Abstract
Mixed connective tissue disease (MCTD) is a complex overlap disease with features of different autoimmune connective tissue diseases (CTDs) namely systemic sclerosis, poly/dermatomyositis and systemic lupus erythematous in patients with antibodies targeting the U1 small nuclear ribonucleoprotein particle. In this narrative review, we summarise the results of a systematic literature research which was performed as part of the European Reference Network on Rare and Complex Connective Tissue and Musculoskeletal Diseases project, aimed at evaluating existing clinical practice guidelines (CPGs) or recommendations. Since no specific CPGs on MCTD were found, other CPGs developed for other CTDs were taken into consideration in order to discuss what can be applied to MCTD even if designed for other diseases. Three major objectives were proposed for the future development of CPGs: MCTD diagnosis (diagnostic criteria), MCTD initial and follow-up evaluations, MCTD treatment. Early diagnosis, epidemiological data, assessment of burden of disease and QOL aspects are among the unmet needs identified by patients.
Keywords: mixed connective tissue disease, european reference networks, ERN reconnet, clinical practice guidelines, unmet needs
Key messages.
What is already known about this subject?
MCTD is a rare autoimmune disorder characterised by features of other connective tissue diseases such as systemic sclerosis, systemic lupus, and polymyositis.
There is non-consensus with regards to its classification criteria, diagnoses and standard of care.
What does this study add?
This work highlights the absence of clinical practice guidelines in the matter of MCTD.
How might this impact on clinical practice?
It does not impact on clinical practice since there is no CPG.
Introduction
Mixed connective tissue disease (MCTD) was initially described by Sharp et al.1 MCTD was first reported as a mild overlap disease with features of different autoimmune connective tissue diseases (CTDs) namely systemic sclerosis, poly/dermatomyositis and systemic lupus erythematous in patients with antibodies targeting the U1 small nuclear ribonucleoprotein particle (U1 snRNP).
Since the initial description, the definition as an independent clinical entity has been discussed, especially since patients with U1 snRNP may meet the classification criteria of other ‘defined’ CTDs in the course of time.2 3 Also, the initial description by Sharp of MCTD as benign disease without organ involvement and with prompt response to low-dose glucocorticoids4 has not been confirmed in all studies. In addition, there are no generally accepted classification criteria.5 Nevertheless, some particularities, for example, pulmonary disease, point to an independent disease pattern.
Clinical practice guidelines (CPGs) are defined as ‘systematically developed statements to assist practitioner and patient decisions about healthcare for specific clinical circumstances’2 and are made to optimise patient care. With regard to CTDs, CPGs should be based on medical high-level evidence and integrate as many themes as possible of each CTD, that is, diagnosis, patients management, treatment, prognosis and complications. In that matter, the European Reference Network on Rare and Complex Connective Tissue and Musculoskeletal Diseases (ERN ReCONNET) launched a cooperative effort to review the existing CPGs on CTDs.
The objective of this review is to identify and evaluate the available CPGs providing specific evidencebased medicine (EBM) recommendations for MCTD.
Methods
Source and search strategy
We carried out a systematic search in PubMed and EMBASE based on controlled terms (MeSH and Emtree) and keywords of the disease and publication type (CPGs). We reviewed all the published articles in order to identify existing CPGs on diagnosis, monitoring and treatment, according to the Institute of Medicine 20113 definition (CPGs are statements that include recommendations intended to optimise patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options3).
The disease coordinator of the ERN-ReCONNET for MCTD has assigned the work on CPGs to the healthcare providers (HCPs) involved. Moreover, in order to implement the list of guidelines provided by MEDLINE and Embase search, the group also performed a hand search. A first screening among papers included in the final list (systematic search+hand search) based on title and abstract selected EBM guidelines to be assessed. A general assessment of the CPGs has been performed following the Appraisal of Guidelines for Research and Evaluation (AGREE) II tool checklist, not for formal appraisal but only to inform discussion.4–6 A discussion group was set for the evaluation of the existing CPGs and to identify the unmet needs.
