Abstract
The incidence of venous thromboembolism (VTE) in adult patients with sickle cell disease (SCD) is high. However, overlapping features between the clinical presentation of VTE and SCD complications and a low index of suspicion for thrombosis can influence patient management decisions. VTE in SCD can therefore present management challenges to the clinical hematologist. Herein, we present 3 distinct clinical vignettes that are representative of our clinical practice with SCD patients. These vignettes are discussed with specific reference to the hypercoagulable state in SCD patients, recent VTE diagnosis and anticoagulant therapy guidelines from the general population, and evaluation of the risk of bleeding as a result of long-term exposure to anticoagulant therapy. We examine current diagnostic and treatment options, highlight limitations of the existing clinical prognostic models that offer personalized guidance regarding the duration of anticoagulation, and propose a clinical approach to guide the decision to extend anticoagulation beyond 3 months.
Introduction
Sickle cell disease (SCD; herein defined as homozygous hemoglobin [Hb] SS, or compound heterozygous HbS/β-thalassemia or HbS/HbC) is the most common inherited blood disorder in the United States, and a major health problem throughout the world.1 Over the last 5 decades, greater disease awareness by patients and improved access to care has reduced overall morbidity and mortality of SCD, especially in high-income countries.2,3 New medical therapies are being developed at a faster pace, and hematopoietic cell transplantation and somatic gene therapy offer curative potential.4-6 Increased life expectancy in adults with SCD is leading to a greater appreciation of organ complications.
SCD has long been considered a disorder primarily of erythrocytes wherein abnormal polymerization of Hb tetramers upon deoxygenation results in intermittent painful episodes, hemolytic anemia, vascular inflammation, and vaso-occlusion, eventually compromising organ function. Hypercoagulability, defined by many biomarkers that denote activation of prothrombotic factors or decreased antithrombotic proteins, is well described in patients with SCD.7,8 The contribution of hypercoagulability to the pathophysiology of common complications (vaso-occlusive crisis [VOC], stroke, acute chest syndrome [ACS]) of SCD is uncertain and therapeutic trials of anticoagulant drugs or platelet inhibitors have shown conflicting results.9
Venous thromboembolism (VTE), defined as deep vein thrombosis (DVT) or pulmonary embolism (PE), is increasingly recognized as a frequent and important clinical complication in adults with SCD, and is likely, at least in part, the result of this hypercoagulable state.10-12 In these reports, up to 12% of patients with SCD have a VTE by 40 years of age.12 Moreover, the VTE recurrence rate in SCD patients is similar to those individuals in the general population with unprovoked VTE, and is associated with increased mortality.10-12 There is no evidence from randomized trials that the management of SCD patients with VTE should be different from that recommended for other adults. However, within the prevailing paradigm, there are unanswered questions. Should SCD, in and of itself, be considered a strong persistent underlying risk factor for recurrent VTE warranting indefinite anticoagulation after a single incident VTE? Alternatively, should SCD be considered a mild thrombophilia, with a shorter duration of secondary pharmacological prophylaxis and further therapy only during exposure to periods of higher risk? How is the clinical paradigm of provoked and unprovoked VTE applicable to this population? Finally, are patients with SCD and VTE at increased risk of bleeding?
We believe that carefully designed randomized clinical trials to identify appropriate primary and secondary prevention strategies for VTE in SCD patients are warranted, given the frequency of this complication in adults and its contribution to mortality. Compared with VTE in the limbs or pulmonary vasculature, the frequency and importance of risk factors associated with VTE in unusual locations (eg, cerebral sinus thrombosis) are possibly different and beyond the scope of this article. In addition, we will not discuss primary VTE prophylaxis for the hospitalized SCD patient, other than propose that such patients be given pharmacological prophylaxis considering their high risk for VTE.
Absent direct evidence, clinicians are left with making management decisions based on extrapolations of general VTE treatment paradigms to SCD patients.13 In the current article, we discuss 3 commonly encountered case scenarios of VTE in SCD in our practices to illustrate how we diagnose and manage this problem. Our goal is to enable hematologists caring for SCD patients to be able to: (1) understand the hypercoagulable state in SCD and quantify VTE risk, (2) discuss the type and duration of anticoagulation for an incident VTE event in SCD patients, and (3) identify situations that warrant extending anticoagulation beyond that required for active treatment of VTE in SCD, weighing the risk of recurrence against that of major bleeding.
Case 1: acute DVT
A 42-year-old African American man with HbSS presents to the hospital with acute-onset left leg swelling. He has no previous history of venous thromboembolic disease, had not been hospitalized for 2 years, and had no recent operations. His mother, who did not have SCD, had an idiopathic lower-extremity DVT at 51 years of age. He has no cardiopulmonary symptoms. D-dimer was elevated, and bilateral Doppler ultrasound revealed an acute occlusive venous thrombosis of the left femoral vein extending from the popliteal trifurcation to the iliac vein. The patient was administered rivaroxaban at a dose of 15 mg orally, twice daily, for 21 days, and then reduced to 20 mg once daily.
Case 2: pregnant SCD patient with a history of DVT
A 32-year-old G1P0 Ghanaian woman with HbSS is 10 weeks pregnant. She developed a left femoral vein deep venous thrombosis at age 26 years during a hospitalization for VOC, and was treated with low-molecular-weight heparin (LMWH) followed by warfarin for 6 months. She has not had recurrent VTE. You are asked to make recommendations for VTE prophylaxis during pregnancy.
Case 3: catheter-related upper-extremity thrombus and its management
A 21-year-old African American woman with SCD on chronic, monthly exchange transfusion therapy for a history of ischemic stroke at 12 years of age presents with sudden-onset pain and swelling of her right upper extremity. She has a double-lumen port-a-cath in the left chest with entry through the left subclavian vein that was placed 2 years ago. She has a progesterone-eluting intrauterine device in place for a year. A Doppler ultrasound reveals an occluding thrombosis of the right axillary, subclavian, and internal jugular vein. Urine pregnancy test is negative, and she is placed on rivaroxaban.
