Abstract
Objective
Given the potential therapeutic effect of vascular disease control timing to reduce dementia risk, we investigated the age-related influences of vascular risk factor burden on brain structure throughout the lifespan.
Methods
We studied participants from the community-based prospective Framingham Heart Study. Overall vascular risk factor burden was calculated according to the Framingham Stroke Risk Profile, a validated algorithm that predicts stroke risk. Brain volume was estimated by MRI. We used cross-sectional data to examine how the strength of association between vascular risk factor burden and brain volume changed across each age decade from age 45–54 years through to 85–94 years (N = 2,887). Second, we leveraged up to 40 years of longitudinal data to determine how the strength of association between vascular risk factor burden and brain volume changed when vascular risk factors were examined at progressively earlier ages (N = 7,868).
Results
In both cross-sectional and longitudinal analyses, higher vascular risk factor burden was associated with lower brain volume across each age decade. In the cross-sectional analysis, the strength of this association decreased with each decade of advancing age (p for trend < 0.0001). In longitudinal analysis, the strength of association between vascular risk factor burden and brain volume was stronger when vascular risk factors were measured at younger ages. For example, vascular risk factor burden was most strongly associated with lower brain volume in later life when vascular risk factors were measured at age 45 years.
Conclusion
Vascular risk factors at younger ages appear to have detrimental effects on current and future brain volume.
Vascular risk factors are important determinates of silent cerebrovascular disease and clinical stroke, which are known risk factors for vascular cognitive impairment and dementia.1,2 Clarifying the effect of age on the association between vascular risk and brain health is important for identifying target populations, in which the screening, management, and treatment of vascular risk factors may have the greatest benefits for protecting against vascular related brain injury.
The Framingham Heart Study (FHS) has obtained brain MRIs on a large group of community-dwelling participants who, for decades, have undergone routine testing for vascular risk factors. We leveraged this unique data set to characterize the age-dependent association between vascular risk factor burden and brain volume. First, we examined the cross-sectional association between vascular risk factor burden and brain volume on MRI, stratified by each age decade from 45–54 years to 85–94 years. We hypothesized that the magnitude of association would diminish with advancing age. Second, we used longitudinal data to examine the relationship between past vascular risk factor burden and current brain volume when vascular risk factors were measured at progressively younger ages in the same participants. For example, for an adult aged 85 years at the time of brain MRI, we examined how vascular risk factor burden at ages 45, 55, 65, 75, and 85 years related to brain volume at age 85 years. We hypothesized that the association between vascular risk factor burden and brain volume would become progressively stronger as exposure to vascular risk factor burden became available from earlier in life.
Methods
The FHS is a prospective, community-based study from the town of Framingham, MA. The Original cohort began in 1948 with the enrollment of 5,209 participants, with surviving members examined approximately every 2 years. In 1971, offspring of the Original cohort and spouses of these offspring were invited to form the Offspring cohort (N = 5,124). The Offspring cohort has been examined across 9 examination cycles, with one approximately every 4 years. The Third Generation cohort comprises grandchildren of the Original cohort (children of the Offspring cohort) and was established in 2002, with the third examination cycle currently ongoing (N = 4,095). In addition to the examination cycles, surviving participants are under constant surveillance for incident events such as myocardial infarction, stroke, and dementia.
Standard protocol approvals, registrations, and patient consents
All participants provided written informed consent. The study was approved by the institutional review board and Boston University Medical Center.
