Abstract
Objectives
TNFAIP3 encodes A20 that negatively regulates nuclear factor kappa light chain enhancer of activated B cells (NF-κB), the major transcription factor coordinating inflammatory gene expression. TNFAIP3 polymorphisms have been linked with a spectrum of inflammatory and autoimmune diseases and, recently, loss-of-function mutations in A20 were found to cause a novel inflammatory disease ‘haploinsufficiency of A20’ (HA20). Here we describe a family with HA20 caused by a novel TNFAIP3 loss-of-function mutation and elucidate the upstream molecular mechanisms linking HA20 to dysregulation of NF-κB and the related inflammasome pathway.
Methods
NF-κB activation was studied in a mutation-expressing cell line using luciferase reporter assay. Physical and close-proximity protein–protein interactions of wild-type and TNFAIP3 p.(Lys91*) mutant A20 were analysed using mass spectrometry. NF-κB -dependent transcription, cytokine secretion and inflammasome activation were compared in immune cells of the HA20 patients and control subjects.
Results
The protein–protein interactome of p.(Lys91*) mutant A20 was severely impaired, including interactions with proteins regulating NF-κB activation, DNA repair responses and the NLR family pyrin domain containing 3 (NLRP3) inflammasome. The p.(Lys91*) mutant A20 failed to suppress NF-κB signalling, which led to increased NF-κB -dependent proinflammatory cytokine transcription. Functional experiments in the HA20 patients’ immune cells uncovered a novel caspase-8-dependent mechanism of NLRP3 inflammasome hyperresponsiveness that mediated the excessive secretion of interleukin-1β and interleukin-18.
Conclusions
The current findings significantly deepen our understanding of the molecular mechanisms underlying HA20 and other diseases associated with reduced A20 expression or function, paving the way for future therapeutic targeting of the pathway.
Keywords: inflammation, autoimmune diseases, t cells, behcet's disease
Key messages.
What is already known about this subject?
A20 is a negative regulator of nuclear factor kappa B (NF-κB) having a key role in immune activation and autoimmunity.
Loss-of-function mutations in A20 are reported to lead to an inflammatory disease termed haploinsufficiency of A20 (HA20).
What does this study add?
We report a novel p.(Lys91*) A20 mutation and use it as a model to uncover molecular mechanisms related to the role of A20 in HA20 development.
The p.(Lys91*) fails to suppress NF-κB activity and has severely impaired protein interactome compared with the wild-type A20 in HEK cells, and causes aberrant NLRP3 inflammasome responsiveness in patient peripheral blood mononuclear cells.
How might this impact on clinical practice?
This study significantly deepens our understanding on the role of A20 in rheumatic diseases and may provide novel approaches for the development of therapeutic approaches.
Introduction
Tumour necrosis factor alpha-induced protein 3 (TNAP3, also known as A20), encoded by the TNFAIP3 gene, is a key negative regulator of nuclear factor kappa B (NF-κB) activation downstream a large number of immune receptors, including the TNF and Toll/interleukin(IL)-1 receptor families and the T and B cell antigen receptors.1–5 A20 achieves this via its dual function as a ubiquitin-editing enzyme: an N-terminal ovarian tumour (OTU) domain removes activating K63-linked polyubiquitin from key intermediate NF-κB signalling molecules to destabilise protein–protein interactions, whereas a C-terminal zinc finger domain catalyses the attachment of K48-linked polyubiquitin to induce proteasomal degradation.3 6 7 In addition, A20 inhibits NF-κB activation via a non-catalytic mechanism involving its binding to linear ubiquitin on NF-κB essential modulator (NEMO).8 9 The mRNA expression of A20 is NF-κB-inducible,10 thus generating a negative feedback loop to attenuate NF-κB responses.