The biochemical and clinical evidence for a hypercoagulable state in SCD
Hypercoagulability in the pathophysiology of SCD
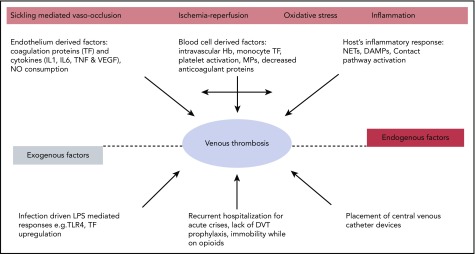
Although HbS polymerization, hemolytic anemia, and impaired microcirculatory blood flow from acute vaso-occlusion are central to disease pathophysiology, combined together they precipitate a cascade of downstream pathologic events that seemingly lead to organ complications and thrombotic vasculopathy (Figure 1). The contribution of coagulation activation, inflammation, and ischemia/reperfusion to the vascular pathobiology of SCD has been recently summarized.14-16
Figure 1.
Hypercoagulability in SCD. DAMP, damage-associated molecular pattern; IL, interleukin; LPS, lipopolysaccharide; MP, microparticle; NET, neutrophil extracellular trap; TF, tissue factor; TLR, Toll-like receptor; TNF, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
There is abundant biomarker evidence for ongoing activation of the coagulation cascade both during steady state (clinically well) and during VOC (Table 1). This may be triggered by the increased expression of tissue factor on monocytes and endothelial cells in the circulation of patients with SCD.17-21 Procoagulant protein activation is further accelerated by phosphatidylserine exposure on platelet and erythrocytes,22,23 and cell-derived microparticles,21,24-27 which serve as a surface for cell-based thrombin generation.28 Cell-free heme, increased in SCD from hemolysis, can induce endothelial tissue factor expression.29 In addition, arginase I released from the red blood cells during hemolysis depletes arginine, which is the substrate for nitric oxide synthesis.30 The resultant decrease in nitric oxide further tilts the hemostatic balance toward thrombosis.
Table 1.
Alterations in the coagulation system in humans with SCD
| Biochemical evidence of coagulation activation | Increased levels | Decreased levels | Steady state | Acute crisis | Reference |
|---|---|---|---|---|---|
| FXa generation | |||||
| Tissue factor pathway | + | + | + | 18, 68 | |
| Intrinsic pathway (FXII, HMWK, prekallikrein) | − | − | 31, 32 | ||
| Thrombin/fibrin generation | |||||
| D-dimer | + | + | + | 69 | |
| Prothrombin fragment F1.2 | + | + | + | 70 | |
| Fibrinopeptide A | + | 71 | |||
| Thrombin antithrombin complexes | + | + | + | 68 | |
| Changes in anticoagulant protein levels | |||||
| Protein C and S | − | − | − | 72, 73 | |
| ATIII and heparin cofactor II | − | − | 74, 75 | ||
| Changes in fibrinolytic protein levels | |||||
| Plasminogen activator inhibitor 1 | + | + | + | 76, 77 | |
| Other plasmatic factors | |||||
| FVIII | + | + | 78 | ||
| VWF | + | + | 79 | ||
| Cellular factors contributing to coagulation | |||||
| Platelet number and size | + | + | * | 22 | |
| Platelet activation and function | + | + | + | 80 | |
| Red blood cell activation (PS exposure and adhesion) | + | + | 81 | ||
| Leukocyte activation (TF exposure) | + | + | + | 20 | |
| Endothelial activation (TF exposure and adhesion) | + | + | + | 17 | |
| Cell-derived microparticles | + | + | + | 21 |
+, increased compared with controls; −, decreased compared with controls; ATIII, antithrombin III; FVIII, factor VIII; FXa, factor Xa; FXII, factor XII; HMWK, high-molecular-weight kininogen; PS, phosphatidylserine; TF, tissue factor; VWF, von Willebrand factor.
Variable findings.
Proximal intrinsic pathway coagulation protein alterations have also been reported in patients with SCD.31-33 Because components of the contact system are mediators of inflammation, activation of this system might play a role in inflammatory pathway perturbations that contribute to a prothrombotic state. Decreases in anticoagulant proteins, such as proteins C and S, have been reported in SCD and likely contribute to the hypercoagulable state.8,14 Finally, the possible role for iron in hypercoagulability has been suggested by decreased ex vivo measures of clotting, as measured by thromboelastography, in the plasma of SCD patients after iron chelation.34
Platelet activation may further promote clot formation. Increased expression of P-selectin on circulating platelets, and plasma soluble factors 3 and 4, β-thromboglobulin, and platelet-derived soluble CD40 ligand are all evidence of ongoing platelet activation in SCD patients.8,14 The clinical relevance of hemostatic activation is suggested by evidence that hydroxyurea treatment lowers many of these markers of hemostatic activation. Therefore, some of the benefit from hydroxyurea in SCD may be through attenuating the hypercoagulable state.35
Recent evidence for increased venous thrombotic events in SCD
For decades, clinicians have suspected that patients with SCD were at an increased risk for VTE but the data were primarily based on limited case series, single-institution studies, and/or case-control studies often confounded by risk factors in the general population.10,36-38 Recently, 2 large retrospective studies, 1 using a natural history cohort and the other using an administrative database from the state of California, have carefully described the incidence of VTE and its sequelae in SCD patients. Retrospectively analyzing data from the Cooperative Study of Sickle Cell Disease (CSSCD) to calculate incidence rates for first-time VTE, Naik and colleagues found an incidence rate of 5.2 events per 1000 person-years (95% confidence interval [CI], 3.8-6.9) in 1523 SCD patients aged ≥15 years with 8862 years of follow-up, with a cumulative incidence of 11.3% (95% CI, 8.3-15.3) by age 40 years.11 Individuals with SS or Sβ0-thalassemia had the highest rate of VTE (7.6 events per 1000 person-years [95% CI, 5.3-10.6]). These incidence rates are comparable with VTE incidence rates observed in prospective cohort studies of patients with inherited thrombophilia. Furthermore, the risk for death of SCD patients with VTE was higher than in those without VTE (adjusted hazard ratio [HR], 2.32; 95% CI, 1.20-4.46). The incidence of PE exceeded that of isolated DVT (3.6 events per 1000 person-years [95% CI, 2.5-5.1] vs 1.6 events per 1000 person-years [95% CI, 0.9-2.7]), although this difference was not statistically significant.
