Description
An 11-year-old girl presented with global developmental delay. As part of her routine work-up for her developmental disability, MRI of the brain was performed and demonstrated a contrast-enhancing lesion with reduced diffusivity in the right parietal lobe (figure 1). A stereotactic robotic-assisted needle biopsy revealed microglial proliferation and lymphocytic inflammation, indicating potential microglioma or gliofibroma; however, a more definitive diagnosis was not established given the limited tissue. Early repeat MRI showed areas of progression, and gross total resection was performed. The pathology demonstrated a moderately cellular proliferation of spindle cells with scattered mitotic figures, and a mixed inflammatory infiltrate of scattered lymphocytes, plasma cells and eosinophils (figure 2A). The areas of nodular spindle cell proliferation were diffusely immunopositive for CD68 and negative for CD30, CD34, smooth muscle actin, desmin, S100, CD1a, CD117, CD3, CD20, CD15, synaptophysin, glial fibrillary acidic protein (GFAP) and leucocyte common antigen (LCA). Activin receptor-like kinase 1 (Alk-1) immunostaining demonstrated diffuse positivity of the spindle cell component best supporting a histopathological classification in the category of inflammatory myofibroblastic tumour (IMT; figure 2B). The spindle cell morphology and architectural pattern raised diagnostic consideration of a tumour arising from microglial cells; however, the Alk-1 immunopositivity would not be expected for this entity. The presence of admixed GFAP-positive islands in the non-spindle cell regions raised differential consideration of a gliofibroma; however, a comprehensive next-generation oncology sequencing panel revealed a novel KIF5b-ALK fusion (K24;A20) most consistent with a diagnosis of atypical central nervous system (CNS) IMT. Our findings are supported by those of Coffin et al, who reported that positive immunohistochemical staining for ALK is relatively specific for IMT in the context of fibroblastic–myofibroblastic tumours.1
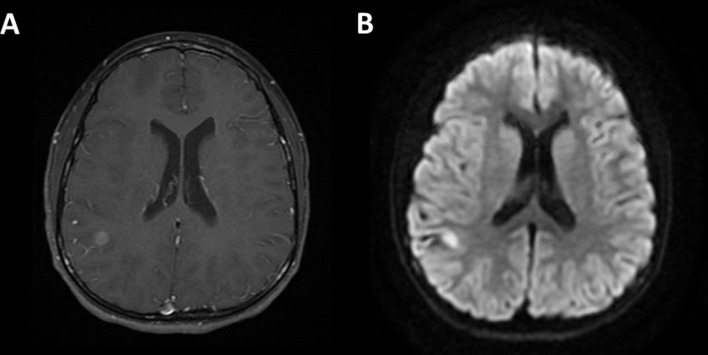
Figure 1.
MRI brain reveals a contrast-enhancing lesion in the right parietal lobe (A) that demonstrated reduced diffusivity on diffusion-weighted sequences (B).
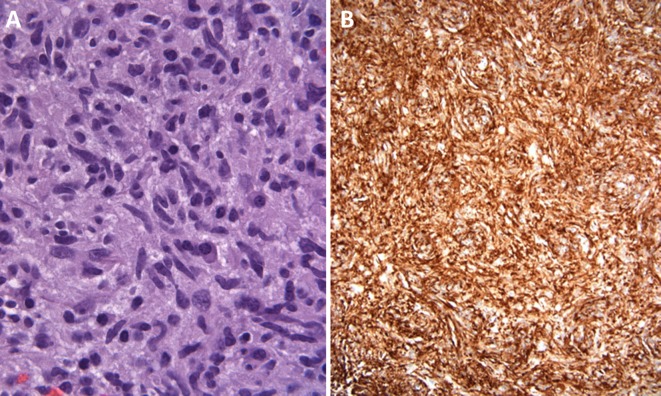
Figure 2.
(A) Neuropathology of the central portion of the tumour (1000×) reveals a moderately cellular proliferation of spindle cells with elongate nuclei tapered at the ends, with scattered mitotic figures and a background infiltrate of scattered lymphocytes and plasma cells. (B) Immunohistochemistry of the spindle cells reveals a strong and diffuse positivity for ALK1.
IMTs occur more often in children and are typically extracranial. Approximately 50% of IMT cases possess ALK fusion events or overexpression.1 CNS IMT expressing ALK are reported to have a significant recurrence rate, even after gross total resection.2 Although various ALK fusion proteins have been previously reported in IMT, the KIF5B-ALK protein has only been described as a rare fusion in lung adenocarcinomas. The presence of this ALK fusion may suggest that recurrences could be treated with ALK inhibitors such as crizotinib and ceritinib. However, there is evidence that the N-terminus domain of KIF5B fusion proteins can activate receptor tyrosine kinases (RTKs) independently of the C-terminus kinase domain in KIF5B-RET fusions, indicating a potential need to combine epidermal growth factor receptor inhibitors with ALK inhibitors in KIF5B-ALK fusions.3 Overall, our case highlights a novel ALK fusion with KIF5B in a rare inflammatory atypical myofibroblastic tumour of the CNS.
Learning points.
Inflammatory myofibroblastic tumour (IMT) should be considered in the diagnosis of contrast-enhancing regions with spindle cell morphology and positive immunostaining for ALK.
ALK in IMT may rearrange with several genes, including KIF5B, mirroring the heterogeneity of ALK fusion proteins observed in lung adenocarcinomas.
ALK rearrangements in IMTs are at high risk of recurrence, even if gross total resection is achieved, and close follow-up is required.
Footnotes
Contributors: All authors contributed for the conception and design, acquisition of data and writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509–20. 10.1097/01.pas.0000213393.57322.c7 [DOI] [PubMed] [Google Scholar]
- 2.Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 2014;4:889–95. 10.1158/2159-8290.CD-14-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das TK, Cagan RL. KIF5B-RET oncoprotein signals through a multi-kinase signaling hub. Cell Rep 2017;20:2368–83. 10.1016/j.celrep.2017.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]