Abstract
Background Autoimmune thrombotic thrombocytopenic purpura (iTTP) is caused by autoantibody-mediated severe a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13 (ADAMTS13) deficiency leading to micro-angiopathic haemolytic anaemia (MAHA) and thrombocytopenia with organ damage. Patients survive with plasma exchange (PEX), fresh frozen plasma replacement and corticosteroid treatment. Anti-CD20 monoclonal antibody rituximab is increasingly used in patients resistant to conventional PEX or relapsing after an acute bout.
Objective This retrospective observational study focused on the relapse rate and possible influencing factors including treatment with rituximab first introduced in 2003.
Patients and Methods Seventy patients treated between January 2003 and November 2014 were evaluated. Number, duration, clinical manifestations, laboratory data and treatment of acute episodes were documented. Diagnostic criteria of acute iTTP were thrombocytopenia, MAHA, increased lactate dehydrogenase and severe ADAMTS13 deficiency.
Results Fifty-four female and 16 male patients had a total of 224 acute episodes over a median observation period of 8.3 years. The relapse rate was 2.6% per month, for women 2.4% and for men 3.5% per month. Since 2003, 17 patients with a first iTTP episode were treated with rituximab, whereas 28 were not. There was a trend towards lower relapse rates after rituximab treatment over the ensuing years. However, this was statistically not significant.
Conclusion This analysis does not show a significant reduction of acute iTTP relapses by rituximab given during an acute bout. Initial episodes are characterized by more severe clinical signs compared with the less severe relapses. Furthermore, men suffer significantly more frequent and considerably more serious acute relapses.
Keywords: ADAMS/ADAMTS13, thrombotic thrombocytopenic purpura (TTP/HUS), clinical studies, autoantibodies
Introduction
Autoimmune thrombotic thrombocytopenic purpura (iTTP) is an acute, life-threatening disorder, and survivors are at risk of disease relapse. Acute episodes are characterized by consumptive thrombocytopenia, micro-angiopathic haemolytic anaemia (MAHA) and spontaneous von Willebrand factor (VWF)-induced platelet clumping. A severe a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13 (ADAMTS13) deficiency (< 10%) due to autoantibodies against ADAMTS13 is associated with acute episodes. ADAMTS13 regulates the size of the newly synthesized and secreted ultra-large (UL) VWF multimers, and cleaves these into less haemostatically active forms. 1 2 Symptoms of iTTP are highly variable. Many patients suffer from neurological symptoms such as confusion, headache, paresis, aphasia and coma. Thrombocytopenia can result in petechiae, epistaxis and gingival bleeding. Further symptoms are abdominal pain, nausea, fatigue, proteinuria and cardiac complications. 3 Patients surviving an acute iTTP bout and showing normalization of the laboratory parameters are often considered to be cured; however, recurrences of acute iTTP attacks are common. 2 4
Since 1991, plasma exchange (PEX) and corticosteroids are the primary standard of care in iTTP. 5 6 However, there is a proportion of iTTP patients having refractory acute episodes, exacerbations or a high tendency to relapse. Since 2002, rituximab, an anti-CD20 monoclonal antibody, was used in the treatment of iTTP and has become an international standard of therapy. 7 PEX removes ADAMTS13 autoantibodies as well as UL VWF multimers and fresh frozen plasma (FFP) replacement supplies ADAMTS13. Efficacy of PEX reducing mortality in acute episodes from 90 to 20 to 30% is indisputable 3 4 6 ; nevertheless, PEX and corticosteroids are not as efficient in suppressing autoantibodies as rituximab. Rituximab suppresses the disease-associated ADAMTS13 inhibitor production by depleting B lymphocytes. 8 Despite widespread application in iTTP, rituximab is still an off-label use.
In the current retrospective observational study, we investigated iTTP patients focusing on the relapse rate and possible influencing factors including treatment with rituximab.
Patients and Methods
In this systematic retrospective study, we analysed all iTTP patients referred to the University Medical Center (UMC) Mainz from January 2003 to November 2014. Subjects had an acute iTTP bout or were consulting our institution having survived an earlier acute iTTP episode. Inclusion criteria were the clinical diagnosis of iTTP, defined as thrombocytopenia (< 150,000/µL), MAHA, increased lactate dehydrogenase (LDH; > 1.5× upper limit of normal values) with or without ischaemic organ damage. Since 2003, severe ADAMTS13 deficiency (< 10%) caused by an ADAMTS13 autoantibody during an acute bout is an additional diagnostic requirement for iTTP.
Acute iTTP episodes were treated according to a standard procedure of the UMC Mainz and in accordance with the international guidelines. All iTTP patients received PEX using FFP or Octaplas SD (Octapharma, Vienna, Austria) daily from admission until platelet count of > 150,000/µL was reached for longer than 48 hours. From the first day of acute iTTP corticosteroids, usually prednisolone, 1 to 2 mg/kg body weight, were given daily.
In off-label use, rituximab was administered for the first time in 2003. In this iTTP cohort, rituximab (MabThera; Roche, Grenzach-Wyhlen, Germany) was only used in acute iTTP bouts (1–4 weekly infusions of 375 mg/m 2 each). In most cases, rituximab was administered to patients with thrombocytopenia persisting under daily PEX for ≥5 days. A second indication for rituximab treatment was in relapsing iTTP patients without severe organ manifestation where rituximab was given to avoid PEX.