Ascertainment of the vascular risk factor burden
Vascular risk factor burden was calculated according to the Framingham Stroke Risk Profile (FSRP), an algorithm of vascular risk factor burden that estimates the 10-year risk of stroke using clinical information.3 Higher FSRP scores are also associated with smaller brain volumes, silent brain injury, and poorer cognitive function.4–6 We calculated the FSRP in accordance with recently updated criteria.3 The FSRP score combines information on age, sex, current smoking status, prevalent cardiovascular disease, prevalent atrial fibrillation, diabetes, hypertension treatment, and systolic blood pressure. This updated algorithm has been validated in 3 large community samples.3
Ascertainment of brain volume on MRI
Attendees of the Original cohort at examination 25, Offspring cohort at examination 7, and the Third Generation cohort at examination 2 were invited to participate in an MRI study. Total brain parenchymal volume was expressed as a percentage of total cranial volume, thus adjusting for differences in head size. As intracranial volume provides an estimate of the largest brain size achieved during life, the percentage of brain volume relative to intracranial volume provides a proxy for overall brain health and brain atrophy and also associates with dementia risk in older persons.7 Measurements were completed using a Siemens 1T or 1.5T field strength machine. Earlier scans used a T2-weighted double spin-echo coronal imaging sequence in contiguous slices of 4 mm, whereas later scans used 3-dimensional T1-weighted coronal spoiled gradient-recalled echo acquisition and fluid-attenuated inversion recovery sequences. Analysis of MRI images was completed by a neurologist (C.D.), blinded to vascular risk factor burden and subject demographics. Further details have been described in detail previously.8
Statistical methods
Statistical analysis was performed using SAS Version 9.4. FSRP scores were examined as an untransformed continuous variable. All results were adjusted for age within each decade of life, sex (except where results are stratified by sex), and the time interval between vascular risk factor assessment and the brain MRI. Participants with missing data were excluded from analysis.
Statistical methods: Cross-sectional analysis
For our cross-sectional analysis, we obtained vascular risk factor data and brain MRI at approximately the same time (within an average of 1 year). There were 6,574 participants with available FSRP scores. Of these participants, 3,922 also had a brain MRI between 2005 and 2008. We excluded participants with stroke (n = 57), participants with known other major neurologic diseases such as MS (n = 135), and participants younger than 45 years or older than 95 years (n = 843), generating an analysis sample of 2,887 participants (Original cohort = 166, Offspring cohort = 1,537, and Third Generation cohort = 1,184).
We examined the association between vascular risk factor burden (FSRP scores) and brain volume with linear regression. We confirmed the assumption of linearity by plotting the predicted values against the residuals for each linear model. All plots showed random scatter around the horizontal axis, indicating that the assumption of linearity was upheld. Results were stratified by each age decade from 45–54 years to 85–94 years. Beta estimates and standard errors were extracted and graphed to observe how the strength of the association between vascular risk factor burden and brain volume changed with age. Differences across the age groups were investigated by testing for a linear trend across the age decades by examining for an interaction between FSRP scores and age as a continuous variable. We then explored for interactions by sex within each age decade.
Statistical methods: Longitudinal analysis
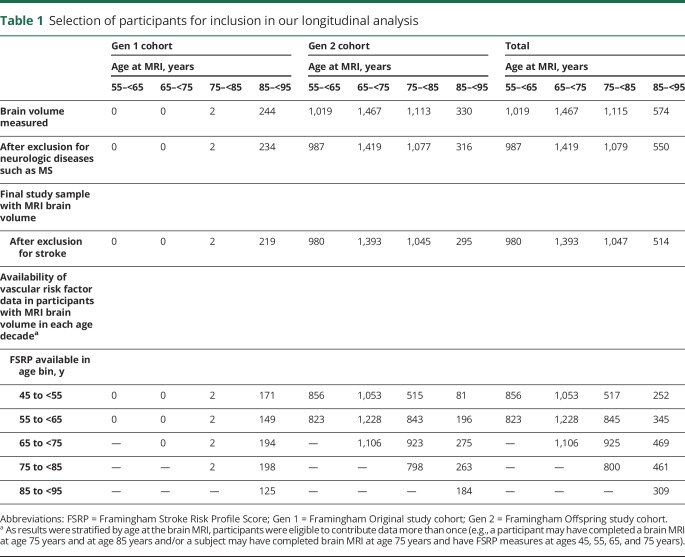
For our longitudinal analysis, we grouped participants into 4 age bins at the time of brain MRI (mean age of 55, 65, 75, and 85 years). For each participant, we then extracted their current and past vascular risk factor data from FHS examination cycles. We used these data to quantify overall vascular risk factor burden at 10-year intervals before brain MRI, extending back to age 45 years. This enabled us to examine how vascular risk factors at different points across the lifespan corresponded to future brain volume. The maximum time interval between past vascular risk factor burden and current brain volume was 40 years. As results were stratified by age at the brain MRI, participants were eligible to contribute data to more than one age bin. Our longitudinal analysis of vascular risk and brain volume is based on 7,868 brain MRIs, including 3,887 unique participants (table 1). Third Generation participants were not included in our longitudinal analysis, given the limited number of examination cycles available for this cohort.