Similarly, Brunson and colleagues, using a population-based administrative database from the state of California, found that by age 40 years, the cumulative incidence of VTE among all SCD patients was 12.5% (95% CI, 11.5-13.6).12 Fifty-two percent presented as PE (±DVT), 25% isolated lower-extremity DVT, and 23% as isolated upper-extremity DVT. Overall, 60% of the VTE events occurred ≤90 days of a prior inpatient hospital discharge, with 94% of these with antecedent inpatient admission lasting >3 days. Among SCD patients, nonpregnant women (HR, 1.18; 95% CI, 1.01-1.38) and those with severe disease (defined as an average of 3 or more hospitalization per year) had an increased risk of VTE (HR, 2.86; 95% CI, 2.42-3.37). Among patients with severe SCD, the 5-year recurrence rate was 36.8%. Overall, VTE was associated with an increased risk of death (HR, 2.88; 95% CI, 2.35-3.52). Taken together, these 2 large studies demonstrated an increased rate of thrombotic events in SCD patients and the similarity of the estimates for incidence and VTE-related mortality are reassuring regarding robustness of the results.
Pregnancy is a well-established risk factor for VTE for women but this risk is magnified in pregnant women with SCD. In 1 study, SCD was an independent risk factor for pregnancy-related VTE with an odds ratio of 6.7 (95% CI, 4.4-10.1).39 Seaman and colleagues examined inpatient hospital discharge data from 212 hospitalized deliveries in African American women with SCD: 6 (2.8%; 95% CI, 1.0%-5.9%) had VTE compared with 0.05% to 2.0% in the general population.40 Overall, the prevalence of VTE among hospitalized deliveries in SCD women with pneumonia, VOC, and/or ACS was significantly greater than among those without these conditions (6.6% vs 2.2%; P < .001). They concluded that pregnancy-related VTE in women with SCD appears to be 1.5 to 5 times greater than pregnancy-related VTE in the general population. Porter and colleagues examined the relationship between sickle hemoglobinopathies and VTE risk during pregnancy or the puerperium.41 Of 103 women with HbSS, HbSC, or HbSβ-thalassemia, 3 women (2.9%) experienced VTE. Compared with women with normal Hb status, the relative risk was 32.2 (95% CI, 9.7-107). The relationship between sickle cell trait, SCD, and VTE in pregnancy has recently been reviewed.42
In the management of pregnancy and SCD, some groups suggest low-dose aspirin as prophylaxis against preeclampsia, and consideration of LMWH when additional risk factors are present. These risk factors include but are not limited to previous VTE, family history of VTE, known thrombophilia, older age, obesity, severe varicose veins, preeclampsia, immobility, and frequent hospitalization.43 Related to pregnancy is the use of oral contraceptives in women with SCD, which is reviewed elsewhere.44-46 Suffice it to say that progesterone-only methods of contraception are the least thrombogenic and are routinely considered first-line.44
Children with SCD are also affected by VTE. Using the Pediatric Health Information System database to investigate all pediatric patients with SCD admitted to 48 participating institutions between January 2009 and September 2015, Kumar and colleagues identified index VTE events and chronic medical conditions known to be associated with VTE using billing codes.47 Of 10 454 eligible subjects with SCD identified, 181 (1.7%) developed an index VTE event at a median age of 15.9 (±7.4) years. On multivariable logistic regression analysis, central venous catheter placement, chronic renal disease, history of stroke, female sex, length of hospitalization, admission to the intensive care unit, and older age were associated with VTE. After adjusting for other variables, VTE was independently associated with death.
Other clinical manifestations potentially involving hypercoagulability in SCD
Recent studies demonstrate the association of thrombosis in situ in the large pulmonary vessels in ∼20% of patients diagnosed with ACS, and post mortem studies of SCD patients diagnosed with ACS showing thrombi in the small pulmonary vessels support a role for thrombosis in disease pathophysiology.48-50 However, the nature of these studies makes it hard to ascertain a cause-effect relationship; that is, whether the presence of thrombosis was a primary ACS-inciting event or whether it occurred as a result of ACS. Results from an ongoing trial of anticoagulation in ACS patients (NCT02580773) could provide evidence or lack thereof regarding the impact of thrombosis on this complication.
Diagnosis of VTE in SCD
Risk factors associated with development of VTE
As with any patient in whom a diagnosis of VTE is being considered, the pretest probability of disease should be assessed. As previously noted, the diagnosis of SCD is an ongoing risk factor for VTE. VTE risk varies with genotype, with HbSS and Sβ0-thalassemia patients having the highest risk compared with HbSC or Sβ+-thalassemia,11 although the same investigators reported a higher incidence with other genotypes when studying a smaller, single-institution cohort of patients.10 Patients who have undergone splenectomy for other diseases,51 especially hemolytic anemias, have increased risk of VTE,52 and this is also likely for SCD patients. SCD patients averaging more than 3 admission per year, and women (even when accounting for pregnancy) were at higher risk for VTE.12,47 As noted previously, 60% of the California cohort were hospitalized within 90 days of incident VTE.12 Those with an elevated tricuspid regurgitant jet velocity on cardiac echocardiography were also at increased risk for VTE in the cohort series from Johns Hopkins.10 Upper-extremity VTE also occurs frequently in patients and is often associated with the presence of an indwelling catheter,10,12,47 suggesting that the risks-to-benefit ratio of indwelling catheter placement in SCD patients must be weighed carefully.
Symptoms and signs of VTE may overlap with other clinical complications of SCD. Lower-extremity edema might be attributed to right heart failure, kidney, or liver disease but its unequal distribution may provide a clue indicating VTE. A unilateral painful, swollen leg might be attributed to cellulitis, bony infarct, or complication of leg ulcers. Shortness of breath, pleuritic chest pain, fever, and hypoxemia are often attributed to ACS and/or pneumonia. Pulmonary emboli may be also associated with wheezing and pulmonary infiltrates. Therefore, a high clinical suspicion for VTE in patients with SCD with a low threshold for diagnostic evaluation is recommended.