Complete remission was defined as full resolution of the clinical manifestations, especially neurological symptoms, with normalized platelet count for more than 30 consecutive days after the last PEX. 9
This retrospective study was approved by German law (Landeskrankenhausgesetz §36 and §37) in accordance with the Declaration of Helsinki and by the Ethics Committee of “Landesärztekammer Rheinland-Pfalz” [837.506.15 (10274)].
Assays
If plasma ADAMTS13 activity was measured between 1996 and 2002, the VWF multimer degradation method (immunoblotting) was used. 1 4 Since January 2003, ADAMTS13 activity was examined by the residual ristocetin co-factor-based method. 10 Since April 2010, it was examined by the fluorescence resonance energy transfer system (FRETS-VWF73) method 11 modified according to Kremer Hovinga et al. 12 ADAMTS13 activity was expressed as percentage relative to that of pooled normal plasma. The normal range of ADAMTS13 activity in the ristocetin co-factor-based method was defined as 52 to 134% in 80 healthy controls with a detection limit of 6.25%. The normal range for the FRETS-VWF73 assay in healthy donors is > 50% with a limit of detection of 1%. 4 12
ADAMTS13 inhibitor was detected by incubating a mixture of heat-inactivated patient plasma with pooled normal plasma (1:1; v:v) for 60 minutes at 37°C and then measuring the ADAMTS13 activity by the FRETS-VWF73 assay. 12 Inhibition of 50% of normal plasma ADAMTS13 activity by undiluted patient plasma was defined as 1 Bethesda unit (BU) per mL. An ADAMTS13 inhibitor was diagnosed when ≥0.5 BU/mL were found.
Variables/Covariates
The following data of iTTP patients were collected: date of birth, sex, number of biological children, body height and weight, calculated body mass index, smoking status and possible co-morbidities.
Data concerning the acute iTTP bouts of each patient was collected such as number of acute bouts, clinical symptoms, beginning and end of the respective bout (admission into and discharge from a hospital), calculated time of an acute episode, calculated age at a bout and calculated whole observation time. Therapeutic procedures (PEX, immune adsorption or splenectomy) and medication (corticosteroids, rituximab, other) given during an acute episode were documented.
Furthermore, we collected laboratory data such as platelet count, LDH, haemoglobin, presence of schistocytes, ADAMTS13 activity and ADAMTS13 inhibitors.
Clinical Severity Score
To classify the severity of an iTTP bout, we established a score based on clinical and laboratory data ( Table 1 ). It divides all iTTP bouts into five categories. Category 0 defines acute episodes characterized by all laboratory abnormalities listed in Table 1 only without manifest clinical signs, usually encountered in known iTTP patients during outpatient visits. Category 1 involves mild clinical symptoms and all the laboratory abnormalities as in category 0. Category 2 and category 3 include more severe clinical signs and symptoms. Acute iTTP episodes with fatal outcome were grouped in category 4 ( Table 1 ).
Table 1. Clinical severity score.
| 0 = laboratory abnormalities only | All four laboratory abnormalities listed must be present • thrombocytopenia (< 150,000/µL) • increased LDH (> 1.5× upper limit of normal) • decreased haemoglobin (< 12 g/dL in females, < 14 g/dL in males) • presence of schistocytes |
| 1 = mild | Laboratory abnormalities plus at least one of the following clinical manifestations • haematoma, petechiae, ecchymoses • cephalgia, vertigo, nausea • fatigue, drowsiness, weakness • (sub-)febrile temperatures, shivering • pain, especially abdominal pain |
| 2 = moderate | Laboratory abnormalities plus at least one of the following clinical manifestations • micro- or macro-haematuria • icterus • tachycardia, dyspnoea, • reversible dys- or paresthesia, visual field defects • impaired consciousness (somnolence, stupor), disorientation |
| 3 = severe | Laboratory abnormalities plus at least one of the following clinical manifestations • stroke with aphasia and/or paresia and/or apraxia and/or ataxia • acute myocardial infarction • acute renal failure, multi-organ failure • coma, seizure • in case of pregnancy abortion or stillbirth |
| 4 = lethal | iTTP episode with fatal outcome |
Abbreviations: iTTP, autoimmune thrombotic thrombocytopenic purpura; LDH, lactate dehydrogenase.
Statistical Analysis
We organized all data in a SPSS file pseudonymizing patient names according to the guidelines of the ethics committee.
For descriptive analysis, median and interquartile range (IQR), as well as minimum and maximum were calculated for continuous variables. In addition, absolute and relative frequencies were computed for categorical variables and visualized via bar charts. For interpreting the recurrent events, a graphic is created with one line for each patient on the y -axis with dots for each recurring event with observation time on the x -axis. Kaplan–Meier estimates were used to describe the relapse-free survival times for patients who did or did not receive rituximab. The log-rank test was used to compare the curves. Additionally, a Cox proportional hazard regression model was used to evaluate the effect of explorative variables on relapse-free survival.
To model the number of events for each patient and specifically to estimate the relapse rate in different sub-groups, a Poisson model with the log-transformed observation time incorporated as offset variable was performed.