Table 1.
Selection of participants for inclusion in our longitudinal analysis
Separate linear regressions were performed to examine the association between vascular risk factor burden and brain volume, with FSRP scores obtained in 10-year intervals extending into the past. As before, we confirmed that the assumption of linearity was upheld for each linear model. Beta estimates and standard errors were extracted and graphed to observe how the strength of association between vascular risk factor burden and brain volume changed as the FSRP was measured at progressively earlier time points. Differences across time were examined by testing for a linear trend across time using an age by risk factor burden interaction, taking into account that participants may have repeated measures of the FSRP. Based on the results from our cross-sectional analysis, results were stratified by each decade of life at the time of brain MRI.
Data availability
The FHS makes phenotypic and genetic data available through the online repositories BioLINCC and dbGap, respectively. In addition, investigators can request data for specific projects through the FHS website for a fee.
Results
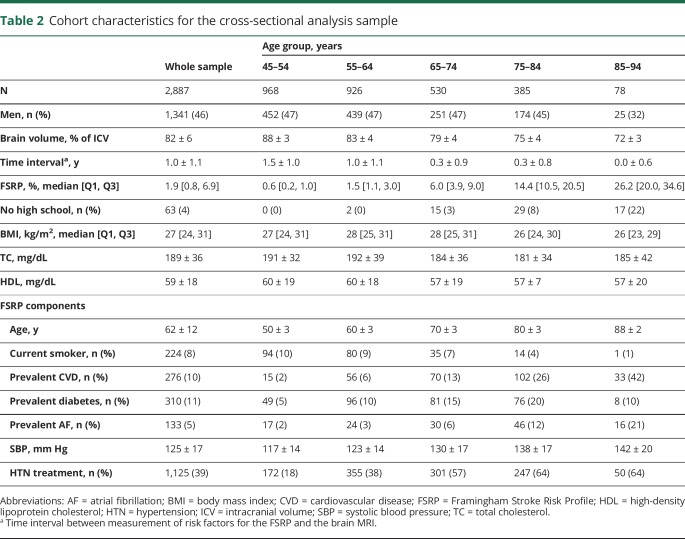
Table 2 summarizes the cohort characteristics for our cross-sectional analysis sample. As expected, brain volume decreased, whereas vascular risk factor burden increased with each decade of advancing age.
Table 2.
Cohort characteristics for the cross-sectional analysis sample
Cross-sectional association between vascular risk factor burden and brain volume
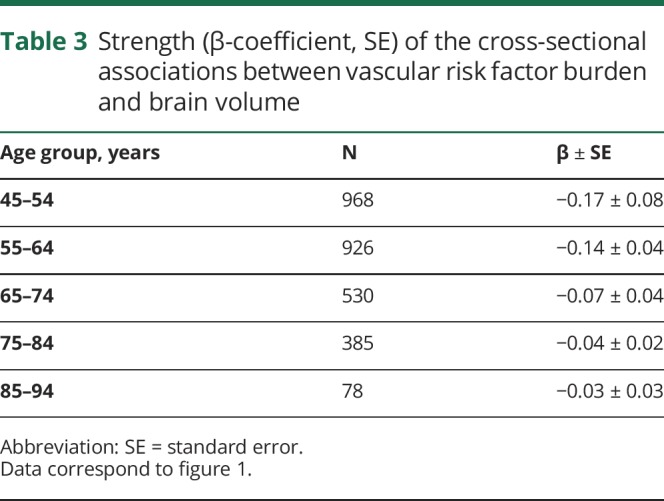
Higher vascular risk factor burden was associated with lower brain volume in each decade of life (figure 1 and table 3), and this association became progressively weaker with each decade of advancing age (p for trend < 0.0001). When predicting brain volume, we observed a significant interaction between sex and vascular risk factor burden, but only for participants aged 45–54 years (p = 0.01). In this age group, a higher vascular risk factor burden was associated with statistically significant smaller brain volumes in both sexes, although this effect was larger in women (β ± standard error, −0.15 ± 0.08 for men vs −1.32 ± 0.49 for women).
