Abstract
Objective
We applied direct cortical stimulation (DCS) to the orbitofrontal cortex (OFC) in neurosurgical patients implanted with intracranial electrodes to probe, with high anatomic precision, the causal link between the OFC and human subjective experience.
Methods
We administered 272 instances of DCS at 172 OFC sites in 22 patients with intractable focal epilepsy (from 2011 to 2017), none of whom had seizures originating from the OFC.
Results
Our observations revealed a rich variety of affective, olfactory, gustatory, and somatosensory changes in the subjective domain. Elicited experiences were largely neutral or negatively valenced (e.g., aversive smells and tastes, sadness, and anger). Evidence was found for preferential left lateralization of negatively valenced experiences and strong right lateralization of neutral effects. Moreover, most of the elicited effects were observed after stimulation of OFC tissue around the transverse orbital sulcus, and none were seen in the most anterior aspects of the OFC.
Conclusions
Our study yielded 3 central findings: first, a dissociation between the “silent” anterior and nonsilent middle/posterior OFC where stimulation clearly elicits changes in subjective experience; second, evidence that the OFC might play a causal role in integrating affect and multimodal sensory experiences; and third, clear evidence for left lateralization of negatively valenced effects. Our findings provide important information for clinicians treating OFC injury or planning OFC resection and scientists seeking to understand the brain basis for the integration of sensation, cognition, and affect.
The orbitofrontal cortex (OFC), consisting of the ventral portions of the prefrontal cortex (figure 1), figures prominently in many higher-order theories of emotion,1–3 decision making,4 reward processing,5 and taste and olfaction.6,7 Damage to the OFC often results from both cerebrovascular accidents8 and traumatic brain injury9 and can have debilitating effects on patients' quality of life. A more detailed understanding is therefore critical for neurologists treating OFC injury, neurosurgeons planning OFC resection (e.g., for intractable epilepsy), and psychiatrists and neuroscientists seeking to understand the OFC's role in higher cognitive-affective abilities.
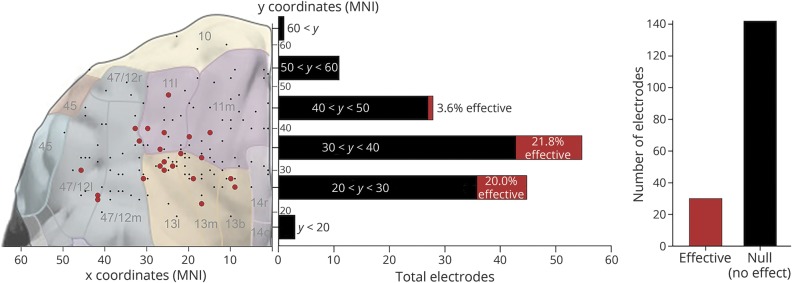
Figure 1. Summary of all effective vs null results.
Summary of effective (red) and null (black) results. The overall elicitation rate was 17.4%: stimulation at n = 30 electrodes yielded some reliable subjective effect, whereas stimulation at 142 electrodes elicited no effects (right panel). The elicitation rate differed significantly along an anterior-posterior axis (results displayed for 143 electrodes, where Montreal Neurological Institute coordinates were available; see Methods): between y-coordinates 20 and 40, the rate was approximately 20% effective, whereas anterior to y = 40, the elicitation rate dropped nearly to zero. Electrodes from both sides of the brain have been projected onto a single hemisphere for clarity in demonstrating the gradient of the elicitation rate; for effects displayed bilaterally, see figures 2 and 3. Approximate Brodmann areas are indicated in gray numerals. Brain with Brodmann areas adapted from figure 2 in reference 5, modified and extended from the work of Öngür and Price.42
Investigations in nonhuman primates have shown that the OFC receives multimodal sensory inputs, including olfactory,10 gustatory,11 and somatosensory information,12 and has widespread connections throughout the brain, including to the amygdala, anterior cingulate, insula, and hypothalamus.5 The OFC therefore appears well placed to represent both affective and multimodal sensory information. This prediction is corroborated by neurophysiologic investigations of the OFC in animals: individual OFC neurons can encode stimulus modality13 and even identity,14 as well as reward value.15 In humans, functional neuroimaging has shown that the OFC is activated by stimuli of every sensory modality,5 abstract reinforcers such as money,16,17 and a wide variety of emotions.18,19 Moreover, humans with OFC lesions show impaired stimulus-reward learning, impaired identification of emotional expression, and exaggerated emotional experience.20,21 Direct cortical stimulation (DCS) in humans with intracranial electrodes can provide a unique contribution to understanding regional function.22 Here, we conduct a comprehensive investigation of the subjective phenomena elicited by DCS of the OFC.