Recently Zhou et al reported six families with heterozygous loss-of-function mutations in the TNFAIP3 gene that led to haploinsufficiency of A20 (HA20) and caused an early-onset Behçet-like disease.11 Studies in mouse models have previously demonstrated a crucial role for A20 in autoinflammation and autoimmunity as mice with a germline deletion of Tnfaip3 spontaneously develop severe multiorgan inflammation and tissue damage resulting in perinatal death,12 and cell-specific ablation of Tnfaip3 results in diverse symptoms of immune dysregulation.13 14 Similar to Tnfaip3 deficiency in mice,12–15 human HA20 led to exaggerated NF-κB responses and increased secretion of NLR family pyrin domain containing 3 (NLRP3) inflammasome target cytokines.11 Additional reports from these16 and further identified patients17–21 have expanded the clinical spectrum of HA20 to comprise diseases such as autoimmune lymphoproliferative syndrome, systemic juvenile arthritis, psoriatic arthritis, Crohn’s disease and Hashimoto’s thyroiditis. The increasing number of diseases associated with A20 has emphasised its role in inflammatory diseases and in autoimmunity and raised the possibility that mutations in TNFAIP3 may be an unexpectedly common underlying factor in the pathogenesis of rheumatic and other autoimmune diseases.
We describe here a family with polyautoimmunity and autoinflammatory symptoms caused by a novel heterozygous TNFAIP3 p.(Lys91*) loss-of-function mutation resulting in haploinsufficiency. Our aim was to further elucidate the upstream molecular mechanisms linking HA20 to dysregulated NF-κB activation and NLRP3 inflammasome responses. Thus, we analysed protein–protein interactions of wild-type (wt) and p.(Lys91*) A20 in HEK cells and performed functional experiments in patients’ immune cells to study NLRP3 inflammasome responses. As A20 is rapidly emerging as a key regulator of immune activation and autoimmunity in humans, the detailed characterisation of A20 effector mechanisms paves the way for future therapeutic targeting of the pathway.
Materials and methods
Genetic analyses
DNA samples were extracted from total peripheral blood using standard methods. Whole-exome sequencing and data analysis were performed as previously described.22 23 All the common (frequencies >0.01 in the general population) and non-coding variants were discarded. We searched for rare heterozygous variants, according to the inferred autosomal-dominant inheritance pattern (figure 1A). The variant identified in the TNFAIP3 gene (Ensembl ENSG00000118503:ENST00000433680: exon2:c.A271T: p.(Lys91*) was confirmed using PCR and capillary electrophoresis.
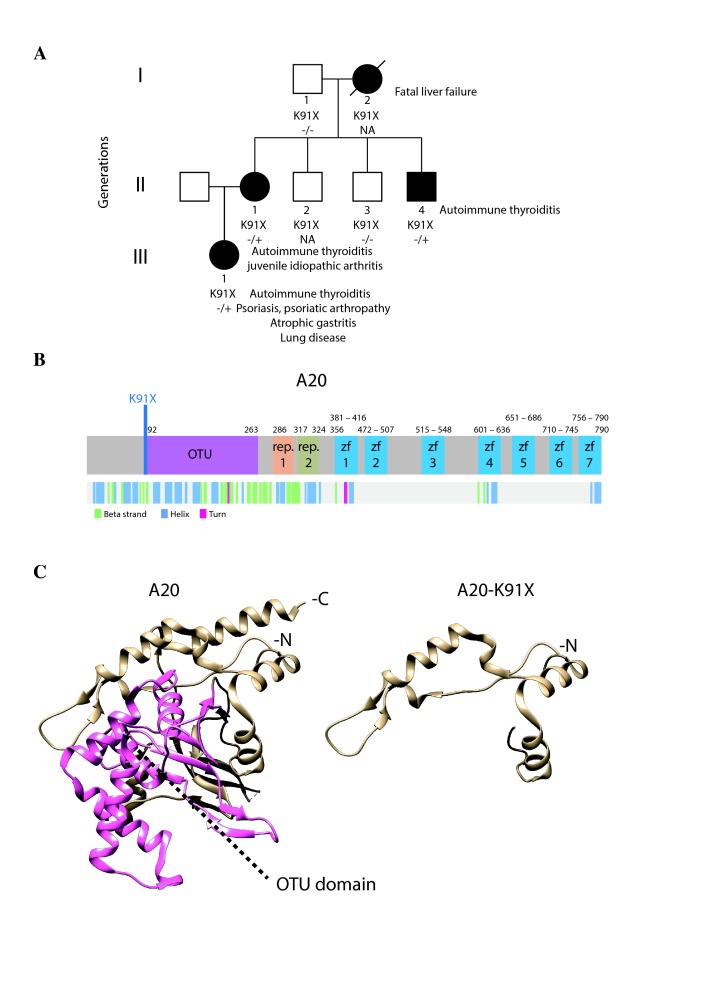
Figure 1.