Figure 1. Age-dependent association between vascular risk factor burden and brain volume.
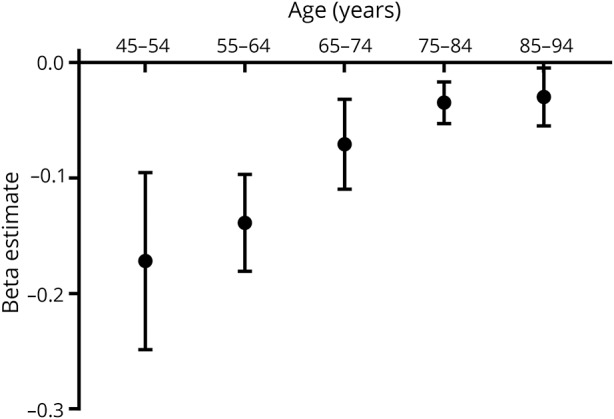
Cross-sectionally, the strength of association between vascular risk factor burden and brain volume is stronger at younger ages (p for trend < 0.0001).
Table 3.
Strength (β-coefficient, SE) of the cross-sectional associations between vascular risk factor burden and brain volume
Longitudinal association between vascular risk factor burden and brain volume
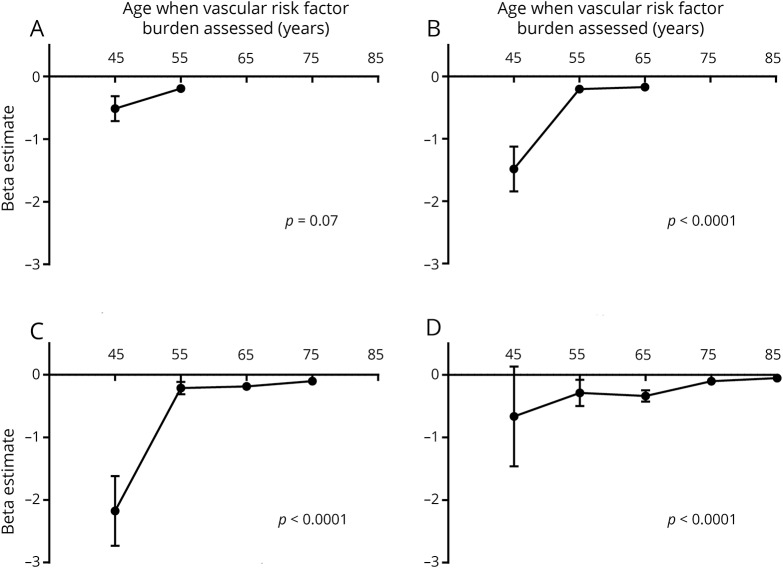
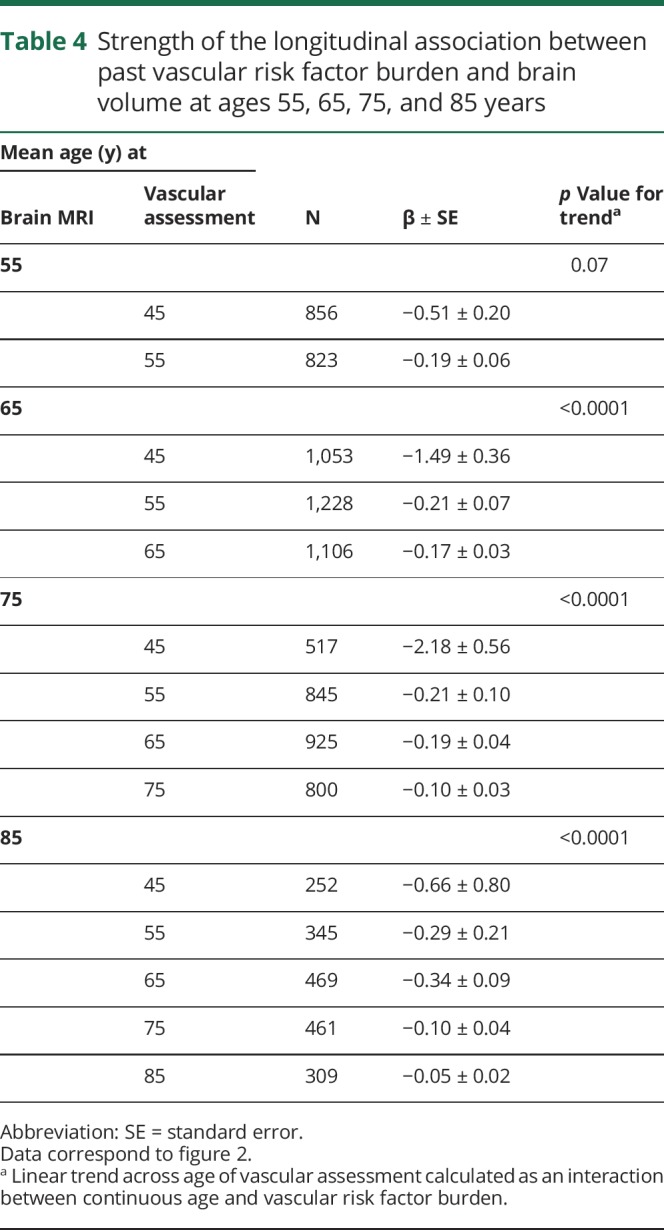
Greater vascular risk factor burden was associated with smaller brain volumes, with the association becoming stronger as risk factors were measured at younger ages (figure 2). This was true when brain volume was measured at the mean ages of 65, 75, and 85 years (p for trend < 0.0001). Early exposure to vascular risk factor burden, therefore, was the strongest predictor of current brain volume. The beta coefficients and standard errors corresponding to figure 2 are shown in table 4.
Figure 2. Past vascular risk factor burden in relation to current brain volume.
Longitudinal association between vascular risk factor burden quantified at different points across the lifespan and brain volume quantified at a mean age of 55 (A), 65 (B), 75 (C), and 85 (D) years.
Table 4.
Strength of the longitudinal association between past vascular risk factor burden and brain volume at ages 55, 65, 75, and 85 years
Discussion
Our study yields many important findings. First, higher vascular risk factor burden was cross-sectionally associated with smaller brain volumes, and this association got progressively weaker with aging, from age 45 years onward. Second, the effect of vascular risk factors on brain volume was most pronounced in young women. Third, early exposure to vascular risk factor burden was the strongest predictor of future brain volume. Therefore, both our longitudinal and cross-sectional data converge on a similar conclusion; vascular risk factors in the fourth and fifth decades of life appear to be critically important for current and future brain volume.