For confirmatory analysis to estimate the rate ratio for patients treated with or without rituximab, adjusting for the variables sex and age, an Anderson–Gill model for the recurrent events was performed. The Poisson model and the Anderson–Gill model take into account the dependency between all iTTP episodes in one individual patient. The significance level was chosen to be 0.05. We present the rate ratio with its 95% confidence interval (CI) and its p -value. All statistical analyses were performed using SPSS version 22.0 (IBM GmbH, Ehningen, Germany) or the statistical program R version 3.4.1.
Results
Recruitment and Characteristics of 70 iTTP Patients
Since January 2003 until November 2014, a total of 88 patients were seen at the UMC Mainz for suspected iTTP. One young woman died during transfer to the UMC in her first acute iTTP episode (ascertained by an autopsy). Seventy of them demonstrated an ADAMTS13 deficiency and an ADAMTS13 inhibiting autoantibody ( Fig. 1 ). Sixty-five of the 70 TTP patients had a documented severe ADAMTS13 deficiency (< 10%) during their acute TTP episode and a detectable ADAMTS13 inhibitor. The other five iTTP patients consulted the UMC Mainz after their first acute episode and suffered from further relapses until 2014. When consulting in remission, they showed ADAMTS13 activities of 16, 19, 15, 17 and 18% with or without weak ADAMTS13 inhibitor titers. The initially treating physicians of those five patients did not have the technical possibilities to measure ADAMTS13 activity or an ADAMTS13 inhibitor on site. Forty-five patients had their first acute iTTP episode during the recruiting period, and 25 iTTP patients had been diagnosed before January 2003. We started our retrospective analysis in 2003, because at that time rituximab was administered for the first time at our institution.
Fig. 1.
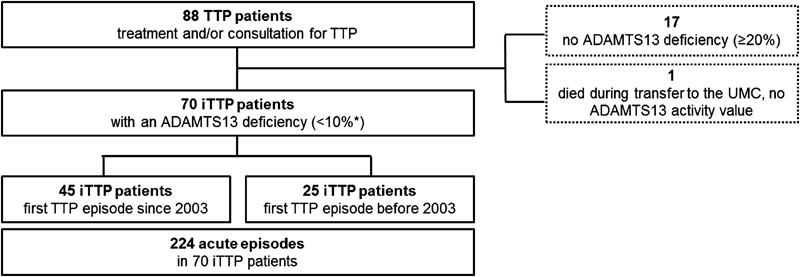
Patients' recruitment. *Five autoimmune thrombotic thrombocytopenic purpura (iTTP) patients consulted the UMC after having survived their first acute episode. At the time of consultation they showed a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13 (ADAMTS13) activities of 16, 19, 15, 17 and 18%, with/ without weak ADAMTS13 inhibitors. All other iTTP patients had documented severe ADAMTS13 deficiency (< 10%) during acute episode.
The characteristics of the 70 iTTP patients are shown in Table 2 . The iTTP cohort consisted predominantly of females (77%) and all patients were Caucasian. Overall, 70 iTTP patients suffered from 224 acute episodes over an observation time of 8.3 years (median; range, 0.4–31.9 years, IQR, 4.3–14.3 years). The median age at diagnosis is 33 years, ranging from 12 to 64 years (IQR, 26–49 years) ( Table 2 ).
Table 2. Patients' characteristics.
| Characteristics | No. | % |
|---|---|---|
| Total number of iTTP patients | 70 | |
| Gender | ||
| Female | 54 | 77.1 |
| Male | 16 | 22.9 |
| Ethnicity (white Caucasian) | 70 | 100 |
| Total number of acute iTTP episodes | 224 | |
| Age at time of diagnosis of first acute iTTP episode, y | ||
| Median | 33 | |
| Range | 12–64 | |
| Frequency of all acute episodes per patient | ||
| Median, total | 2 | |
| Range | 1–21 | |
| Median, female | 2.0 | |
| Median, male | 2.5 | |
| Observation time, y | ||
| Median | 8.3 | |
| Range | 0.4–31.9 |
Abbreviation: iTTP, autoimmune thrombotic thrombocytopenic purpura.
Severity of Acute Bouts and Therapy
Detailed data for therapy was obtainable in 219 ( Fig. 2 ) and for severity in 213 ( Supplementary Table S1 , available in the online version) of 224 acute episodes in 70 iTTP patients. No severity and therapy data were accessible in 3 iTTP patients in 4 acute bouts. Thus, detailed data for clinical severity as well as therapy in the respective acute bout were available for 211 of 224 acute episodes in 70 iTTP patients.
Fig. 2.
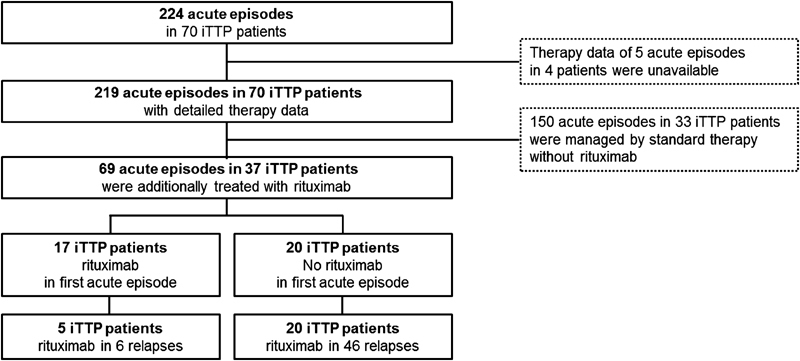
Treatment of acute autoimmune thrombotic thrombocytopenic purpura (iTTP) episodes.