Phenotypic and protein-level characteristics of a family with newly identified TNFAIP3 c.A271T: p.(Lys91*) mutation. (A) A pedigree showing the TNFAIP3 p.(Lys91*) mutation carriers (–/+, solid symbols) in three generations (I–III). Autoimmune thyroiditis was diagnosed in all carriers (I-2, II-1, II-4, III-1) at an early age. In addition, the index patient (II-1) suffered from psoriasis, articular symptoms, atrophic gastritis, severe autoinflammatory lung reaction, anaemia, genital papillomatosis and repeated genital HSV infections. Daughter of the index (III-1) was diagnosed with polyarticular juvenile idiopathic arthritis at the age of 4 years, and mother of the index (I-2) died of liver failure at the age of 46 years. (B) Schematic illustration of A20 structure showing the protein domains (upper panel) and the folding (lower panel). Position of the novel p.(Lys91*) mutation is indicated in blue. (C) 3D structure models of wild-type and mutant p.(Lys91*) A20 (based on PDB accession 5LRX). OTU domain is highlighted with pink. HSV, herpes simplex virus; OTU, ovarian tumour.
Creating A20 expressing Flp-In 293 T-REx cell lines
A20 mutant plasmids were created using QuickChange Site-Directed Mutagenesis kit (Agilent Technologies) to wt TNFAIP3 obtained from the human Orfeome collection (Horizon Discovery). TNFAIP3 constructs were further subcloned into N-terminal MAC-tag Gateway destination vector.24 All generated constructs were confirmed with direct sequencing. The MAC-tagged expression constructs were transfected into Flp-In 293 T-REx cells (Invitrogen, Life Technologies) with FuGENE HD (Promega). The cells were grown according to manufacturer’s instructions under selection with Hygromycin B (Thermo Fisher Scientific) to create stable cell lines. Expression of stable transgenes was confirmed by western blotting using anti-hemagglutin (HA) primary (Biolegend, MMS-101R) and horseradish peroxidase-conjugated secondary antibody.
Luciferase assay
For reporter assays, HEK293 cells were transfected with in total 50 ng A20 mutant or wt MAC-tagged construct and GFP or empty MAC-tag-vector,24 40 ng of NF-κB luciferase reporter plasmid (Cignal 45), and 1.4 ng Renilla luciferase reporter plasmid (pRL-SV40). Transfected cells were stimulated with indicated amounts of tumour necrosis factor alpha TNF-α (R&D Systems) for 16 hours, lysed with 1× passive lysis buffer (Promega) and luciferase assays performed with Dual-Glo Luciferase Assay System according to manufacturer’s instructions (Promega).
Affinity purification and mass spectrometry
For each pull-down approximately 5×107 cells (5×15 cm dishes) were induced with 2 µg/mL tetracycline (Thermo Fisher Scientific) for 24 hours (for BioID additional 50 µM biotin was added). After induction, cells were washed, harvested, pelleted by centrifugation, snap-frozen and stored at −80°C until analysis. Affinity purification of AP-MS and BioID was performed as described in Turunen et al 25 and Heikkinen et al,26 respectively. Sample preparation for mass spectrometry and LC-MS/MS analysis on Orbitrap Elite ETD mass spectrometer was performed as in Heikkinen et al.26 After peptide reconstitution, 4 µL was analysed from both Strep-Tag and BioID-samples.
Culture and stimulation of peripheral blood mononuclear cells (PBMCs)
PBMCs were isolated by density gradient centrifugation in Ficoll-Paque PLUS (GE Healthcare) and seeded at 1.5×106 cells/mL in Macrophage-SFM medium supplemented with penicillin-streptomycin or in RPMI 1640 supplemented with GlutaMAX, penicillin-streptomycin and 10% FBS (all from Gibco). PBMCs were allowed to rest for a minimum of 3 hours before starting the stimulations with 1 µg/mL lipopolysaccharides (LPS) from Escherichia coli O111:B4 (Sigma), 1 µg/mL Pam3Cys-SKKKK (Pam3Cys; EMC microcollections), 10 µg/mL polyinosinic-polycytidylic acid (poly(I:C); InvivoGen) and 5 mM adenosine 5′-triphosphate (ATP; Sigma) for the indicated times. Inhibitors Z-YVAD-FMK (20 µM; R&D Systems), Z-IETD-FMK (5 µM; BD Bioscience) and necrostatin-1 (30 µM; Enzo Life Sciences) were added to the cells simultaneously with LPS in the 16-hour stimulations or 30 min before LPS in the LPS 6 hour+ATP 45 min stimulations.