Midlife vascular risk factors are known to be associated with later-life vascular brain injury,9 cognitive impairment,10 and incident dementia.11 However, most studies tend to focus on vascular risk factors individually when, in reality, multiple vascular risk factors often coexist. Individual studies also tend not to compare the effect of vascular risk factors at different ages. The comparison of different studies can offer insight into the age-dependent association between vascular risk factors, such as blood pressure,12 and brain volume, although the comparison of different studies is confounded by differences in cohort characteristics and by definitions of midlife itself. The present study extends the literature by systematically investigating the age windows in which overall vascular risk most strongly related to brain volume.
There are many plausible explanations for why vascular risk factors are most strongly associated with brain volume at younger ages. Age is the biggest known risk factor for dementia13 and aging is also associated with the development of several traditional cardiovascular disease risk factors.14 Having a high vascular risk factor burden at younger ages may be indicative of early vascular aging15 and overall poor health, whereas having a high vascular risk profile in the later years of life is more in keeping with the patterns of aging observed in the general Western population. Similarly, the strong association between vascular risk factor burden and brain volume in young women may be because a high vascular risk factor burden is more uncommon in women who are premenopausal. In other words, young women with high vascular risk are unhealthy for their chronological age and advanced regarding their biological age. Another potential explanation is that the co-occurrence of multiple brain pathologies is common in old age,16,17 and this may overshadow the effect of vascular risk factor burden in the elderly. Last, persons with the highest vascular risk factor burdens early in life are less likely to make it to old age because of the competing risk of death from cardiovascular disease and stroke.