Summary of the search strategy
MEDLINE (PubMed): (“mixed connective tissue disease”[MeSH Terms] OR (“mixed”[All Fields] AND “connective”[All Fields] AND “tissue”[All Fields] AND “disease”[All Fields]) OR “mixed connective tissue disease”[All Fields]) OR “sharp syndrome”[all fields] AND (“Practice Guideline”[Publication Type] OR “Practice Guidelines As Topic”[MeSH Terms] OR Practice Guideline[Publication Type] OR “Practice Guideline”[Text Word] OR “Practice Guidelines”[Text Word] OR “Guideline”[Publication Type] OR “Guidelines As Topic”[MeSH Terms] OR Guideline[Publication Type] OR “Guideline”[Text Word] OR “Guidelines”[Text Word] OR “Consensus Development Conference”[Publication Type] OR “Consensus Development Conferences As Topic”[MeSH Terms] OR “Consensus”[MeSH Terms] OR “Consensus”[Text Word] OR “Recommendation”[Text Word] OR “Recommendations”[Text Word] OR “Best Practice”[Text Word] OR “Best Practices”[Text Word]). Embase: ('mixed connective tissue disease'/exp OR 'connective tissue disease, mixed' OR 'mctd' OR 'mixed collagen disease' OR 'mixed connective tissue disease' OR 'mixed connective tissue disorder' OR 'sharp syndrome'/exp) AND ('practice guideline'/exp OR ‘practice guideline’ OR ‘practice guidelines’/exp OR ‘practice guidelines’ OR 'clinical practice guideline'/exp OR ‘clinical practice guideline’ OR ‘clinical practice guidelines’/exp OR ‘clinical practice guidelines’ OR 'clinical practice guidelines as topic'/exp OR ‘clinical practice guidelines as topic’ OR ‘guideline'/exp OR ‘guideline’ OR ‘guidelines’/exp OR ‘guidelines’ OR 'guidelines as topic'/exp OR ‘guidelines as topic’ OR ‘consensus development’/exp OR ‘consensus development’ OR ‘consensus development conference’/exp OR ‘consensus development conference’ OR ‘consensus development conferences’/exp OR ‘consensus development conferences’ OR ‘consensus development conferences as topic’/exp OR ‘consensus development conferences as topic’ OR ‘consensus’/exp OR ‘consensus’ OR ‘recommendation’ OR ‘recommendations’) AND embase]/lim NOT [medline]/lim
State of the art on CPGs
Absence of specific recommendations/guidelines for MCTD
The state of the art on CPGs in MCTD is presented in figure 1. A systematic Embase/MEDLINE search identifies 124 references to be screened and analysed by the ERN ReCONNET MCTD group. After screening and full-text analysis, five references were selected.7–11 After final evaluation, no reference was selected as specific CPG for MCTD.
Figure 1.
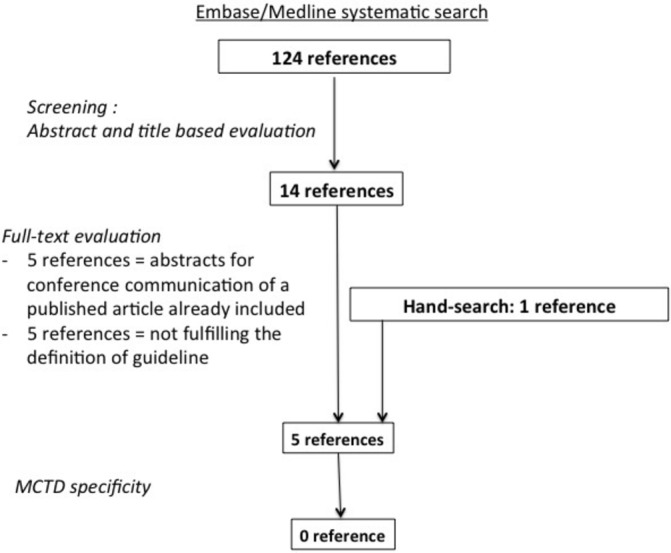
Flow diagram of the systematic literature search on mixed connective tissue disease (MCTD) clinical practice guidelines.
Combined recommendations
Although there was no specific CPG on MCTD, the ERN-ReCONNET MCTD group selected five references that could be used in the context of MCTD. These references were common to other CTD such as systemic sclerosis or vasculitis and included recommendations for the testing of anti-Sm and anti-RNP antibodies,7 for screening pulmonary arterial hypertension,8 for the assessment of autoantibodies to cellular antigens referred to as antinuclear antibodies,9 for the management of skin ulcers,10 and for using cardiovascular MR.11
Unmet needs
This is the first review providing an overview of currently available CPGs for MCTD. We did not identify specific CPGs addressing important issues in MCTD. Efficient and effective guidelines improve patient care and help medical practice and decision-making.12 Therefore, the ERN-ReCONNET MCTD group agrees on the general need for specific recommendations for MCTD. To prioritise what should be done in the matter of MCTD, three major objectives were proposed for CPGs future development:
CPGs on MCTD diagnosis (diagnostic criteria): Although different sets of clinical criteria have been proposed, there is no consensus about the most accurate one.5 MCTD often resembles several conditions and yet can easily be misdiagnosed by numerous rheumatic conditions including systemic sclerosis, rheumatoid arthritis or so-called overlap syndromes. Without a standardised and acknowledged set of criteria, an agreement regarding MCTD existence and disease CPGs is not possible. Moreover, such classification criteria would facilitate the establishment of registers to collect a meaningful number of patients with MCTD to evaluate the course and prognosis. The classification criteria would also enable to evaluate the role of biomarkers, specifically the potential diagnostic/prognostic role of antibodies targeting U1 snRNP and/or in combination with the detection of autoantibodies that could earlier characterise more defined CTDs such as anti-SCL70, anti-CCP, anti-Jo1, antiSm, anti-ds-DNA-associated entities for defining early progression.