Methods
Patient characteristics
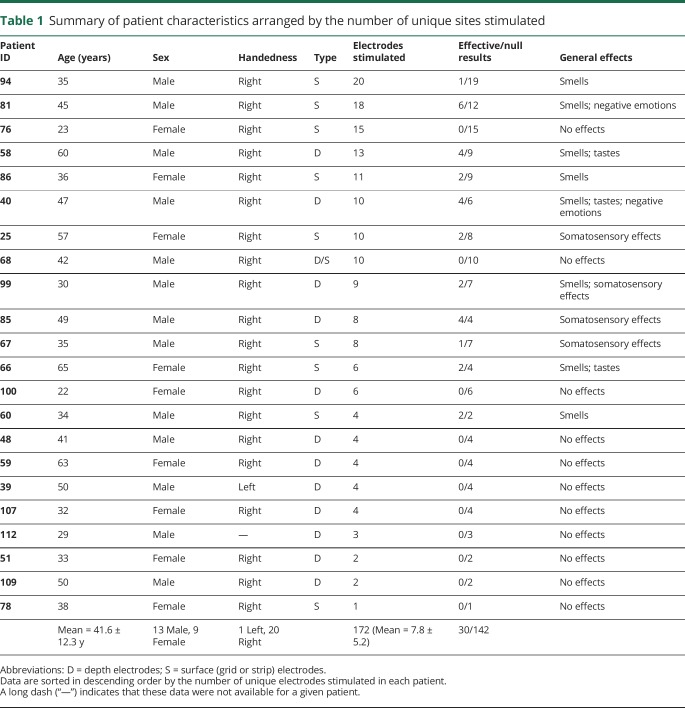
Data were drawn from a pool of 114 patients admitted to the Stanford Hospital for intracranial EEG monitoring of medically refractory epilepsy between 2008 and 2017. We identified patients with electrode coverage in the OFC who also had CT scans and high-resolution T1-weighted MRI scans available for precise reconstruction of electrode locations in standard space. Ultimately, our sample comprised 22 patients tested between 2011 and 2017 (all details are available in table 1).
Table 1.
Summary of patient characteristics arranged by the number of unique sites stimulated
Standard protocol approvals, registrations, and patient consents
All patients provided informed consent in accordance with the Stanford Institutional Review Board for human experiments.
Electrode placement and localization
Patients were implanted with either subdural grid/strip electrode arrays (n = 9), depth electrodes (n = 12), or a mix of both (n = 1), made by Ad-Tech Medical Instruments (Racine, WI). Placement of all electrodes was determined strictly according to clinical criteria. To precisely determine electrode locations for each patient, electrodes localized in a postoperative CT scan were nonlinearly projected to the cortical surface reconstructed from a preoperative T1-weighted MRI scan.23 To pool results for visualization purposes, electrodes in patient-specific space were normalized to Montreal Neurological Institute (MNI) space and displayed on the MNI Colin 27 brain. In total, 18 of 22 patients had all the requisite data and scans to ultimately be displayed (figures 1–3). Data from patients 39, 51, 58, and 85 were not visualized because of missing files that precluded accurate transformation into standard (MNI) space but were included in the summary of subjective effects. Data not visualized in figures 1–3 include 19 null effects, 1 olfactory effect, 2 gustatory effects, 1 smell + taste effect, and 4 somatosensory effects.
Figure 2. Anatomic location and category of subjective phenomena elicited.
Summary of 30 elicited effects and 142 null effects after electrical stimulation of the orbitofrontal cortex (OFC) bilaterally. Electrodes on the ventral surface are indicated by circles; those at some depth are indicated with diamonds (projected onto the ventral surface). The right hand panel shows a histogram of effect types. Note that all stimulation sites located within the OFC have been translated ventrally to be displayed on the ventral orbitofrontal surface. Sites that appear to lie on the temporal pole are actually situated just superior to temporopolar cortex in the OFC. Note that it was not possible to visualize electrode location data from 4 patients; for details of missing data, see Methods.
Figure 3. Evidence for lateralization of effect valence.