Diagnostic testing
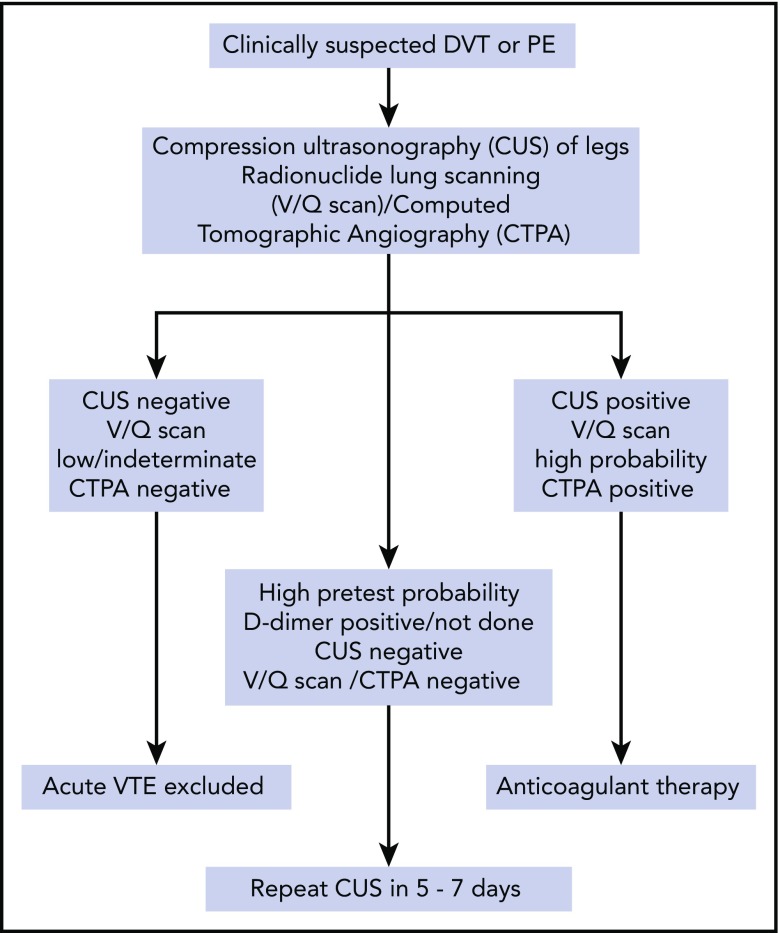
There are no studies that directly address the diagnostic algorithm for VTE in patients with SCD but we follow the algorithm shown in Figure 2. The use of D-dimer levels in diagnosis is uncertain, given baseline elevations even when patients are clinically well.8,14 In the general population, D-dimer testing, particularly when adjusted for age, can guide clinical decisions because of its high negative predictive value to rule out VTE and when tested serially, could help individualize the decision to extend anticoagulation duration.53 Normal D-dimer levels may be useful to rule out VTE, but it is rather unusual in our experience, and if the patient has a high pretest probability, one usually proceeds to imaging studies. D-dimer testing does not have a clear role in diagnosis or treatment of VTE in SCD and further research could clarify its utility.
Figure 2.
Algorithm for diagnosis of SCD patients with an episode of suspected VTE.
Our initial imaging includes the use of compression ultrasound Doppler for those suspected of upper- or lower-extremity DVT. We are not aware of any evidence that interpretation of compression ultrasound Doppler should be any different for a patient with SCD.
Multidetector computerized tomographic pulmonary angiography (CTPA) is currently a widely used test to detect PE. However, results may be confounded in patients with SCD experiencing ACS due to the high prevalence of in situ pulmonary thrombosis (17% [95% CI, 10%-23%]).48 Even in the absence of ACS, there is the possibility that subsegmental (or smaller) filling defects on CTPA may represent in situ sickling rather than a classic fibrin-rich clot. A further concern is the occurrence of contrast-induced acute kidney injury with CTPA, although we have not observed this with the newer nonionic low-osmolality contrast agents.
Radionuclide scanning (ventilation-perfusion [V/Q] scan) offers practical advantages over CTPA for the diagnosis of PE in SCD specifically by minimizing radiation exposure, absence of kidney injury, and well-defined diagnostic criteria. In one of our experiences (A.S.S.), diagnostic testing with V/Q scanning offers advantages over CTPA in patients who undergo frequent testing and offers specific advantages in establishing the diagnosis of chronic thromboembolic pulmonary hypertension.54 As with other populations, V/Q scanning is less useful in those individuals exhibiting pulmonary parenchymal abnormalities on plain chest radiographs.
Treatment of VTE in SCD
Treatment of acute VTE calls for urgent anticoagulation therapy to prevent extension, potentially fatal PE, and early recurrence of the thrombotic process, with no less than 3 months of anticoagulation because shorter treatment periods are associated with higher risk of recurrence.13 To date, the treatment of VTE in SCD relies on clinical guidelines established for VTE management in the general population because there are no clinical trials conducted to specifically inform anticoagulation practices in SCD. This includes consideration for thrombolytic therapy in appropriate situations. Currently, anticoagulation practice for the general population, as described in the American College of Chest Physicians (ACCP) 2016 guidelines,13 applies to individuals with SCD (summarized in Table 2). Nonetheless, there are special considerations for SCD patients that may modify the general recommendations, based on clinical judgement.
Table 2.