Most common were mild symptoms or signs such as fatigue and drowsiness (43.5%), headaches (26.8%), petechiae (27.3%), haematoma (23.4%), vertigo (12.9%), nausea (7.2%) and abdominal pain (6.7%). Among the more severe symptoms, impairment of consciousness (15.8%), dyspnoea (12.9%), acute renal failure (9.6%) and haematuria (11.5%) were most frequent. When counting neurological abnormalities together, they appeared in 34.4% of all bouts.
During the first iTTP manifestation, the proportion of severe episodes (50%) was higher than during 1st relapse (19%) and all subsequent relapses (13%) ( Fig. 3 ). Similarly, moderately severe bouts were more common during 1st acute episodes (32%) as compared with 1st (22%) and later (10%) relapses. In contrast, mild bouts and mere laboratory abnormalities were more common during 1st relapse (56%) and further relapses (76%) than during initial manifestation (17%) ( Fig. 3 and Supplementary Table S1 , available in the online version).
Fig. 3.
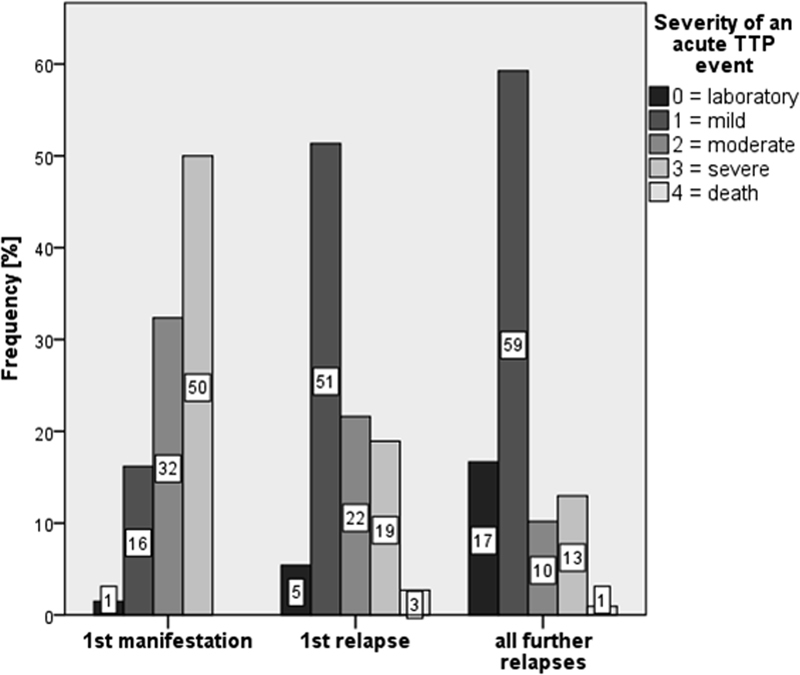
Frequency of the different clinical severity scores at first, second and all following bouts in 70 autoimmune thrombotic thrombocytopenic purpura (iTTP) patients. During the first iTTP manifestation, the proportion of severe episodes was higher than during 1st relapse and all subsequent relapses. Absolute numbers in a total of 219 bouts: 1st manifestation: laboratory (0): n = 1, mild (1): n = 11, moderate (2): n = 22, severe (3): n = 34; 1st relapse: laboratory (0): n = 2, mild (1): n = 19, moderate (2): n = 8, severe (3): n = 7, death (4): n = 1; all further relapses: laboratory (0): n = 18, mild (1): n = 64, moderate (2): n = 11, severe (3): n = 14, death (4): n = 1.
Plasma products were used in 191 of the 219 acute episodes (87%), prednisolone was administered in 188 (86%) and rituximab in 69 (32%) acute bouts. Overall, patients received 12 PEX procedures (median; range, 1–100, IQR, 6–23) for an acute bout. In the first acute episode, 62 (91.2%) iTTP patients received 21.5 PEX procedures (median; range, 2–100, IQR, 12–30). An acute iTTP bout lasted for 29 days (median; range, 1–160 days, IQR, 14–30 days).
Sixty-nine of of 219 acute bouts in 37 iTTP patients were additionally or exclusively (8 acute bouts in 3 different iTTP patients) treated with rituximab, whereas 150 acute bouts in 33 iTTP patients were not ( Fig. 2 ). Therefore, 31.5% of all acute TTP bouts were treated with rituximab in 53% of iTTP patients.
In this cohort, rituximab has exclusively been used in acute iTTP bouts.
The main indication for rituximab therapy was a refractory episode (58 acute episodes in 37 patients); a second indication was a high tendency to relapse (8 acute bouts in 4 patients).
Rituximab therapy was applied in acute iTTP events of all different clinical severity scores. Forty-three per cent of all bouts with severity score of 0, 26% of all mild bouts, 22% of all moderate bouts, 43% of all severe bouts and one of the two lethal bouts were treated with additional rituximab ( Supplementary Table S2 , available in the online version).