Collapsing across the effect type for all subjective experiences (n = 30), valence was mostly negative (n = 11) or neutral (n = 15), with a handful of positive effects (n = 4; right panel). Montreal Neurological Institute (MNI) coordinates were available for electrode sites for 22 of these effects (see Methods for details of missing data). Of these 22 electrodes sites, neutral effects (n = 13) showed evidence of right-hemisphere preference (mean x = 21, light green diamond), whereas negatively valenced effects (n = 9) showed a tendency toward left lateralization (mean x = −9, dark green diamond; left panel). This difference was significant (t[20] = 2.998, p = 0.007), and the size of the effect was large (Cohen d = 1.3). In contrast, the difference in the anterior-posterior position (mean y-coordinates) was not significant (p = 0.21). Coordinates are in standard (MNI) space; the orbitofrontal surface is shown for illustrative purposes only, and electrode locations are not exact (see figure 2 for precise locations). Note that it was not possible to visualize electrode location data from 4 of 22 patients; for details of missing data, see Methods.
Delineating the OFC
We defined the OFC according to standard neuroanatomic landmarks for each patient, identified in the patient's native brain space. Broadly speaking, the OFC includes the entire ventral surface of the frontal lobe; electrodes in grids or strips along this ventral surface were included in the study, as were some depth electrodes with contacts near the ventral surface. Depth electrodes were visualized on coronal brain sections for each patient, and only electrode contacts that fell within the gray matter of the gyri along the ventral surface of the frontal lobe were included in our analyses.
Direct cortical stimulation
Patients underwent DCS as part of a routine clinical mapping procedure to determine localization of function. The specifics of stimulation were at the discretion of the clinician administering the DCS session. Typically, bipolar stimulation was delivered using an alternating square wave current applied across 2 adjacent electrodes at 50 Hz, 2–8 mA current, and pulse width of 200–300 ms. Further details of stimulation methods and parameters are described extensively in our previous work24,25 (data available from Dryad, table e-1, doi.org/10.5061/dryad.3h3k08d). Occasional sham stimulations were also randomly delivered to control for demand characteristics. During sham stimulation, the experimenter behaved exactly as during veridical stimulation, adjusting settings on the stimulator and pressing the same buttons, followed by the same standardized questions about any changes in subjective experience, the only difference being that no current was actually delivered. Because some patients had few OFC electrodes and received correspondingly few administrations of DCS, or never reported subjective effects (table 1), sham stimulations were delivered only in 12 patients, rather than every patient in our sample.
Evaluating and classifying subjective effects of stimulation
We considered a subjective effect of stimulation to the OFC valid only if (1) the anatomic location was confirmed via electrode localization as being within the OFC; (2) the tissue stimulated was not later determined to be pathologic or resected; (3) stimulation at a given site did not result in seizure(s) or after-discharges; (4) repeated stimulation at the same site in the same patient produced the same or a very similar effect (e.g., a more or less intense instance of the same smell); and (5) sham stimulation at a given site did not result in subjective phenomena. Repeated stimulation at sites yielding positive effects was conducted whenever possible, but time and other considerations precluded replication of every observed effect; for details of effects and replications, see the detailed annotated data available from Dryad (table e-1, doi.org/10.5061/dryad.3h3k08d).
After DCS or sham stimulation, patients were asked standardized, open-ended questions about any experiences evoked (e.g., “Did you notice anything?” or “Any change?”), with follow-up questions, as needed, to further clarify the character and emotional valence of effects (e.g., “Is it something you would prefer to approach or avoid?”).
Specific DCS parameters and elicited subjective experiences (or lack thereof) were logged for each stimulation. Raters blinded to the aims of the study (S.L.P., L.E.L., and D.D.M.) subsequently viewed and coded digitized DCS reports and video-EEG recordings to confirm results. After reviewing these accounts, we noticed that the elicited subjective experiences could be classified in part within the following data-driven categories: (1) smells, (2) tastes, (3) somatosensations (e.g., tingling and pain), and (4) emotional experiences. The valence of experiences (positive, negative, or neutral) was also classified, and occasional multimodal experiences we had not anticipated were considered a separate category: (5) combined smell and taste. When active stimulations did not elicit any subjective effects, they were classified as (6) “null” results or “no effect.”