Summary of the approach to diagnosis and treatment of VTE in SCD
| VTE diagnosis and treatment approaches in SCD | |
|---|---|
| Diagnosis | • Compression ultrasonography (±Doppler) for deep venous thrombosis |
| • CTPA with nonionic low-osmolality contrast media | |
| o We do not routinely recommend red cell transfusion prior to contrast | |
| o Although less frequently performed V/Q scanning has clinical utility, especially when tested serially | |
| • D-dimer is routinely elevated in SCD precluding the high negative predictive value advantage this biomarker has in other settings | |
| Treatment | • Treatment as per ACCP 2016 guidelines with full-dose anticoagulation |
| o Potential for increased risk of bleeding in patients with MRA evidence for Moya Moya syndrome | |
| • Heparin, DOAC, or vitamin K antagonists are therapeutic options | |
| • In line with ACCP 2016 guidelines, our initial choice of anticoagulant is a DOAC if not contraindicated | |
| • Anticoagulate for at least 3 mo for VTE event | |
| • Consider extended anticoagulation in those with low bleeding risk even if the event was provoked by hospitalization for medical illness | |
| • Continue anticoagulation for catheter-associated upper-extremity thrombosis until catheter removal |
Adapted from Wun and Brunson.9
MRA, magnetic resonance angiography.
Type and intensity of anticoagulant therapy for VTE in SCD
As in the general population, there is no direct clinical evidence that the type of anticoagulant for the treatment of VTE should differ in patients with SCD. We typically treat SCD patients with acute VTE with direct oral anticoagulants (DOACs).13 However, estimation of glomerular filtration rate by serum creatinine can be inaccurate in patients with SCD potentially warranting the use of cystatin C, when available.55 Accurately estimating glomerular filtration rate and/or creatinine clearance guides appropriate selection of heparin and DOAC agents, all of which have varying degrees of renal clearance that can impact efficacy. For example, edoxaban may be less efficacious for nonvalvular atrial fibrillation when the creatinine clearance is >95 mL/min, which is not unusual in patients with SCD. Conversely, for varying creatinine clearance thresholds below 50 mL/min, dose reduction, and/or even use of alternative agents, is recommended for LMWH and DOACs.
For SCD patients with recurrent VTE, or bleeding on standard doses of nonwarfarin anticoagulants, we evaluate medication adherence, routinely check anti-Xa levels (for heparin), and consider testing DOAC drug levels. In SCD patients at high risk for bleeding, access to and availability of agents that can reverse the anticoagulant effect of DOACs may influence anticoagulant choice.56
Duration of anticoagulation
The duration of anticoagulation, beyond the minimum of 3 months for most patients, is determined by weighing the risk and seriousness of recurrent VTE with the risk of major bleeding. Data from the California cohort show the risk of recurrent VTE at 5 years to be nearly 37% in those averaging >3 admissions a year,12 and others have also reported a high rate of recurrent VTE.10 Interestingly, unpublished analysis of the California cohort reveals a high recurrence rate even in those with less severe disease (18% at 5 years, similar to men with unprovoked VTE13) with no difference whether the incident event was within or >90 days of a hospital admission. Therefore, a provoked VTE in a patient with SCD may be associated with a much higher risk of recurrence than a provoked VTE event in the general population.
The risk of major bleeding on therapeutic anticoagulation for VTE has typically been reported as low for patients without risk factors for bleeding.13 It is unknown whether SCD is associated with increased risk of bleeding on anticoagulation, absent other known risk factors, when compared with the general population of patients with VTE. However, preliminary data from the California cohort revealed a surprisingly high cumulative incidence of major bleeding of 2.9% at 6 months, and 5.0% at 1 year in SCD patients after incident VTE. Most of these episodes were gastrointestinal bleeding. This compares with 4% to 6% major bleeding incidence in patients treated for cancer-associated thrombosis with various anticoagulants on clinical trials57-59 and is higher than generally reported in large studies of non-SCD, noncancer patients, thereby stratifying SCD patients into the moderate to high risk for bleeding category.13 This information should also inform therapeutic considerations regarding the use of thrombolytic therapy for higher clot burden VTE in SCD patients.
The observation of a high risk of recurrent VTE, coupled with a relatively high risk of bleeding, is similar to the situation seen in patients with cancer.60-62 The current recommendation is to continue anticoagulation for cancer-associated thrombosis as long as there is active cancer.13,60 In the absence of clinical trials of anticoagulation in patients with SCD, we propose a conceptually similar approach. However, this must be carefully weighed against the increased risk of bleeding in these patients, which varies over time. This calculus would make one consider indefinite anticoagulation for a non–catheter-related VTE in a SCD patient regardless of whether the event was assessed to be provoked or not. Further work needs to be done to determine whether SCD patients with incident VTE can be risk stratified for recurrence, with duration of anticoagulation accordingly adjusted. Patient choice, health care costs associated with indefinite anticoagulation, and the potential long-term use of aspirin63 or lower doses of DOACs64,65 are also considerations that must be factored into making this decision.
For catheter-related VTE, we use the approach taken in other patients (Table 2). For symptomatic, upper-extremity catheter-related thrombosis, we recommend a minimum of 3 months of anticoagulation.66 If the catheter is functional and is required, we would continue therapeutic anticoagulation until catheter removal. Attention to the location of the catheter tip, and routine catheter care including anticoagulation flushes is also important to maintain catheter function and prevent mural thrombosis.
Cases revisited
Case 1
The patient’s symptoms resolved and after 3 months of treatment, he was reevaluated in clinic to discuss the risk and benefits of extending anticoagulation beyond 3 months to reduce his risk of recurrence. Despite a possible greater risk of recurrence with his family history, on the basis of severity of VTE (ie, DVT), and infrequent hospitalizations for SCD, he elected to discontinue anticoagulation after 3 months of therapy, due to bleeding risk-related concerns. Aspirin to reduce VTE recurrence, albeit less effectively than either warfarin or a DOAC but with a more favorable bleeding risk profile, was declined because he did not want an additional medication for an indefinite period.
Case 2
It was recommended to the patient that she start prophylactic-dose LMWH at the beginning of her second trimester through 6 weeks postpartum, with short interruption around the time of delivery. She did so, and had an uneventful spontaneous normal delivery. There was also discussion about the need for indefinite anticoagulation, given her prior VTE history and SCD. She decided against extended anticoagulation beyond the 6 weeks postpartum period, based on the provoked nature of her prior event, the time elapsed, her infrequent hospitalizations, and the increased risk for bleeding.
