Abstract
Patients with Churg-Strauss syndrome often suffer from unusual cardiac manifestations and sudden cardiac death. This differs from patients with other autoimmune disorders, who typically present with premature ischaemic heart disease. We report the case of a 56-year-old man with recurrent coronary vasospasm, including an inferoposterior ST-elevation myocardial infarction, complicated by bradycardic arrest. There was only minor coronary artery disease on coronary angiography. An elevated eosinophil count was noted. His medical history included allergic rhinitis with polyposis, adult-onset asthma and biopsy-proven eosinophilic oesophagitis. Review of his sinus biopsies demonstrated blood vessels with marked accumulation of eosinophils in extravascular areas. The patient, therefore, met the American College of Rheumatology criteria for Churg-Strauss syndrome. The patient was commenced on immunosuppression, with the return of the eosinophil count to within normal limits, and remains free of cardiovascular events over 24 months.
Keywords: immunology, cardiovascular medicine
Background
Cardiovascular manifestations of Churg-Strauss syndrome (CSS) include myocarditis, heart failure, pericarditis, valvular insufficiency, pericardial effusions and arrhythmias. These complications are a common cause of morbidity and mortality in patients with CSS. However, an association between eosinophilia and coronary artery vasospasm is not widely recognised. This is a rare but an important illustration of a vasospastic acute coronary syndrome (ACS) in a male patient, which subsequently led to the diagnosis of CSS. The spasm is thought to be mediated by vasoactive compounds produced by eosinophils. In addition, this case illustrates that achieving remission with immunosuppression can prevent recurrent vasospastic events. This case highlights the need to consider coronary vasospasm as a cause of cardiac morbidity or unexplained mortality in patients with CSS.
Case presentation
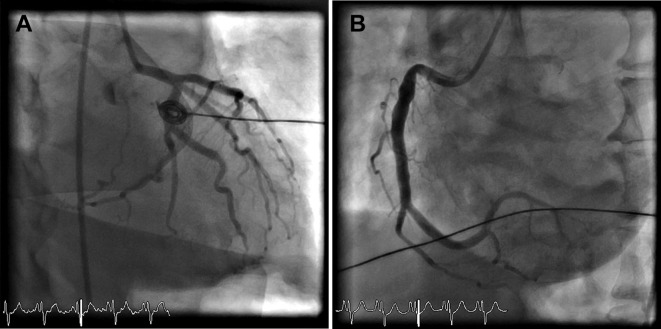
A 56-year-old male ex-smoker presented with an inferoposterior ST-elevation myocardial infarction, after two similar episodes of chest pain in the previous 12 months. His history included dyslipidaemia, adult-onset asthma, allergic rhinitis with polyposis and eosinophilic oesophagitis. While awaiting primary percutaneous coronary intervention, the patient suffered a bradycardic arrest, necessitating cardiopulmonary resuscitation, thrombolysis and electrical cardioversion for ventricular fibrillation, resulting in the return of spontaneous circulation. ST elevation normalised and coronary angiography demonstrated only minor coronary artery disease (figure 1). Findings were therefore in keeping with coronary artery vasospasm, and a calcium channel blocker and nitrate were commenced. An automated implantable cardioverter defibrillator was inserted. The toxicology screen was deemed clinically unnecessary.
Figure 1.
Coronary angiogram of the patient showing minor coronary artery disease in (A) the left coronary system and (B) the right coronary artery.
Investigations
A significant peripheral eosinophilia (4.0×109/L) was noted, and further investigations demonstrated an elevated immunoglobulin E and negative antineutrophil cytoplasmic antibodies. Respiratory function tests confirmed a moderately severe obstructive defect. Review of earlier imaging revealed extensive opacification of the sinuses and nasal polyposis; repeat imaging confirmed chronic sinus mucosal thickening (figure 2), with diffuse oesophageal thickening (figure 3), consistent with his known history of eosinophilic oesophagitis. Review of his sinus biopsies demonstrated vessels with lymphocytic infiltrates and marked accumulation of eosinophils in extravascular areas (figure 4).
Figure 2.