To examine the possibility of functional-anatomic gradients (e.g., a left-right or anterior-posterior axis of effects), the mean x- and y-coordinates in MNI space for electrodes where effects were elicited were computed for both negative and neutral valence (collapsing across effect category) but not for positive valence (which was represented only in 4 effects, and for which MNI coordinates were not available in the patients reporting these effects). Mean x-coordinates for each valence category were used to provide an indication of any hemispheric preference and y-coordinates to determine whether any anterior-posterior gradients were present. As data were approximately normally distributed, parametric independent t tests were conducted to determine whether mean laterality of effects was significantly different using SPSS 20 (IBM Corp, Armonk, NY). Because negative emotions are not necessarily equivalent to negatively valenced sensory effects, these analyses were also conducted with all negative emotion effects excluded to ensure that these were not driving or biasing any lateralization effects.
Visual inspection of our data further suggested that more anterior regions of the OFC almost always yielded null effects, suggesting anterior-posterior gradients in the effective rate of stimulation (regardless of the effect type). To formally examine this possibility, we therefore divided our data into six 10 mm bins (y = 11–20, 21–30, 31–40, 41–50, 51–60, and 61–70) to visualize the elicitation rate throughout the OFC (figure 1). Rates were used to control for the density of electrodes in each bin. Because of our relatively small sample size of electrodes, an analysis of variance comparing all bins would have been severely underpowered. Therefore, to provide a more statistically powerful test, we pooled all data into 2 broader bins based on the midpoint of our data set (y ≤ 40 vs y > 40). Visual inspection of the binarized data (effective or null) revealed that the elicitation rate was not normally distributed; a nonparametric Mann-Whitney U test was therefore conducted to compare the elicitation rate for each bin using SPSS 20.
Controlling for ictal phenomena and other potential confounds
To preclude the confounding effects of any ictal phenomena, we ensured that none of the patients had an epileptic focus within, or required resection of, the OFC. Some patients had electrode grids placed over the ventral surface of the OFC; any smell-related effects elicited by stimulation of electrodes along the midline of the ventral surface were excluded from all analyses as potentially confounded by stimulation of the olfactory nerve.
Data availability
Data on patient demographics and electrode coverage and stimulation are available in table 1. Summary data on elicited subjective effects are available in table 2 and displayed in figures 1–3. For full details of all trials leading to elicitation of subjective effects, including stimulation parameters, exact MNI coordinates, and summaries of first-person reports, see data available from Dryad (table e-1, doi.org/10.5061/dryad.3h3k08d).
Table 2.
Summary of subjective effects elicited

Results
Overall rates and functional-anatomic gradients of effective stimulation
Pooling all 22 patients, a total of 272 electrical stimulations were delivered throughout 172 unique electrodes in the OFC (86 in the right hemisphere and 92 in the left; M ± SD = 7.8 ± 5.2 electrodes per patient). Stimulation of about one-fifth (n = 30; 17.4%) of the electrodes elicited some subjective experience according to patients' first-person reports (figure 1 and table 2). Although only 11 of our 22 patients (50%) reported DCS-elicited effects, most patients who never reported effects had more anterior rather than posterior OFC coverage and relatively few (≤6) electrodes in the OFC stimulated during the clinical mapping procedure (table 1). Of 143 electrodes for which MNI coordinates were available, more posterior (y ≤ 40) OFC sites showed a markedly higher rate of elicitation (M = 20.4%) than more anterior (y > 40) sites (M = 2.5%); the distributions of the 2 bins differed significantly (Mann-Whitney U = 1,691.5, n1 = 103, n2 = 40, p = 0.008, 2-tailed).
Categories of elicited subjective effects
The most commonly elicited effects were olfactory phenomena (n = 13), most of which were either unpleasant (n = 6) or neutral (n = 6), but one report was rated as pleasant (n = 1). A variety of somatosensory (n = 8) and taste (n = 3) effects were also elicited in several participants, and multimodal experiences of smell + taste effects (n = 3) were also reported. Negative emotions (anger and sadness/despair) were also occasionally elicited (n = 2). In one additional case, a mix of negative emotions (both anger and sadness) was associated with recall of an old memory event (see patient 40, data available from Dryad, table e-1, doi.org/10.5061/dryad.3h3k08d).
To control for any demand characteristics, we randomly administered a total of 43 sham stimulations in 12 patients, followed by the same standardized questions about any changes in subjective experience. Of these, just 3 yielded false-positive reports (all from patient 66), whereas 40 (93%) had no effect.