Case 3
The patient’s symptoms resolved with reduction in swelling and pain following the initiation of anticoagulation. The catheter position was confirmed to be appropriately located at the superior vena cava–right atrial junction. A V/Q scan was performed and ruled out the presence of PE. Due to concerns about the potential for dislodging a clot if erythrocytapheresis was performed, after 3 weeks of anticoagulation a repeat ultrasound was performed, which revealed organizing clot. Red cell exchange was then carried out without complication. Her anticoagulation will continue with a plan to stop therapy if the catheter is ever removed. An important area for future research is to prospectively assess the risk of catheter-associated thrombosis in SCD patients because it is known to be higher in patients with inherited thrombophilia.67
In conclusion, VTE is a frequent underrecognized clinical event that can complicate clinical manifestations adversely impacting the morbidity and mortality of SCD. The sickle genotypes HbSS and Sβ0-thalassemia, female sex, >3 hospitalization per year, functional hyposplenism, and presence of indwelling catheters are factors associated with increased risk. Restricting the use of central venous catheters and minimizing the exposure of patients to known risk factors for thrombosis could help prevent incident VTE. A high index of suspicion for VTE is required for early recognition and initiation of appropriate anticoagulation therapy. Diagnostic testing with compression ultrasonography for DVT, and V/Q scanning or CTPA for PE, is standard for objective confirmation of VTE in SCD. The diagnostic and prognostic value of D-dimer testing for VTE in SCD patients is yet to be clarified. Heparin, vitamin K antagonists, and DOACs are all effective agents in the treatment of VTE in SCD patients. A non–catheter-related VTE event in SCD justifies consideration of indefinite anticoagulation regardless of whether the event was provoked. However, the possible increased risk of bleeding in an SCD patient indicates that the choice of extended duration anticoagulation must be weighed carefully. Randomized clinical trials are warranted to identify optimal primary and secondary prevention strategies for VTE in SCD patients and to prospectively ascertain the risk or recurrence and bleeding.
Authorship
Contribution: A.S.S. and T.W. participated equally in the drafting and revision of the manuscript.
Conflict-of-interest disclosure: T.W. is a steering committee member and receives research funding from Janssen and Pfizer. A.S.S. declares no competing financial interests.
Correspondence: Arun S. Shet, Sickle Cell Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Building 10, Room 6S241 MSC 1589, 10 Center Dr, Bethesda, MD 20892-1589; e-mail: arun.shet@nih.gov.
REFERENCES
- 1.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. [DOI] [PubMed] [Google Scholar]
- 2.Gardner K, Douiri A, Drasar E, et al. Survival in adults with sickle cell disease in a high-income setting. Blood. 2016;128(10):1436-1438. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi S, DeBaun MR. Evolution of sickle cell disease from a life-threatening disease of children to a chronic disease of adults: the last 40 years. Am J Hematol. 2016;91(1):5-14. [DOI] [PubMed] [Google Scholar]
- 4.Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeil JA, Hacein-Bey-Abina S, Payen E, et al. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017;376(9):848-855. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman E, Cappelli B, Bernaudin F, et al. ; Eurocord, the Pediatric Working Party of the European Society for Blood and Marrow Transplantation, and the Center for International Blood and Marrow Transplant Research. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129(11):1548-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ataga KI, Key NS. Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology Am Soc Hematol Educ Program. 2007;2007:91-96. [DOI] [PubMed] [Google Scholar]
- 8.Pakbaz Z, Wun T. Role of the hemostatic system on sickle cell disease pathophysiology and potential therapeutics. Hematol Oncol Clin North Am. 2014;28(2):355-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wun T, Brunson A. Sickle cell disease: an inherited thrombophilia. Hematology Am Soc Hematol Educ Program. 2016;2016:640-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naik RP, Streiff MB, Haywood C Jr, Nelson JA, Lanzkron S. Venous thromboembolism in adults with sickle cell disease: a serious and under-recognized complication. Am J Med. 2013;126(5):443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naik RP, Streiff MB, Haywood C Jr, Segal JB, Lanzkron S. Venous thromboembolism incidence in the Cooperative Study of Sickle Cell Disease. J Thromb Haemost. 2014;12(12):2010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunson A, Lei A, Rosenberg AS, White RH, Keegan T, Wun T. Increased incidence of VTE in sickle cell disease patients: risk factors, recurrence and impact on mortality. Br J Haematol. 2017;178(2):319-326. [DOI] [PubMed] [Google Scholar]
- 13.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315-352. [DOI] [PubMed] [Google Scholar]
- 14.Noubouossie D, Key NS, Ataga KI. Coagulation abnormalities of sickle cell disease: relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev. 2016;30(4):245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127(3):750-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17(11):1391-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337(22):1584-1590. [DOI] [PubMed] [Google Scholar]
- 18.Solovey A, Gui L, Key NS, Hebbel RP. Tissue factor expression by endothelial cells in sickle cell anemia. J Clin Invest. 1998;101(9):1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setty BN, Key NS, Rao AK, et al. Tissue factor-positive monocytes in children with sickle cell disease: correlation with biomarkers of haemolysis. Br J Haematol. 2012;157(3):370-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragab SM, Soliman MA. Tissue factor-positive monocytes expression in children with sickle cell disease: clinical implication and relation to inflammatory and coagulation markers. Blood Coagul Fibrinolysis. 2016;27(8):862-869. [DOI] [PubMed] [Google Scholar]