Relapse Rate and Influencing Factors
Fifty-nine per cent of all 70 iTTP patients had at least one relapse independent of their therapy in acute episodes. Relapse rate ( Fig. 4 ) is defined as acute recurrent disease episodes in survivors of an initial iTTP bout per 100 patient-months of follow-up. The relapse rate of the whole group was 2.6% per month. Fifty-four women had a relapse rate of 2.4% per month and 16 men of 3.5% per month. Thus, men have a 1.5 (95% CI, 1.11–2.01; p = 0.009) times higher risk to relapse than women.
Fig. 4.
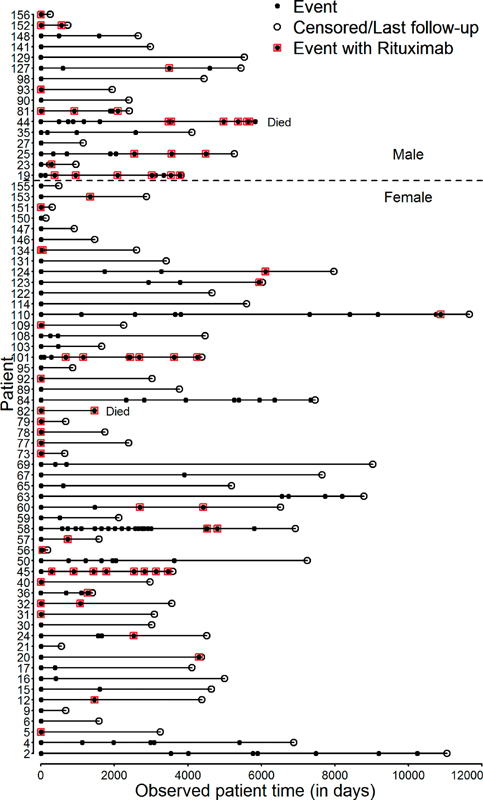
Event history of all autoimmune thrombotic thrombocytopenic purpura (iTTP) patients with detailed therapy data. Acute iTTP episodes of all 70 patients from the first day of first acute thrombotic thrombocytopenic purpura (TTP) episode until end of observation time in days. Patients were pseudonymized with a number code. Men are listed (above dotted horizontal line no. 19–156) in the upper part and women in the lower part of the figure (below dotted line no. 2–155). Acute iTTP episodes are represented by a black dot. If being treated by rituximab, this circle is bordered by red rectangles. Empty circles represent the last day of observation. Two patients died during a relapse (Died): including one woman following her first acute TTP relapse (no. 82). She had denied plasma products for religious beliefs. One man did not survive his 13th acute TTP episode (no. 44).
We analysed 219 acute episodes concerning possible risk factors for relapses. Smoking status seemed to be associated with higher relapse rate, whereas co-morbidities, including other autoimmune diseases and obesity, did not have an influence on the relapse rate when corrected for gender. With increasing age relapse rate declined.
Relapse rate after acute episodes treated with rituximab was 2.3% per month in comparison to 2.6% per month in acute bouts not treated with rituximab ( Fig. 4 , Anderson–Gill model, p = 0.729, relative risk, 0.945, 95% CI, 0.687–1.30). Accordingly, rituximab had no significant influence on the relapse rate.
Relapse-Free Survival in 45 Patients with First iTTP Manifestation Since 2003
For this analysis, we considered all 45 patients with an initial acute iTTP bout since 2003, the time point when rituximab was first used for this indication at our institution. Seventeen iTTP patients received rituximab during their first acute episode and 20 iTTP patients only during relapses ( Fig. 2 ). We investigated the relapse-free survival time of these 45 iTTP patients, 17 receiving rituximab compared with 28 not receiving rituximab during their first acute iTTP bout ( Fig. 5 ). Assessing the relapse-free survival after a first acute episode using Kaplan–Meier analysis, no significant difference between both groups of patients, those treated versus those not treated with rituximab (log-rank test, p -value = 0.131) was evident ( Fig. 5 ). Relapse rate in iTTP treated with rituximab in the initial acute episode was 29% (5 out of 17) and 50% (14 out of 28) in those who did not. The event-free survival in patients treated or not treated with rituximab after 1 year was 94 and 82%, respectively, and 79 versus 57%, respectively, after 1,000 days. The median event-free time until first relapse was 1,337 days in patients not treated with rituximab. This value cannot be determined for patients treated with rituximab because less than half of them had a relapse.
Fig. 5.
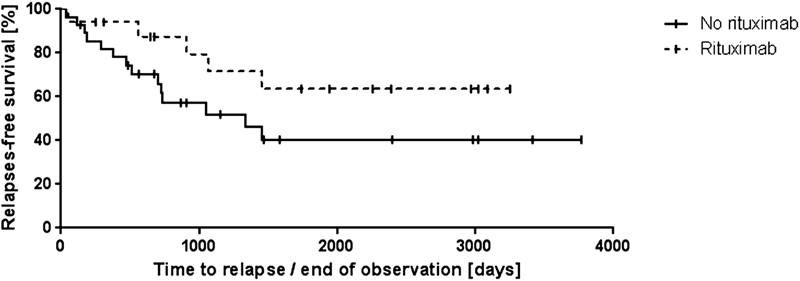
Kaplan–Meier estimates of relapse-free survival of 45 autoimmune thrombotic thrombocytopenic purpura (iTTP) patients with a first disease bout since 2003. Relapse-free survival of iTTP patients receiving rituximab ( n = 17) (upper dotted curve) compared with iTTP patients who did not receive rituximab ( n = 28) (lower curve) during their first acute thrombotic thrombocytopenic purpura (TTP) bout. Vertical bars denote censored patients not having suffered from relapse. There was no statistical difference between these groups ( p -value = 0.131).