Lateralization of effect valence
Collapsing across all effect types, we found that negatively valenced effects tended to be left lateralized (mean x = −9), whereas neutral effects tended to be right lateralized (mean x = 21) (figure 3). This difference was significant (t[20] = 2.998, p = 0.007), and the size of the effect was large (Cohen d = 1.30). In contrast, the difference in the mean anterior-posterior position between negative (mean y = 35) and neutral (mean y = 31) effects was not significant (p = 0.21). As noted in the Methods section, because negative emotions might not necessarily be equivalent to negatively valenced smells and tastes, we also conducted this analysis with all negative emotion effects excluded. With these 3 emotion effects removed, our observed effect remained consistent (and in fact was more prominent): negatively valenced effects were left lateralized (mean x = −19), neutral effects were right lateralized (mean x = 21), and the difference remained significant (t[17] = 4.108, p < 0.001); again, the mean anterior-posterior position (y-coordinates) did not differ significantly (p = 0.76).
Discussion
Our study yields 3 important findings. First, we provide evidence for a dissociation between “silent” anterior OFC areas that do not respond to DCS and middle/posterior OFC areas that often yield subjective experiences (figure 1)—a pattern of OFC functional specialization that mirrors recent findings of “silent” and “nonsilent” areas in the human posteromedial cortex.25 Second, we provide evidence that the OFC could play a causal role in integrating affect and multimodal sensory experiences in humans (figure 2)—replicating and expanding on electrophysiologic work in rodents14 and nonhuman primates,13,15 and corroborating correlational findings from human neuroimaging and inferences from lesion patients.2 Third, we show that negatively valenced effects are strongly left lateralized (figure 3), providing unique DCS data from humans that contributes to the fraught debate over lateralization of valence effects in the prefrontal cortex26; tellingly, our results corroborate anatomically specific findings from functional neuroimaging27 but contradict findings from lower-resolution scalp EEG.26
The majority of effects were elicited at more posterior (y ≤ 40) sites within the range of our OFC electrodes (figure 1). In contrast, stimulation of more anterior electrodes (y > 40) yielded only a single effect (patient 81, negative emotion effect at y = 48). These results show evidence for a possible anatomic boundary differentiating “silent” vs “nonsilent” parts of the OFC, although this finding must be interpreted cautiously, given the relatively small number of anterior OFC electrodes in our cohort. Of note, our findings are broadly consistent with patterns of anatomic connectivity: more rostral prefrontal regions (e.g., Brodmann area 10) receive few if any direct inputs from unimodal or multimodal sensory areas, but are instead connected predominantly, and perhaps exclusively, to other “transmodal” association areas in both prefrontal cortex and anterior temporal cortex that primarily encode and process more abstract information.28,29 The direct anatomic connections between more posterior aspects of the OFC and various limbic and sensory areas5 could explain why stimulation here yields subjective effects far more frequently. The differential elicitation rate we observed in the OFC parallels results recently reported in the posteromedial cortex.25 These intriguing patterns of “silent” and “nonsilent” cortex warrant careful exploration in further investigations to extend these findings to other parts of the brain, particularly transmodal cortical areas of the default25,30 and executive networks that are distant from sensory inputs.31 Our interpretation of an anterior-posterior gradient in the elicitation rate is based on the assumption that stimulation at further electrode sites in the most posterior OFC areas (y < 20) is likely to yield subjective effects. However, subjective effects may be induced primarily by stimulation of a cluster of OFC tissue in the central region near y ≅ 30 (i.e., the approximate location of the transverse orbital sulcus, which bisects the OFC). This would align well with data from neuroimaging, which suggest that neural recruitment related to olfaction is considerably more anterior in the OFC of humans than was expected based on electrophysiologic and anatomic tracing data from animals.7 Indeed, a meta-analysis of 13 neuroimaging investigations found that the peak of activation across studies was centered on the transverse orbital sulcus and roughly equidistant from the medial and lateral boundaries of the frontal lobe—closely in line with our own findings (cf. figure 6 in reference 7).
In the subjective domain, we observed a rich variety of olfactory, gustatory, and somatosensory effects, as well as multimodal (smell + taste) experiences. The finding that smell and taste experiences could occur both in isolation and also conjointly is consistent with neurophysiologic evidence that both unimodal and bimodal neurons are present in the OFC13—although, given the spread of electrical charge to neighboring neuronal populations,24 these bimodal results are much more parsimoniously explained by simultaneous stimulation of multiple unimodal units. Spread of electrical charge could also potentially have activated other nearby sensory and limbic areas beyond the OFC, yielding some of the elicited subjective effects—a limitation that should be kept in mind when interpreting our results. Further research examining the relationship between delivered current, spread of electrical charge, and corresponding changes in subjective experience—as examined recently, for instance, in the visual cortex24—could mitigate these concerns in the future.