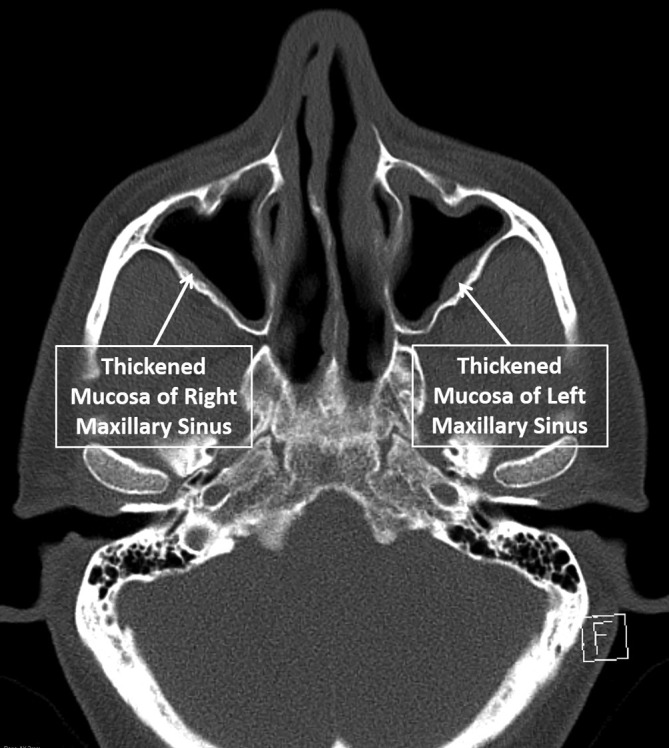
CT scan showing changes of chronic sinusitis.
Figure 3.
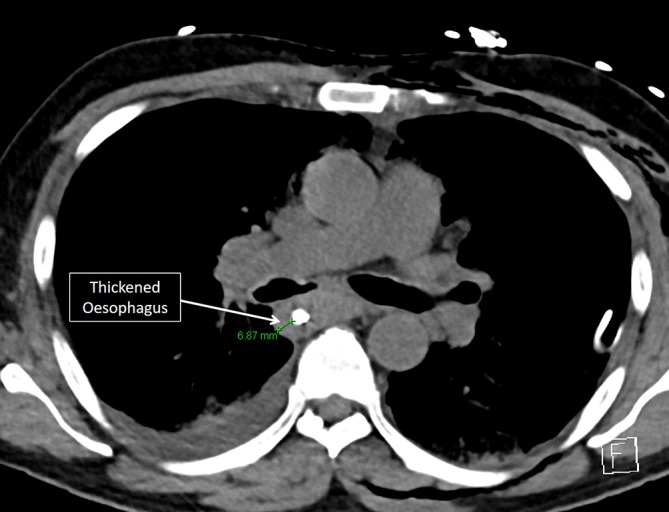
CT scan showing thickened oesophagus.
Figure 4.
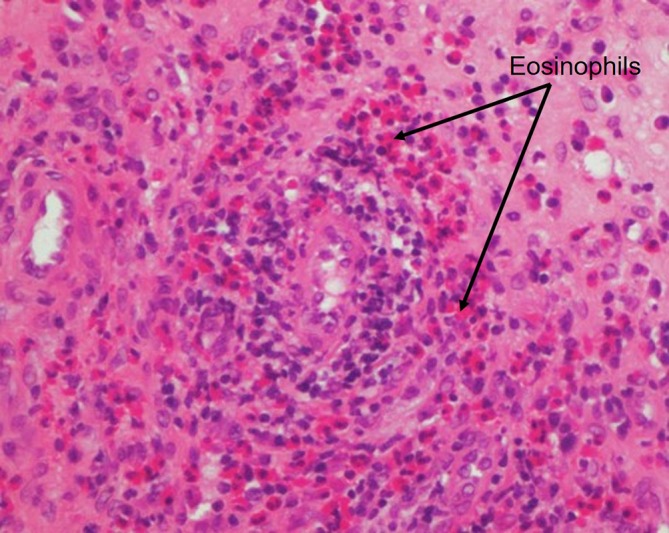
Sinus biopsy demonstrating vessels with lymphocytic infiltrates and marked accumulation of eosinophils in extravascular areas (cells with intensely pink cytoplasm, arrow).
Differential diagnosis
A diagnosis of CSS was made based on the patient meeting four out of six American College of Rheumatology criteria. Other hypereosinophilic disorders were excluded or thought unlikely. The FIP1L1-PDGFRA fusion was not detected.
Treatment
The patient was commenced on immunosuppression with prednisolone and azathioprine, with almost complete control of asthma, rhinitis and oesophageal symptoms, associated with normalisation of the eosinophil count.