Discussion
Since 2003, rituximab was used in 69 acute episodes as second-line medication in 37 of our 70 patients, generally because they were refractory to standard treatment or showed early relapses. Rituximab has been increasingly used in iTTP over the past 15 years, 7 13 14 albeit the indications (refractory disease, upfront treatment in all patients, pre-emptive treatment in survivors with recurring severe ADAMTS13 deficiency) are heterogeneous and still debated. 15 Rituximab was initially used for patients with refractory acute TTP and those with early relapses, and was generally considered to be effective. 14 15 In our retrospective cohort, 59% of all 70 iTTP patients had a relapse, independent of whether rituximab was added or not. This is higher than the 40% reported by Coppo and Froissart and Ferrari et al. 16 17 Relapse rate was 29% in 17 iTTP patients treated with rituximab during their first acute episode, and 50% in those who did not. Chemnitz et al observed a relapse rate of 25% in their 12 rituximab-treated patients over a 50-month period. 18 Scully et al found a relapse rate of only 10% in her upfront-rituximab-treated cohort, which compared favourably with a rate of 57% in a historical control group not having received rituximab. 19 Similar data, relapse rates of 43% without versus 12.5% with rituximab, were reported by Page et al in a smaller cohort but with simultaneously treated control group. 20 According to accumulated data from various cohorts, rituximab may decrease the frequency of subsequent relapses; nonetheless, we can detect only a weak tendency and no significant effect in our cohort. The influence of rituximab seems to be greater in the first year after the initial episode. Our data are similar to that reported by Froissart et al finding no significant difference in the relapse rate of their refractory patients treated versus not treated with rituximab. 21 Advantage of rituximab seems to lie in a faster recovery and possibly less recurrences during the first year. 21 22 23 24 25 We can confirm that, in our iTTP patients the event-free-survival after 1 year was 94% (treated with rituximab) versus 82% (not treated with rituximab).
Severe ADAMTS13 deficiency is pathophysiologically strongly linked to the development of clinical disease manifestations. Furthermore, high anti-ADAMTS13 immunoglobulin G (IgG) antibody titers are associated with increased mortality. 26 In turn, decrease of anti-ADAMTS13 IgG is directly associated with rituximab therapy, 25 suppression of B-lymphocytes being detectable for 9 to 15 months. Hence, relapse rate must be carefully watched after this time period. 23
As it is necessary to achieve remission and reduce relapse frequency, it is desirable to prevent life-threatening relapses at all. In our patients, the initial iTTP bouts were more severe than the later occurring relapses, which have been reported by earlier investigators as well. 27 This may not be due to the disease itself but rather to increased awareness following the initial diagnosis. Our patients were regularly seen and amenable to early treatment. In a few patients with frequent relapses, rituximab was given after detection of defined laboratory abnormalities even before clinical symptoms arose which led to reduced need of PEX. Early administration of rituximab leads to faster remission and lower number of necessary PEX sessions. 22 This finally raises the question whether rituximab should be given to any patient with persisting or reappearing severe ADAMTS13 deficiency even before any fall in platelet count or LDH increase is apparent. Hie et al showed that pre-emptive rituximab treatment in asymptomatic survivors of at least one iTTP bout seemed to reduce relapses as compared with patients not receiving rituximab 28 however, this pre-emptive treatment has been called into question by others. 15
Moreover, gender plays an important role regarding relapse rate and severity of acute bouts. Generally, men are affected by severe autoimmune diseases more rarely than women. 29 30 According to our results, men suffered significantly more and also more serious relapses than women. This is similar to other autoimmune diseases such as multiple sclerosis and in systemic lupus erythematous with men suffering from more severe disease course as compared with women. 30 31 A significantly higher tendency to relapse for men has also been described by Fakhouri et al. 23 Further investigation needs to evaluate whether gender-adapted treatment is necessary.
Strengths and Limitations of this Study
Our study has several limitations. One is the retrospective data acquisition. In hindsight, missing data or further desirable information could not be collected. On the other side, the long observation time was an advantage over cohorts with shorter follow-up periods. This enabled us to generate relevant results regarding relapse rates. Another limitation is a treatment bias regarding the use of rituximab. Rituximab was predominantly used in iTTP bouts, resistant to conventional PEX and corticosteroid treatment or in patients with a high tendency to relapse. Strength of our study is that iTTP patients given rituximab were compared with simultaneously recruited control iTTP patients, although the two cohorts are small for statistical analysis and not matched by age or sex. A randomized, prospective controlled trial would still be desirable to clarify the role of rituximab but it seems rather unlikely that such a trial will be performed shortly.
