAPE) on spinal cord ischemia/reperfusion injury in rabbits. Euro J Cardiothorac Surg. 1999;16(4):458–63. doi: 10.1016/s1010-7940(99)00246-8. [DOI] [PubMed] [Google Scholar]