Outcome and follow-up
The patient remains on maintenance immunosuppression with low-dose prednisolone and azathioprine, without any major cardiovascular events over the past 24 months.
Discussion
CSS, now known as eosinophilic granulomatosis with polyangiitis, is a multisystem disorder involving allergic rhinosinusitis, asthma and eosinophilia.1 It is a vasculitis of the small-sized and medium-sized arteries, although vasculitis often manifests late in the disease. Pulmonary involvement is common, however, any organ system can be affected, including the cardiovascular, gastrointestinal, renal and central nervous systems.
Cardiac abnormalities are the most serious manifestation of CSS, accounting for 50% of mortality relating to this disorder.2 Clinical manifestations include signs of heart failure or pericarditis and cardiac rhythm abnormalities.2–5
Reports of coronary artery vasospasm associated with CSS are rare.6–8 Vasospastic angina is caused by sudden occlusive vasoconstriction of an epicardial coronary artery, causing transmural myocardial ischemia. This may be accompanied by ST-segment elevation, with or without ventricular arrhythmias causing cardiac arrest, which was present in this case. Vasospasm can develop in normal or diseased vessels and can usually be reversed with nitrates or calcium-channel blockers.9
Coronary artery vasospasm in patients with CSS may be due to eosinophilic infiltration of the coronary artery wall.10 It is suggested that eosinophilic basic proteins or vasoactive cytokines directly stimulate constriction of vascular smooth muscle.10 Previous studies have demonstrated eosinophilic infiltration around vasa vasorum and nerve fibres, and stimulation of adventitial nerve fibres may also cause coronary vasospasm.11 Eosinophils also damage tissues, predisposing to dissection,12 aneurysmal dilatation7 and thrombosis,13 as well as subsequent fibrosis of the intima and media of the coronary artery wall.11 The association between asthma and vasospastic angina may be due to eosinophils provoking smooth muscle constriction in both the coronary arteries and bronchi.14
In contrast to patients with atherosclerotic coronary artery disease-related vasospasm, which responds well to vasodilators and portends a good prognosis,15 patients with coronary artery vasospasm and eosinophilia are at a high risk of recurrent coronary events, including death, despite treatment with calcium channel antagonists and nitrates.10 Most patients improve with corticosteroids, which have both anti-inflammatory and immunosuppressive effects. High oral doses are initially commenced (eg, 40–80 mg) to achieve remission quickly.6 On clinical improvement, treatment is gradually tapered down to a maintenance dose, although recurrent events can occur during this weaning process. Many patients require long-term therapy. Treatment with a steroid-sparing immunosuppressant drug, such as cyclophosphamide, azathioprine, mycophenolate or ciclosporin, can allow steroid doses to be reduced, thereby minimising side effects.16 In our case, the patient has remained free of any major cardiovascular events for over 24 months on low-dose prednisolone (5 mg daily) and azathioprine.
CSS is known to ‘evolve’, typically starting with IgE-spectrum disorders, such as allergic rhinitis and atopic asthma, both of which were present in our patient. The next phase of the disease is marked by peripheral and tissue eosinophilia, with the cardiac disease being the most frequent complication of sustained eosinophilia.6 The third and final phase of CSS is end organ vasculitis, which can affect the heart, skin, lungs, kidney and nervous system. As described above, vasculitic lesions in the heart can result in a variety of clinical manifestations, including ACS, as was the case in our patient. In patients presenting with vasospastic ACS and eosinophilia, CSS should be considered in the differential diagnosis. Conversely, although coronary vasospasm may be an unusual clinical manifestation of CSS, it should be suspected in CSS patients presenting with ACS and may be a pathological mechanism underlying sudden cardiac death in these patients.
Learning points.
Churg-Strauss syndrome (CSS) is associated with a number of unusual cardiac manifestations, including coronary vasospasm.
These manifestations may be mediated by vasoactive compounds produced by eosinophils, which often infiltrate the heart.
In patients presenting with cardiac pathology and eosinophilia, CSS should be considered in the differential diagnosis.
Treatment with immunosuppression and resolution of eosinophilia may prevent further cardiovascular events.
Footnotes
Contributors: SJT, DT and DS: involved in the inpatient and outpatient management of the patient. SJT, DT and DS: planned and conceptualised the design of the case report. SJT: wrote the first draft of the manuscript. DT and DS:reviewed and edited the manuscript. All authors have approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This article has been corrected since it published Online. The authors noticed a spacing error in the article title.