In conclusion, we did not detect a significant advantage of rituximab regarding relapse rate, neither during the time between initial acute episode and the first relapse, nor in long-term observation including all acute episodes. Nevertheless, we confirm that rituximab can help to achieve remission in refractory iTTP. Interestingly, we found that men suffer significantly more frequent and considerably more serious acute relapses. Furthermore, initial episodes are characterized by more severe clinical signs compared with the less severe relapses.
Funding Statement
Funding This study (BMBF 01EO1503) as well as Tanja Falter (BMBF 01EO1003) were supported by the Federal Ministry of Education and Research.
Conflict of Interest The authors declare that they have no conflicts of interest relevant to the manuscript. I. Scharrer is a member of the Data Safety Monitoring Board in the BAX 930 study (investigating recombinant ADAMTS13 infusion in hereditary TTP). She received travel and accommodation support for participating at scientific congresses or meetings from Bayer and NovoNordisk. B. Lämmle is chairman of the Data Safety Monitoring Board in the BAX 930 study (investigating recombinant ADAMTS13 infusion in hereditary TTP). He is on the Advisory Board of Ablynx for the development of caplacizumab. He holds a patent on ADAMTS13 and received travel and accommodation support for participating at scientific congresses or meetings from Baxalta, Siemens, Alexion, Ablynx and Bayer and speaker's fees from Siemens, Bayer and Alexion.
Authors' Contributions
T. Falter: Study concept and design, data analysis, writing of the manuscript and approval. S. Herold: Data acquisition and analysis, writing of the manuscript and approval. V. Weyer: Statistical advice, revision of the manuscript and approval. C. Scheiner: Statistical advice, revision of the manuscript and approval. V. Schmitt: Data acquisition, revision of the manuscript and approval. X. Messmer: Data acquisition and approval. C. von Auer: Revision of the manuscript and approval. P. Wild: Revision of the manuscript and approval. K. Lackner: Revision of the manuscript and approval. B. Lämmle: Data compilation, writing of the manuscript, revision of the manuscript and approval. I. Scharrer: Study initiation, revision of the manuscript, writing of the manuscript and approval.
Tanja Falter and Stephanie Herold are first co-authors of the study.
What is known about this topic?
Rituximab has been increasingly used in iTTP over the past 15 years.
The first and commonly accepted indication concerns patients refractory to standard treatment with PEX and corticosteroids. Recently, upfront rituximab in any patient with acute iTTP has been proposed based on data suggesting faster remission and shorter hospital stay. Lastly, prophylactic rituximab has been given to survivors of an acute iTTP with recurrent or persistent severe ADAMTS13 deficiency to avoid relapses. No formal indication has been approved.
What does this paper add?
First large German cohort of 70 iTTP patients examined for factors influencing the relapse rate.
Rituximab has no significant effect on the long-term relapse rate in our cohort.
We report that men suffer significantly more frequent and considerably more serious acute episodes than women.
Initial acute episodes are characterized by more severe clinical signs compared with the less severe relapses.
Supplementary Material
References
- 1.Furlan M, Robles R, Lämmle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87(10):4223–4234. [PubMed] [Google Scholar]
- 2.Coppo P, Veyradier A. Thrombotic microangiopathies: towards a pathophysiology-based classification. Cardiovasc Hematol Disord Drug Targets. 2009;9(01):36–50. doi: 10.2174/187152909787581318. [DOI] [PubMed] [Google Scholar]
- 3.Scully M, Hunt B J, Benjamin S et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158(03):323–335. doi: 10.1111/j.1365-2141.2012.09167.x. [DOI] [PubMed] [Google Scholar]
- 4.Kremer Hovinga J A, Vesely S K, Terrell D R, Lämmle B, George J N. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(08):1500–1511. doi: 10.1182/blood-2009-09-243790. [DOI] [PubMed] [Google Scholar]
- 5.George J N. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116(20):4060–4069. doi: 10.1182/blood-2010-07-271445. [DOI] [PubMed] [Google Scholar]
- 6.Rock G A, Shumak K H, Buskard N A et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325(06):393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 7.Scully M. Rituximab in thrombotic thrombocytopenic purpura: medical and financial benefits. Acta Haematol. 2015;134(03):168–169. doi: 10.1159/000381413. [DOI] [PubMed] [Google Scholar]
- 8.Elliott M A, Heit J A, Pruthi R K, Gastineau D A, Winters J L, Hook C C. Rituximab for refractory and or relapsing thrombotic thrombocytopenic purpura related to immune-mediated severe ADAMTS13-deficiency: a report of four cases and a systematic review of the literature. Eur J Haematol. 2009;83(04):365–372. doi: 10.1111/j.1600-0609.2009.01292.x. [DOI] [PubMed] [Google Scholar]
- 9.Scully M, Cataland S, Coppo P et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(02):312–322. doi: 10.1111/jth.13571. [DOI] [PubMed] [Google Scholar]