- 21.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102(7):2678-2683. [DOI] [PubMed] [Google Scholar]
- 22.Tomer A, Harker LA, Kasey S, Eckman JR. Thrombogenesis in sickle cell disease. J Lab Clin Med. 2001;137(6):398-407. [DOI] [PubMed] [Google Scholar]
- 23.Styles L, de Jong K, Vichinsky E, et al. Increased RBC phosphatidylserine exposure in sickle cell disease patients at risk for stroke by transcranial Doppler screening [abstract]. Blood. 1997;90 Abstract 604. [Google Scholar]
- 24.Nebor D, Bowers A, Connes P, et al. Plasma concentration of platelet-derived microparticles is related to painful vaso-occlusive phenotype severity in sickle cell anemia. PLoS One. 2014;9(1):e87243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tantawy AA, Adly AA, Ismail EA, Habeeb NM, Farouk A. Circulating platelet and erythrocyte microparticles in young children and adolescents with sickle cell disease: relation to cardiovascular complications. Platelets. 2013:24(8):605-614. [DOI] [PubMed] [Google Scholar]
- 26.Gerotziafas GT, Van Dreden P, Chaari M, et al. The acceleration of the propagation phase of thrombin generation in patients with steady-state sickle cell disease is associated with circulating erythrocyte-derived microparticles. Thromb Haemost. 2012;107(6):1044-1052. [DOI] [PubMed] [Google Scholar]
- 27.van Beers EJ, Schaap MC, Berckmans RJ, et al. ; CURAMA Study Group. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94(11):1513-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJ. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: an isolated defect in platelet procoagulant activity. J Biol Chem. 1989;264(29):17049-17057. [PubMed] [Google Scholar]
- 29.Setty BN, Betal SG, Zhang J, Stuart MJ. Heme induces endothelial tissue factor expression: potential role in hemostatic activation in patients with hemolytic anemia. J Thromb Haemost. 2008;6(12):2202-2209. [DOI] [PubMed] [Google Scholar]
- 30.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller R, Verma P, Adams R. Studies of the kallikrein-kinin system in patients with sickle cell disease. J Nat Med Assoc. 1983;75(6):551-556. [PMC free article] [PubMed] [Google Scholar]
- 32.Verma P, Adams R, Miller R. Reduced plasma kininogen concentration during sickle cell crisis. Res Comm Chem Path Pharm. 1983;41(2):313-322. [PubMed] [Google Scholar]
- 33.Gordon EM, Klein BL, Berman BW, Strandjord SE, Simon JE, Coccia PF. Reduction of contact factors in sickle cell disease. J Pediatr. 1985;106(3):427-430. [DOI] [PubMed] [Google Scholar]
- 34.Shah N, Welsby IJ, Fielder MA, Jacobsen WK, Nielsen VG. Sickle cell disease is associated with iron mediated hypercoagulability. J Thromb Thrombolysis. 2015;40(2):182-185. [DOI] [PubMed] [Google Scholar]
- 35.Colella MP, De Paula EV, Conran N, et al. Hydroxyurea is associated with reductions in hypercoagulability markers in sickle cell anemia. J Thromb Haemost. 2012;10(9):1967-1970. [DOI] [PubMed] [Google Scholar]
- 36.Stein PD, Beemath A, Meyers FA, Skaf E, Olson RE. Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease. Am J Med. 2006;119(10):897. [DOI] [PubMed] [Google Scholar]
- 37.Novelli EM, Huynh C, Gladwin MT, Moore CG, Ragni MV. Pulmonary embolism in sickle cell disease: a case-control study. J Thromb Haemost. 2012;10(5):760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Hamel Parsons V, Gardner K, Patel R, Thein SL. Venous thromboembolism in adults with sickle cell disease: experience of a single centre in the UK. Ann Hematol. 2016;95(2):227-232. [DOI] [PubMed] [Google Scholar]
- 39.James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194(5):1311-1315. [DOI] [PubMed] [Google Scholar]
- 40.Seaman CD, Yabes J, Li J, Moore CG, Ragni MV. Venous thromboembolism in pregnant women with sickle cell disease: a retrospective database analysis. Thromb Res. 2014;134(6):1249-1252. [DOI] [PubMed] [Google Scholar]
- 41.Porter B, Key NS, Jauk VC, Adam S, Biggio J, Tita A. Impact of sickle hemoglobinopathies on pregnancy-related venous thromboembolism. Am J Perinatol. 2014;31(9):805-809. [DOI] [PubMed] [Google Scholar]
- 42.Noubouossie D, Key NS. Sickle cell disease and venous thromboembolism in pregnancy and the puerperium. Thromb Res. 2015;135(suppl 1):S46-S48. [DOI] [PubMed] [Google Scholar]
- 43.Koh MB, Lao ZT, Rhodes E. Managing haematological disorders during pregnancy. Best Pract Res Clin Obstet Gynaecol. 2013;27(6):855-865. [DOI] [PubMed] [Google Scholar]
- 44.Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI. Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. BMJ. 2012;345:e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith-Whitley K. Reproductive issues in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2014;2014:418-424. [DOI] [PubMed] [Google Scholar]
- 46.Altshuler AL, Gaffield ME, Kiarie JN. The WHO’s medical eligibility criteria for contraceptive use: 20 years of global guidance. Curr Opin Obstet Gynecol. 2015;27(6):451-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar R, Stanek J, Creary S, Dunn A, O’Brien SH. Prevalence and risk factors for venous thromboembolism in children with sickle cell disease: an administrative database study. Blood Adv. 2018;2(3):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mekontso Dessap A, Deux JF, Abidi N, et al. Pulmonary artery thrombosis during acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2011;184(9):1022-1029. [DOI] [PubMed] [Google Scholar]
- 49.Anea CB, Lyon M, Lee IA, et al. Pulmonary platelet thrombi and vascular pathology in acute chest syndrome in patients with sickle cell disease. Am J Hematol. 2016;91(2):173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch Pathol Lab Med. 2001;125(11):1436-1441. [DOI] [PubMed] [Google Scholar]