References
- 1.Keogh KA, Specks U. Churg-Strauss syndrome. Semin Respir Crit Care Med 2006;27:148–57. 10.1055/s-2006-939518 [DOI] [PubMed] [Google Scholar]
- 2.Neumann T, Manger B, Schmid M, et al. Cardiac involvement in Churg-Strauss syndrome: impact of endomyocarditis. Medicine 2009;88:236–43. 10.1097/MD.0b013e3181af35a5 [DOI] [PubMed] [Google Scholar]
- 3.Dennert RM, van Paassen P, Schalla S, et al. Cardiac involvement in Churg-Strauss syndrome. Arthritis Rheum 2010;62:627–34. 10.1002/art.27263 [DOI] [PubMed] [Google Scholar]
- 4.Moosig F, Bremer JP, Hellmich B, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis 2013;72:1011–7. 10.1136/annrheumdis-2012-201531 [DOI] [PubMed] [Google Scholar]
- 5.Mehta AK, Langford CA, Taylor DO, et al. A 39-year-old postpartum woman with foot drop and shortness of breath. Chest 2016;149:e61–e64. 10.1016/j.chest.2015.10.051 [DOI] [PubMed] [Google Scholar]
- 6.Petrakopoulou P, Franz WM, Boekstegers P, et al. Vasospastic angina pectoris associated with Churg-Strauss syndrome. Nat Clin Pract Cardiovasc Med 2005;2:484–9. quiz 90 10.1038/ncpcardio0299 [DOI] [PubMed] [Google Scholar]
- 7.Drogue M, Vergnon JM, Wintzer B, et al. Prinzmetal’s angina pectoris revealing aneurysm of the right coronary artery during evolution of Churg-Strauss syndrome. Chest 1993;103:978 10.1378/chest.103.3.978a [DOI] [PubMed] [Google Scholar]
- 8.Wagner AD, Meyer GP, Rihl M, et al. Acute coronary syndrome associated with Churg-Strauss syndrome. Vasc Health Risk Manag 2007;3:775–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Okumura K, Yasue H, Matsuyama K, et al. Diffuse disorder of coronary artery vasomotility in patients with coronary spastic angina. Hyperreactivity to the constrictor effects of acetylcholine and the dilator effects of nitroglycerin. J Am Coll Cardiol 1996;27:45–52. 10.1016/0735-1097(95)00432-7 [DOI] [PubMed] [Google Scholar]
- 10.Wong CW, Luis S, Zeng I, et al. Eosinophilia and coronary artery vasospasm. Heart Lung Circ 2008;17:488–96. 10.1016/j.hlc.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 11.Kajihara H, Kato Y, Takanashi A, et al. Periarteritis of coronary arteries with severe eosinophilic infiltration. A new pathologic entity (eosinophilic periarteritis)? Pathol Res Pract 1988;184:46–52. 10.1016/S0344-0338(88)80190-0 [DOI] [PubMed] [Google Scholar]
- 12.Zagelidou H, Leodari R, Roupa Z, et al. Death from spontaneous coronary artery dissection in a healthy postmenopausal woman. Am J Forensic Med Pathol 2004;25:176–7. 10.1097/01.paf.0000127396.59458.03 [DOI] [PubMed] [Google Scholar]
- 13.Kubota T, Yamaguchi J, Higashitani M, et al. [Survivor of cardiogenic shock following acute myocardial infarction with Churg-Strauss syndrome: first angiographic documention of coronary recanalization of infarct-related arteries: a case report]. J Cardiol 2004;44:153–9. [PubMed] [Google Scholar]
- 14.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–9. 10.1126/science.1100283 [DOI] [PubMed] [Google Scholar]
- 15.Bory M, Pierron F, Panagides D, et al. Coronary artery spasm in patients with normal or near normal coronary arteries. Long-term follow-up of 277 patients. Eur Heart J 1996;17:1015–21. 10.1093/oxfordjournals.eurheartj.a014996 [DOI] [PubMed] [Google Scholar]
- 16.Hellmich B, Gross WL. Recent progress in the pharmacotherapy of Churg-Strauss syndrome. Expert Opin Pharmacother 2004;5:25–35. 10.1517/14656566.5.1.25 [DOI] [PubMed] [Google Scholar]