- 10.Böhm M, Vigh T, Scharrer I. Evaluation and clinical application of a new method for measuring activity of von Willebrand factor-cleaving metalloprotease (ADAMTS13) Ann Hematol. 2002;81(08):430–435. doi: 10.1007/s00277-002-0502-3. [DOI] [PubMed] [Google Scholar]
- 11.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(01):93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 12.Kremer Hovinga J A, Mottini M, Lämmle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost. 2006;4(05):1146–1148. doi: 10.1111/j.1538-7836.2006.01904.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M, Fujimura Y, Wada H et al. Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) 2017 in Japan. Int J Hematol. 2017;106(01):3–15. doi: 10.1007/s12185-017-2264-7. [DOI] [PubMed] [Google Scholar]
- 14.Froissart A, Veyradier A, Hié M, Benhamou Y, Coppo P; French Reference Center for Thrombotic Microangiopathies.Rituximab in autoimmune thrombotic thrombocytopenic purpura: a success story Eur J Intern Med 20152609659–665. [DOI] [PubMed] [Google Scholar]
- 15.Lim W, Vesely S K, George J N. The role of rituximab in the management of patients with acquired thrombotic thrombocytopenic purpura. Blood. 2015;125(10):1526–1531. doi: 10.1182/blood-2014-10-559211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppo P, Froissart A; French Reference Center for Thrombotic Microangiopathies.Treatment of thrombotic thrombocytopenic purpura beyond therapeutic plasma exchange Hematology (Am Soc Hematol Educ Program) 20152015637–643. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari S, Scheiflinger F, Rieger M et al. Prognostic value of anti-ADAMTS 13 antibody features (Ig isotype, titer, and inhibitory effect) in a cohort of 35 adult French patients undergoing a first episode of thrombotic microangiopathy with undetectable ADAMTS 13 activity. Blood. 2007;109(07):2815–2822. doi: 10.1182/blood-2006-02-006064. [DOI] [PubMed] [Google Scholar]
- 18.Chemnitz J M, Uener J, Hallek M, Scheid C. Long-term follow-up of idiopathic thrombotic thrombocytopenic purpura treated with rituximab. Ann Hematol. 2010;89(10):1029–1033. doi: 10.1007/s00277-010-0968-3. [DOI] [PubMed] [Google Scholar]
- 19.Scully M, McDonald V, Cavenagh J et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118(07):1746–1753. doi: 10.1182/blood-2011-03-341131. [DOI] [PubMed] [Google Scholar]
- 20.Page E E, Kremer Hovinga J A, Terrell D R, Vesely S K, George J N. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2016;127(24):3092–3094. doi: 10.1182/blood-2016-03-703827. [DOI] [PubMed] [Google Scholar]
- 21.Froissart A, Buffet M, Veyradier A et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Crit Care Med. 2012;40(01):104–111. doi: 10.1097/CCM.0b013e31822e9d66. [DOI] [PubMed] [Google Scholar]
- 22.Westwood J P, Webster H, McGuckin S, McDonald V, Machin S J, Scully M. Rituximab for thrombotic thrombocytopenic purpura: benefit of early administration during acute episodes and use of prophylaxis to prevent relapse. J Thromb Haemost. 2013;11(03):481–490. doi: 10.1111/jth.12114. [DOI] [PubMed] [Google Scholar]
- 23.Fakhouri F, Vernant J P, Veyradier A et al. Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13-deficient thrombotic thrombocytopenic purpura: a study of 11 cases. Blood. 2005;106(06):1932–1937. doi: 10.1182/blood-2005-03-0848. [DOI] [PubMed] [Google Scholar]
- 24.Clark W F, Rock G, Barth D et al. A phase-II sequential case-series study of all patients presenting to four plasma exchange centres with presumed relapsed/refractory thrombotic thrombocytopenic purpura treated with rituximab. Br J Haematol. 2015;170(02):208–217. doi: 10.1111/bjh.13408. [DOI] [PubMed] [Google Scholar]
- 25.Miyakawa Y, Imada K, Ichinohe T et al. Efficacy and safety of rituximab in Japanese patients with acquired thrombotic thrombocytopenic purpura refractory to conventional therapy. Int J Hematol. 2016;104(02):228–235. doi: 10.1007/s12185-016-2019-x. [DOI] [PubMed] [Google Scholar]
- 26.Alwan F, Vendramin C, Vanhoorelbeke K et al. Presenting ADAMTS13 antibody and antigen levels predict prognosis in immune-mediated thrombotic thrombocytopenic purpura. Blood. 2017;130(04):466–471. doi: 10.1182/blood-2016-12-758656. [DOI] [PubMed] [Google Scholar]
- 27.Rose M, Eldor A. High incidence of relapses in thrombotic thrombocytopenic purpura. Clinical study of 38 patients. Am J Med. 1987;83(03):437–444. doi: 10.1016/0002-9343(87)90753-4. [DOI] [PubMed] [Google Scholar]
- 28.Hie M, Gay J, Galicier L et al. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood. 2014;124(02):204–210. doi: 10.1182/blood-2014-01-550244. [DOI] [PubMed] [Google Scholar]
- 29.Boodhoo K D, Liu S, Zuo X. Impact of sex disparities on the clinical manifestations in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95(29):e4272. doi: 10.1097/MD.0000000000004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortona E, Pierdominici M, Maselli A, Veroni C, Aloisi F, Shoenfeld Y. Sex-based differences in autoimmune diseases. Ann Ist Super Sanita. 2016;52(02):205–212. doi: 10.4415/ANN_16_02_12. [DOI] [PubMed] [Google Scholar]
- 31.Quintero O L, Amador-Patarroyo M J, Montoya-Ortiz G, Rojas-Villarraga A, Anaya J-M.Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity J Autoimmun 201238(2-3):J109–J119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