Several instances of negative emotion were elicited in an idiosyncratic fashion across patients, including high-intensity emotions such as anger (patient 40), as well as less-arousing emotions described as “despair” (accompanied by a negatively valenced smell; patient 81) and “sadness” (patient 40). Of note, 1 instance of intense, mixed anger and sadness (patient 40) was accompanied by long-term memory recall of a traumatic car accident the patient had experienced some 15–20 years prior, and the patient explicitly described experiencing the same emotional state at the time of the accident (data available from Dryad, table e-1, doi.org/10.5061/dryad.3h3k08d). Although anecdotal, this effect is suggestive of mood-dependent memory evocation.32 Furthermore, although not identified explicitly as emotions, several instances of disgust at the smells elicited were evident from the reports of patient 81 (data available from Dryad, table e-1, doi.org/10.5061/dryad.3h3k08d). The variety of negative emotions elicited supports the notion that discrete emotions are not localized to specific brain regions, but rather that the OFC processes general affective significance, in particular goal relevance3 and valence.18 Our findings are consistent with the OFC's putative role in emotion processing and its anatomic connections with various limbic areas, especially the amygdala.
Research has long suggested that emotional processing in the frontal lobe is lateralized,26 and we found that negatively valenced effects tended to be left lateralized, whereas neutral effects were right lateralized. Moreover, this trend remained (indeed, was strengthened) when only negatively valenced sensory effects were included (and negative emotion effects excluded). Our DCS findings parallel the results of a meta-analysis of 65 neuroimaging investigations, which found that activations for negatively valenced emotions were left lateralized in the OFC and vice versa for positive affect.27 Although our directly elicited and anatomically precise findings contribute unique data to the ongoing discussion regarding lateralization of affective valence in the frontal lobe,26 the effects we observed here tended to be quite simple and cannot speak to the debate on lateralization of higher-order processes in the OFC. Moreover, our valence lateralization effect should be interpreted with caution, given our relatively small sample size and the paucity of positively valenced effects (which precludes examination of the double dissociations reported by others27).
Laterality effects aside, there is an apparent overall negativity bias to experiences elicited by OFC stimulation. Only 4 positively valenced experiences were elicited (figure 3), and moreover, some “neutral” experiences, although not explicitly identified by patients as negative or unpleasant, were by no means pleasant or rewarding: reports of “saline” tastes, “damp” and “smoky” smells, and “tingling” somatosensations (data available from Dryad, table e-1, doi.org/10.5061/dryad.3h3k08d) could reasonably be categorized as negative using more liberal criteria. This apparent negativity bias needs to be qualified, however, by the finding that elicitation of positively valenced experiences of any kind is extremely rare with DCS (for some exceptions, references 33–36), whereas elicitation of pain, negative emotions, and aversive sensory phenomena is common.37 A facile interpretation of these findings might be that the human brain is predisposed toward interpreting the effect of delivered electrical pulses as negative rather than positive. This suggests that the negativity bias observed here (figure 3), and extensively in previous research,37 could be an artifact of specific stimulation parameters or the manner in which artificial stimulation propagates throughout the brain—neither of which is well understood.24,38
Several other studies have previously reported findings of subjective effects elicited by DCS to the OFC.39–41 Mahl et al.39 reported memory recall, hallucinations, illusions, motor expression, and even changes in personality after stimulation of the OFC in a single patient, but few of these effects were replicated in our larger cohort. Begum et al.41 reported tingling sensations and muscle twitching in the lower face after OFC stimulation in a single patient. Although somatosensory experiences are consonant with our own findings, we observed no motor effects in any of our patients, and the authors themselves suggested that motor twitching was likely elicited via spreading of current to the facial nerve. Most recently, Mulak et al.40 reported that 3 stimulations to the ventromedial aspects of the OFC resulted in epigastric and retrosternal sensations (the number of unique patients in whom these effects were elicited is not clear but is ≤3). However, this study reported exclusively on “digestive sensations” elicited from a large cohort (N = 339) over 15 years; because other effects of stimulation to the OFC in this cohort were not reported, assessing the importance of these findings—or of the fact that we found no comparable results in our sample—is problematic. Overall then, while findings from our 22 patients partially corroborate previous reports, we also expand greatly on them, clarifying where stimulation tends to elicit effects (figure 1), the relative frequency of different effect types (figure 2), and general tendencies in effect valence (figure 3).