CPGs on MCTD initial and follow-up evaluations: At present, the initial approach, the type and the frequency of follow-up assessments of patients with MCTD are often handled very differently or performed according to the management of other rheumatic diseases. On one hand, MCTD is described as a potentially mild and often treatable condition.13 On the other hand, the disease is incurable and can be deleterious or even life-threatening with development of pulmonary, kidney, gastrointestinal and central nervous system involvement. Here, early and targeted intervention will definitively be associated with a better outcome. The worst prognosis and high mortality are associated with the presence of pulmonary disease.14 15 To improve management, it would certainly be helpful to develop and validate a (composite) disease activity score for MCTD that takes into account all relevant signs and symptoms.16 Agreement on the frequency of follow-up assessments during the course of disease on the basis of guidelines would facilitate the management of patients for clinicians.
CPGs on MCTD treatment: There is no agreement about the initial or long-term treatment of MCTD, especially on the usefulness of low-dose glucocorticoids, antimalaria and immunosuppressive therapies in various clinical situations.17 To date, there has been no randomised controlled trial in MCTD. Usually, patients with MCTD are treated based on similarities with other CTDs or based on organ-based management.17 Classification criteria would therefore also enable to perform clinical trials on therapeutic interventions. In addition, the management of comorbidities (such as osteoporosis, atherosclerosis, etc) and specific situations (such as pregnancy, family planning, etc) should be addressed. Overall, the ERN-ReCONNET MCTD group agrees on the need for evidence-based CPGs on MCTD treatment. Such guidelines undoubtedly require careful data collection from a larger number of patients and collaboration between experts and registers from different countries.
Last but not the least, CPGs on MCTD should also take into account patients’ point of view in order to improve patients’ daily quality of care and life. Patient-reported outcomes for patients with MCTD should therefore be developed and evaluated.
Patients’ unmet needs
This paragraph intends to highlight the unmet needs of the MCTD community. The content of this paragraph has been realised by the ERN ReCONNET European Patient Advocacy Group that carefully collected the voices and the points of view of the whole European community of the disease they represent.
Early diagnosis is the primal need for patients with MCTD as it often takes a long time to establish the diagnosis. Besides that, when a diagnosis is finally made, patients are traditionally vaguely told that they have ‘some kind of’ rheumatic disease or autoimmune disease which clearly does not help understanding what is wrong with them and what they are expected to face during the lifelong course of the disease. The understanding how this disease develops and what outcomes are realistic for these patients, is essential.
Furthermore, prevalence of the disease is not well defined, and patients and doctors have no precise information on the number of MCTD cases at present. Eventually, the European Reference Networks will be able to help somehow with the most fundamental measure of the burden of the disease to plan appropriately for patient healthcare needs. Also, there’s a lack of knowledge regarding this disease among physicians as patients often feel that they do not know how to address the reported symptoms.
Life style and its impact in patients quality of life (QOL) is often disregarded, thus the need to measure it or understand if it is ever measured would be useful to build evidence-based patient-focused guidelines on lifestyle issues. There is also a need for a more holistic approach to disease management, as doctors may be treating symptoms and organ to organ, while overlooking the individual beyond the patient. Patient education may also contribute to improve QOL.
In addition, pain management and fatigue are huge challenge, as pain is still treated insufficiently, and fatigue is still inadequately assessed. Non-pharmacological approaches and specific programme, such as exercise, patient education, psychological support or emotional burden management, are needed.
Treatment options are limited to hydroxychloroquine, corticosteroids and immunosuppressive drugs, so there is also a need for alternative treatments with less side effects.
Information and awareness are undoubtfully very important unmet needs among all stakeholders involved: HCPs, public, employers, families, care givers and patients. Furthermore, information on available clinical trials is missing so there is definitely a gap to fill.
Last but not least, tax exemption on access to care needs reflection, as we need to understand how it works in different European countries.
Conclusions
This review highlights the absence of specific CPG on MCTD. There is a need for high-quality evidence-based guidelines to assist practitioner and patient decisions about MCTD healthcare. Further CPGs should focus on MCTD diagnosis, evaluations, treatment and patients’ needs. International registries are particularly important to collect solid data that can serve as input for CPGs.
Acknowledgments
Thanks to all the members of the Steering Committee of the ERN ReCONNET for the huge commitment during this work. A special thank goes to all the members of the ERN ReCONNET team for providing support during all the phases of the Work Package 3.