There are also plausible explanations to suggest why past rather than current vascular risk factor burden was a superior predictor of brain volume. In addition to the fact that past vascular risk factor burden captures health at a younger age, assessment of past risk factor burden may provide an estimate of cumulative exposure.2 In the FHS, time-averaged exposure to high blood pressure predicts stroke18 and cardiovascular disease19 independent of current blood pressure levels. In the absence of clinical stroke, vascular risk factors likely have an insidious detrimental effect on the brain.8 Therefore, an older adult who has been living with a high vascular risk factor burden since midlife may have a higher likelihood of subclinical brain injury compared with someone who first developed a high vascular risk factor burden in old age.
Total brain volume, relative to intracranial volume, is a proxy for brain atrophy and an indicator of overall brain integrity. In the FHS, total brain volume is an important determinate of cognitive function5 and a powerful predictor of dementia in older persons.7 Although our observational study cannot establish causality, vascular risk factors are known to increase the risk of brain injury and vascular cognitive impairment1 with the management and treatment of vascular risk factors cornerstone to the primary prevention of cerebrovascular disease.20 Consequently, our results have potential implications for public health and clinical practice. First, our results underscore the importance of limiting, treating, and managing vascular risk factors from at least the fourth and fifth decades of life. This appears to be especially true for women as recently reported.21 Therefore, screening for vascular risk factors in young to middle-aged women should be a priority. Our findings also underscore the importance of knowing past vascular risk factor burden. Where patient history is available, considering past rather than current vascular risk factor profiles may be more informative for evaluating vascular cognitive impairment. Such an approach may be particularly relevant in the era of electronic medical records where this information may become more readily available. Moreover, knowing current vascular risk factor profiles may aid in risk stratification for later-life brain atrophy and consequent cognitive impairment. It is unclear, however, whether more aggressive treatment of these individuals will alter brain health. Only randomized clinical trials examining the effect of attaining ideal vascular risk will be sufficient to address this question.22
Our results suggest that studies using cross-sectional data or short follow-up periods may grossly underestimate the true association between vascular risk factors and brain volume. Our results are timely, given current interest surrounding the long-preclinical phase of neurodegenerative diseases, whereby Alzheimer disease is known to begin decades before clinical dementia.23 The early preclinical phase of dementia is of current interest because treatments administered late in the Alzheimer disease process have failed.24 It is therefore important to understand how vascular and other risk factors earlier in life affect later-life neurologic end points. Future studies will need to merge parallel streams of research and examine how early life vascular risk factors interact with early Alzheimer disease pathology to ultimately contribute to dementia years later. The relevance of these findings to increased dementia risk for women is also of extreme interest and should be further studied.
Strengths of our study include the large community-based sample and the repeated assessments of vascular risk factors across the lifespan, extending 40 years into the past. However, our study is not without limitations. Our findings are derived from an overwhelmingly white sample, which means that it is unclear how our results will generalize to other racial and ethnic groups. We had few participants older than 85 years and were underpowered to examine this age group. Although we had participants younger than 45 years, we did not examine these participants, given the low vascular risk factor burden (i.e., narrow range of FSRP scores) observed in this age group.
Vascular risk factors during the fourth and fifth decades of life appear to be important determinates of brain volume. We also found that the magnitude of the association between higher vascular risk factor burden and smaller brain volumes was particularly pronounced in young women. Our findings suggest a need for vigilance in screening for and treating vascular risk factors in young adults, particularly women. When considering the effect of vascular risk factors on overall brain integrity, both researchers and physicians should consider a patient's history of vascular risk burden and be mindful that current vascular risk factor profiles may underestimate the true association between vascular risk factor burden and brain integrity. As the brain is an end organ of vascular disease,25 our results underscore the importance of minimizing vascular risk factor burden early in life.