Although our study contributes a large pool of patients to the sparse literature on OFC stimulation, we advise caution in the interpretation of our findings. First, as already noted, DCS can result in current spread to nearby parts of the brain (in millimeter space), as well as interregional signal propagation to areas that are anatomically connected with the OFC.22 Therefore, the subjective effects produced by DCS most likely are due to engagement of a distributed network rather than a small area of the OFC alone. Second, DCS likely perturbs activity in hundreds of thousands of neurons, so detailed understanding of effects at the local circuit or single-cell level is not possible. Third, the mechanisms of DCS—i.e., whether it potentiates, inhibits, or otherwise perturbs ongoing neuronal activity, or some combination thereof—remain poorly understood.22 Finally, our investigation mostly explored the affective and sensory experiences elicited by stimulation of the OFC and did not investigate the effects of stimulation on either enhancing or disrupting higher-order cognitive-affective processes or task performance. We hope that future studies can investigate the causal role of precisely located OFC neuronal populations in higher-order cognitive-affective abilities, such as decision making and reward processing, using targeted electrical stimulation of these populations during experimental tasks known to recruit the OFC.
Acknowledgment
The authors are grateful to the many patients who participated in the study, without whom this research would not have been possible. They also thank Amy L. Daitch and Ren Na for assistance in preparing figures and transforming coordinate data from subject-specific to standard MNI space.
Glossary
- DCS
direct cortical stimulation
- OFC
orbitofrontal cortex
- MNI
Montreal Neurological Institute
Footnotes
CME Course: NPub.org/cmelist
Author contributions
K.C.R. Fox: study concept and design, acquisition of data, analysis and interpretation of data, writing of the manuscript, and critical revision of the manuscript for intellectual content. J. Yih: study concept and design, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for intellectual content. O. Raccah: analysis and interpretation of data and critical revision of the manuscript for intellectual content. S.L. Pendekanti: acquisition of data and analysis and interpretation of data. L.E. Limbach and D.D. Maydan: analysis and interpretation of data. J. Parvizi: study concept and design, acquisition of data, analysis and interpretation of data, writing of the manuscript, and critical revision of the manuscript for intellectual content.
Study funding
Study funded by the US National Science Foundation (BCS1358907) to J. Parvizi. K.C.R. Fox is supported by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council (NSERC) of Canada. S.L. Pendekanti was supported by the Stanford Institutes of Medicine Summer Research (SIMR) program. J. Parvizi acknowledges support from the US National Science Foundation (BCS1358907).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology February 8, 2018. Accepted in final form July 13, 2018.
References
- 1.Damasio AR. Descartes' Error: Emotion, Reason and the Human Brain. New York: Putnam; 1994. [Google Scholar]
- 2.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004;72:341–372. [DOI] [PubMed] [Google Scholar]
- 3.Dixon ML, Thiruchselvam R, Todd RM, Christoff K. Emotion and the prefrontal cortex: an integrative review. Psychol Bull 2017;143:1033–1081. [DOI] [PubMed] [Google Scholar]
- 4.Rushworth M, Behrens T, Rudebeck P, Walton M. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci 2007;11:168–176. [DOI] [PubMed] [Google Scholar]
- 5.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005;6:691–702. [DOI] [PubMed] [Google Scholar]
- 6.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn 2004;55:11–29. [DOI] [PubMed] [Google Scholar]
- 7.Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Res Rev 2005;50:287–304. [DOI] [PubMed] [Google Scholar]
- 8.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000;123:2189–2202. [DOI] [PubMed] [Google Scholar]
- 9.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol 1988;150:663–672. [DOI] [PubMed] [Google Scholar]
- 10.Morecraft R, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol 1992;323:341–358. [DOI] [PubMed] [Google Scholar]
- 11.Baylis L, Rolls E, Baylis G. Afferent connections of the caudolateral orbitofrontal cortex taste area of the primate. Neuroscience 1995;64:801–812. [DOI] [PubMed] [Google Scholar]
- 12.Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol 1988;276:313–342. [DOI] [PubMed] [Google Scholar]
- 13.Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci 1994;14:5437–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol 1995;74:733–750. [DOI] [PubMed] [Google Scholar]