Footnotes
Contributors: CB, SCA, TR, MM and F-BR: substantial contributions to the conception and design of the work; the acquisition, analysis and interpretation of data; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. ATo, AZ, ATa, BL, DA, GA, GV, HÉ, KT, LD, LG, MP, M-LU, RS, RA, TSW, vVRF, VA: substantial contributions to the analysis and interpretation of data; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. BS, FJE, GI, SM, SV, CM: revising the work critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This publication was funded by the European Union’s Health Programme (2014-2020).
Disclaimer: ERN ReCONNET is one of the 24 European Reference Networks (ERNs) approved by the ERN Board of Member States. The ERNs are co-funded by the European Commission. The content of this publication represents the views of the authors only and it is their sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the Consumers, Health, Agriculture and Food Executive Agency (CHAFEA) or any other body of the European Union. The European Commission and the Agency do not accept any responsibility for use that may be made of the information it contains.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Sharp GC, Irvin WS, Tan EM, et al. . Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an Extractable Nuclear Antigen (ENA). Am J Med 1972;52:148–59. 10.1016/0002-9343(72)90064-2 [DOI] [PubMed] [Google Scholar]
- 2. Institute of Medicine (US) Committee to Advise the Public Health Service on Clinical Practice Guidelines In: Field MJ, Lohr KN, Clinical practice guidelines: directions for a new program. Washington (DC): National Academies Press (US), 1990. [PubMed] [Google Scholar]
- 3. Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines In: Graham R, Mancher M, Miller Wolman D, Clinical practice guidelines we can trust. Washington (DC): National Academies Press (US), 2011. [PubMed] [Google Scholar]
- 4. Brouwers MC, Kho ME, Browman GP, et al. . AGREE II: advancing guideline development, reporting and evaluation in health care. Can Med Assoc J 2010;182:E839–42. 10.1503/cmaj.090449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brouwers MC, Kho ME, Browman GP, et al. . Development of the AGREE II, part 1: performance, usefulness and areas for improvement. Can Med Assoc J 2010;182:1045–52. 10.1503/cmaj.091714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brouwers MC, Kho ME, Browman GP, et al. . Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ 2010;182:E472–78. 10.1503/cmaj.091716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benito-Garcia E, Schur PH, Lahita R, et al. . Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-Sm and anti-RNP antibody tests. Arthritis Rheum 2004;51:1030–44. 10.1002/art.20836 [DOI] [PubMed] [Google Scholar]
- 8. Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903–75. 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 9. Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. . International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis 2014;73:17–23. 10.1136/annrheumdis-2013-203863 [DOI] [PubMed] [Google Scholar]
- 10. Fujimoto M, Asano Y, Ishii T, et al. . The wound/burn guidelines - 4: guidelines for the management of skin ulcers associated with connective tissue disease/vasculitis. J Dermatol 2016;43:729–57. 10.1111/1346-8138.13275 [DOI] [PubMed] [Google Scholar]
- 11. Mavrogeni SI, Kitas GD, Dimitroulas T, et al. . Cardiovascular magnetic resonance in rheumatology: current status and recommendations for use. Int J Cardiol 2016;217:135–48. 10.1016/j.ijcard.2016.04.158 [DOI] [PubMed] [Google Scholar]
- 12. Keiffer MR. Utilization of clinical practice guidelines: barriers and facilitators. Nurs Clin North Am 2015;50:327–45. 10.1016/j.cnur.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 13. Lundberg IE. The prognosis of mixed connective tissue disease. Rheum Dis Clin North Am 2005;31:535–47. 10.1016/j.rdc.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 14. Sullivan WD, Hurst DJ, Harmon CE, et al. . A prospective evaluation emphasizing pulmonary involvement in patients with mixed connective tissue disease. Medicine 1984;63:92–107. 10.1097/00005792-198403000-00003 [DOI] [PubMed] [Google Scholar]
- 15. Burdt MA, Hoffman RW, Deutscher SL, et al. . Long-term outcome in mixed connective tissue disease: longitudinal clinical and serologic findings. Arthritis Rheum 1999;42:899–909. [DOI] [PubMed] [Google Scholar]
- 16. Musters A, Tas SW. How to monitor safety and efficacy of biologic treatment in rare, therapy-refractory immune-mediated inflammatory diseases? Rheumatology 2018;57:591–3. 10.1093/rheumatology/kex016 [DOI] [PubMed] [Google Scholar]
- 17. Kim P, Grossman JM. Treatment of mixed connective tissue disease. Rheum Dis Clin North Am 2005;31:549–65. 10.1016/j.rdc.2005.04.008 [DOI] [PubMed] [Google Scholar]