- 51.Boyle S, White RH, Brunson A, Wun T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with immune thrombocytopenia. Blood. 2013;121(23):4782-4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.L’Acqua C, Hod E. New perspectives on the thrombotic complications of haemolysis. Br J Haematol. 2015;168(2):175-185. [DOI] [PubMed] [Google Scholar]
- 53.Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D-dimer. J Am Coll Cardiol. 2017;70(19):2411-2420. [DOI] [PubMed] [Google Scholar]
- 54.Ruggiero A, Screaton NJ. Imaging of acute and chronic thromboembolic disease: state of the art. Clin Radiol. 2017;72(5):375-388. [DOI] [PubMed] [Google Scholar]
- 55.Airy M, Eknoyan G. The kidney in sickle hemoglobinopathies. Clin Nephrol. 2017;87(2):55-68. [DOI] [PubMed] [Google Scholar]
- 56.Pollack CV Jr, Reilly PA, Weitz JI. Dabigatran reversal with idarucizumab. N Engl J Med. 2017;377(17):1691-1692. [DOI] [PubMed] [Google Scholar]
- 57.Lee AY, Levine MN, Baker RI, et al. ; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153. [DOI] [PubMed] [Google Scholar]
- 58.Hull RD, Pineo GF, Brant RF, et al. ; LITE Trial Investigators. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119(12):1062-1072. [DOI] [PubMed] [Google Scholar]
- 59.Raskob GE, van Es N, Verhamme P, et al. ; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. [DOI] [PubMed] [Google Scholar]
- 60.Lee AY. Anticoagulation in the treatment of established venous thromboembolism in patients with cancer. J Clin Oncol. 2009;27(29):4895-4901. [DOI] [PubMed] [Google Scholar]
- 61.Lee AY. Treatment and secondary prophylaxis of VTE in the cancer patient: current status. Pathophysiol Haemost Thromb. 2003;33(suppl 1):42-43. [DOI] [PubMed] [Google Scholar]
- 62.Lee AYY, Kamphuisen PW, Meyer G, et al. ; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686. [DOI] [PubMed] [Google Scholar]
- 63.Brighton TA, Eikelboom JW, Mann K, et al. ; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979-1987. [DOI] [PubMed] [Google Scholar]
- 64.Agnelli G, Becattini C. Risk assessment for recurrence and optimal agents for extended treatment of venous thromboembolism. Hematology Am Soc Hematol Educ Program. 2013;2013:471-477. [DOI] [PubMed] [Google Scholar]
- 65.Weitz JI, Lensing AWA, Prins MH, et al. ; EINSTEIN CHOICE Investigators. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211-1222. [DOI] [PubMed] [Google Scholar]
- 66.Rajasekhar A, Streiff MB. How I treat central venous access device-related upper extremity deep vein thrombosis. Blood. 2017;129(20):2727-2736. [DOI] [PubMed] [Google Scholar]
- 67.Dentali F, Gianni M, Agnelli G, Ageno W. Association between inherited thrombophilic abnormalities and central venous catheter thrombosis in patients with cancer: a meta-analysis. J Thromb Haemost. 2008;6(1):70-75. [DOI] [PubMed] [Google Scholar]
- 68.Kurantsin-Mills J, Ofosu FA, Safa TK, Siegel RS, Lessin LS. Plasma factor VII and thrombin-antithrombin III levels indicate increased tissue factor activity in sickle cell patients. Br J Haematol. 1992;81(4):539-544. [DOI] [PubMed] [Google Scholar]
- 69.Francis RB., Jr Elevated fibrin D-dimer fragment in sickle cell anemia: evidence for activation of coagulation during the steady state as well as in painful crisis. Haemostasis. 1989;19(2):105-111. [DOI] [PubMed] [Google Scholar]
- 70.Peters M, Plaat BE, ten Cate H, Wolters HJ, Weening RS, Brandjes DP. Enhanced thrombin generation in children with sickle cell disease. Thromb Haemost. 1994;71(2):169-172. [PubMed] [Google Scholar]
- 71.Hagger D, Wolff S, Owen J, Samson D. Changes in coagulation and fibrinolysis in patients with sickle cell disease compared with healthy black controls. Blood Coagul Fibrinolysis. 1995;6(2):93-99. [DOI] [PubMed] [Google Scholar]
- 72.Tam DA. Protein C and protein S activity in sickle cell disease and stroke. J Child Neurol. 1997;12(1):19-21. [DOI] [PubMed] [Google Scholar]
- 73.Westerman MP, Green D, Gilman-Sachs A, et al. Antiphospholipid antibodies, proteins C and S, and coagulation changes in sickle cell disease. J Lab Clin Med. 1999;134(4):352-362. [DOI] [PubMed] [Google Scholar]
- 74.Porter JB, Young L, Mackie IJ, Marshall L, Machin SJ. Sickle cell disorders and chronic intravascular haemolysis are associated with low plasma heparin cofactor II. Br J Haematol. 1993;83(3):459-465. [DOI] [PubMed] [Google Scholar]
- 75.Onyemelukwe GC, Jibril HB. Anti-thrombin III deficiency in Nigerian children with sickle cell disease. Possible role in the cerebral syndrome. Trop Geogr Med. 1992;44(1-2):37-41. [PubMed] [Google Scholar]
- 76.Nsiri B, Gritli N, Bayoudh F, Messaoud T, Fattoum S, Machghoul S. Abnormalities of coagulation and fibrinolysis in homozygous sickle cell disease. Hematol Cell Ther. 1996;38(3):279-284. [DOI] [PubMed] [Google Scholar]
- 77.Nsiri B, Gritli N, Mazigh C, Ghazouani E, Fattoum S, Machghoul S. Fibrinolytic response to venous occlusion in patients with homozygous sickle cell disease. Hematol Cell Ther. 1997;39(5):229-232. [DOI] [PubMed] [Google Scholar]
- 78.Leslie J, Langler D, Serjeant GR, Serjeant BE, Desai P, Gordon YB. Coagulation changes during the steady state in homozygous sickle-cell disease in Jamaica. Br J Haematol. 1975;30(2):159-166. [DOI] [PubMed] [Google Scholar]
- 79.Krishnan S, Siegel J, Pullen G Jr, Hevelow M, Dampier C, Stuart M. Increased von Willebrand factor antigen and high molecular weight multimers in sickle cell disease associated with nocturnal hypoxemia. Thromb Res. 2008;122(4):455-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stuart MJ, Stockman JA, Oski FA. Abnormalities of platelet aggregation in the vaso-occlusive crisis of sickle-cell anemia. J Pediatr. 1974;85(5):629-632. [DOI] [PubMed] [Google Scholar]
- 81.Wood BL, Gibson DF, Tait JF. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: flow-cytometric measurement and clinical associations. Blood. 1996;88(5):1873-1880. [PubMed] [Google Scholar]