- 15.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex 2000;10:272–283. [DOI] [PubMed] [Google Scholar]
- 16.Thut G, Schultz W, Roelcke U, et al. Activation of the human brain by monetary reward. Neuroreport 1997;8:1225–1228. [DOI] [PubMed] [Google Scholar]
- 17.O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 2001;4:95–102. [DOI] [PubMed] [Google Scholar]
- 18.Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb Cortex 2016;26:1910–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci 2012;35:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornak J, Bramham J, Rolls ET, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 2003;126:1691–1712. [DOI] [PubMed] [Google Scholar]
- 21.Hornak J, O'doherty J, Bramham J, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci 2004;16:463–478. [DOI] [PubMed] [Google Scholar]
- 22.Borchers S, Himmelbach M, Logothetis N, Karnath HO. Direct electrical stimulation of human cortex–the gold standard for mapping brain functions? Nat Rev Neurosci 2012;13:63. [DOI] [PubMed] [Google Scholar]
- 23.Groppe DM, Bickel S, Dykstra AR, et al. iELVis: an open source MATLAB toolbox for localizing and visualizing human intracranial electrode data. J Neurosci Methods 2017;281:40–48. [DOI] [PubMed] [Google Scholar]
- 24.Winawer J, Parvizi J. Linking electrical stimulation of human primary visual cortex, size of affected cortical area, neuronal responses, and subjective experience. Neuron 2016;92:1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster BL, Parvizi J. Direct cortical stimulation of human posteromedial cortex. Neurology 2017;88:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller GA, Crocker LD, Spielberg JM, Infantolino ZP, Heller W. Issues in localization of brain function: the case of lateralized frontal cortex in cognition, emotion, and psychopathology. Front Integr Neurosci 2013;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage 2003;19:513–531. [DOI] [PubMed] [Google Scholar]
- 28.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci 2004;5:184–194. [DOI] [PubMed] [Google Scholar]
- 29.Barbas H, Pandya D. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 1989;286:353–375. [DOI] [PubMed] [Google Scholar]
- 30.Fox KC, Foster BL, Kucyi A, Daitch AL, Parvizi J. Intracranial electrophysiology of the human default network. Trends Cogn Sci 2018;22:307–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margulies DS, Ghosh SS, Goulas A, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci 2016;113:12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis PA, Critchley HD. Mood-dependent memory. Trends Cogn Sci 2003;7:431–433. [DOI] [PubMed] [Google Scholar]
- 33.Meletti S, Tassi L, Mai R, Fini N, Tassinari CA, Russo GL. Emotions induced by intracerebral electrical stimulation of the temporal lobe. Epilepsia 2006;47:47–51. [DOI] [PubMed] [Google Scholar]
- 34.Benedetti F, Colloca L, Lanotte M, Bergamasco B, Torre E, Lopiano L. Autonomic and emotional responses to open and hidden stimulations of the human subthalamic region. Brain Res Bull 2004;63:203–211. [DOI] [PubMed] [Google Scholar]
- 35.Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius MD. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron 2013;80:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005;45:651–660. [DOI] [PubMed] [Google Scholar]
- 37.Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Front Hum Neurosci 2010;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parvizi J, Kastner S. Promises and limitations of human intracranial electroencephalography. Nat Neurosci 2018;21:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahl GF, Rothenberg A, Delgado JM, Hamlin H. Psychological responses in the human to intracerebral electrical stimulation. Psychosom Med 1964;26:337–368. [DOI] [PubMed] [Google Scholar]
- 40.Mulak A, Kahane P, Hoffmann D, Minotti L, Bonaz B. Brain mapping of digestive sensations elicited by cortical electrical stimulations. Neurogastroenterol Motil 2008;20:588–596. [DOI] [PubMed] [Google Scholar]
- 41.Begum T, Ikeda A, Matsuhashi M, et al. Ipsilateral facial sensory and motor responses to basal fronto-temporal cortical stimulation: evidence suggesting direct activation of cranial nerves. Epilepsy Res 2006;71:216–222. [DOI] [PubMed] [Google Scholar]
- 42.Öngür D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 2000;10:206–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data on patient demographics and electrode coverage and stimulation are available in table 1. Summary data on elicited subjective effects are available in table 2 and displayed in figures 1–3. For full details of all trials leading to elicitation of subjective effects, including stimulation parameters, exact MNI coordinates, and summaries of first-person reports, see data available from Dryad (table e-1, doi.org/10.5061/dryad.3h3k08d).
Table 2.
Summary of subjective effects elicited