Acknowledgment
The authors thank the Framingham Heart Study participants for their commitment and dedication.
Glossary
- FSRP
Framingham Stroke Risk Profile
- FHS
Framingham Heart Study
Footnotes
Editorial page 729
Author contributions
Study concept and design: M.P. Pase, K. Davis-Plourde, A.S. Beiser, J.J. Himali, S. Seshadri, and C. DeCarli. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: M.P. Pase, A.S. Beiser, and C. DeCarli. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: K. David-Plourde, A.S. Beiser, and J.J. Himali. Administrative, technical, or material support: S. Seshadri, A.S. Beiser, and C. DeCarli. Obtained funding: M.P. Pase, S. Seshadri, A.S. Beiser, and C. DeCarli. Study supervision: S. Seshadri, A.S. Beiser, and C. DeCarli.
Study funding
Dr. Pase is funded by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1089698). The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute (contracts N01-HC-25195 and HHSN268201500001I), the National Institute of Neurological Disorders and Stroke (NS017950 and UH2 NS100605), and the National Institute on Aging (AG033193, AG033040, AG049505, AG049607, and AG054076). Prof. DeCarli directs the UC Davis Alzheimer's Disease Center with funding from the NIH (P30 AG010182).
Disclosure
M.P. Pase, K. Davis-plourde, J.J. Himali, C.L. Satizabal, H. Aparicio, S. Seshadri, and A.S. Beiser report no disclosures relevant to the manuscript. C. Decarli is a consultant to Novartis on a clinical trial of LCZ696 for heart failure. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology March 1, 2018. Accepted in final form July 9, 2018.
References
- 1.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pase MP, Satizabal CL, Seshadri S. Role of improved vascular health in the declining incidence of dementia. Stroke 2017;48:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dufouil C, Beiser AS, McClure LA, et al. A revised framingham stroke risk profile to reflect temporal trends. Circulation 2017;135:1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaffashian S, Dugravot A, Elbaz A, et al. Predicting cognitive decline: a dmentia risk score vs the Framingham vacular risk scores. Neurology 2013;80:1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology 2004;63:1591–1599. [DOI] [PubMed] [Google Scholar]
- 6.Elias MF, Sullivan LM, D'Agostino RB, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke 2004;35:404–409. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein G, Beiser AS, Decarli C, Au R, Wolf PA, Seshadri S. Brain imaging and cognitive predictors of stroke and Alzheimer disease in the Framingham Heart Study. Stroke 2013;44:2787–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 9.Maillard P, Seshadri S, Beiser A, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 2012;11:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol 2014;71:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitmer RA, Gunderson EP, Quesenberry CP Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 2007;4:103–109. [DOI] [PubMed] [Google Scholar]
- 12.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499. [DOI] [PubMed] [Google Scholar]
- 13.2015 Alzheimer's disease facts and figures. Alzheimer's Dement 2015;11:332–384. [DOI] [PubMed] [Google Scholar]
- 14.Dhingra R, Vasan RS. Age as a risk factor. Med Clin North Am 2012;96:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag 2008;4:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007;357:1821–1828. [DOI] [PubMed] [Google Scholar]
- 17.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology 2015;85:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seshadri S, Wolf PA, Beiser A, et al. Elevated midlife blood pressure increases stroke risk in elderly persons: the Framingham Study. Arch Intern Med 2001;161:2343–2350. [DOI] [PubMed] [Google Scholar]
- 19.Vasan RS, Massaro JM, Wilson PW, et al. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation 2002;105:48–53. [DOI] [PubMed] [Google Scholar]
- 20.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology 2017;89:1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorelick PB, Furie KL, Iadecola C, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Demen 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korczyn AD. Why have we failed to cure Alzheimer's disease? J Alzheimers Dis 2012;29:275–282. [DOI] [PubMed] [Google Scholar]
- 25.Decarli C. Cerebrovascular disease: assessing the brain as an end-organ of vascular disease. Nat Rev Cardiol 2012;9:435–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The FHS makes phenotypic and genetic data available through the online repositories BioLINCC and dbGap, respectively. In addition, investigators can request data for specific projects through the FHS website for a fee.