Measurement of cytokine secretion
TNF-α and the mature, cleaved forms of interleukin (IL)-1β and IL-18 were detected from PBMC culture media supernatants using Human TNF-α DuoSet ELISA, Human IL-1β/IL-1F2 DuoSet ELISA and Human Total IL-18 DuoSet ELISA (all from R&D Systems).
Quantitative real-time PCR
RNA was isolated from PBMCs using RNeasy Plus Mini Kit (Qiagen) and cDNA synthesised with iScript kit (Bio-Rad). Quantitative real-time PCR was performed from 10 ng of cDNA per reaction using LightCycler480 SYBR Green I master (Roche) and LightCycler96 instrument (Roche); primer sequences are listed in online supplementary table 1. Relative gene expression was calculated using the 2(-ΔΔCt) method, normalising to the geometric mean of expression of two housekeeping genes, ribosomal protein lateral stalk subunit P0 and beta-2 microglobulin.
rmdopen-2018-000740supp003.docx (37KB, docx)
Monocyte caspase-1 activity measurement
Aliquots of EDTA-anticoagulated blood were incubated for 6 hours at +37°C under gentle rocking with or without 10 ng/mL LPS (Sigma). ATP at 5 mM (Sigma) was added for 20 min, followed by 2-hour incubation at +37°C with a cell-permeable, irreversibly binding FAM-FLICA fluorescent caspase-1 substrate (ImmunoChemistry Technologies). After red blood cell lysis and staining with the monocyte marker anti-human CD14-APC (Miltenyi Biotec) for 15 min at +4°C, the cells were analysed using BD Accuri C6 flow cytometer (BD Biosciences; instrument maintained by the Biomedicum Flow Cytometry Unit).
Results
Identification of the mutation and the clinical manifestations of the patients
Exome sequencing of the index patient (II-1) identified a novel nonsense mutation in the exon 2 of the TNFAIP3 gene: c.A271T: p.(Lys91*) (ENSG00000118503:ENST00000433680) (figure 1A,B). The mutation is not listed in Genome Aggregation Database (gnomAD, Cambridge, Massachusetts, USA) and was predicted to be pathogenic according to the ACMG Standards and Guidelines.27 The TNFAIP3 p.(Lys91*) mutation was confirmed in the patient’s genomic DNA and screened in the other members of the family. Two other affected family members (II-4, III-1) were observed to also harbour the mutation, (I-2 is an obligate carrier). The clinical manifestations of the carriers included autoimmune thyroiditis, which was diagnosed in all carriers (I-2, II-1, II-4, III-1) at an early age. The index patient suffered from psoriasis, articular symptoms, atrophic gastritis, severe inflammatory lung reaction, anaemia and repeated genital HSV infections. Daughter of the index was diagnosed with polyarticular juvenile idiopathic arthritis at the age of 4. See also the legend of figure 1A.
Effect of the TNFAIP3 p.(Lys91*) mutation on protein structure and expression
The 3D protein structures of wt and p.(Lys91*) A20 were visualised by processing the PDB (http://www.rcsb.org 28) accession number 5LRX (chain A29 with Chimera,30 which yielded a severely truncated protein lacking the complete OTU domain (figure 1C). To further analyse the expression and possible physical and functional changes caused by the TNFAIP3 p.(Lys91*) mutation, stable Flp-In 293 T-REx cell lines expressing wt and p.(Lys91*) A20 were created. The wt HA-tagged A20 was expressed in western blot, but the p.(Lys91*) mutant was detected only with an N-terminal tag (figure 2A), suggesting that only an N-terminal fragment is produced and no alternative start codons are used. Peptide identification using mass spectrometry confirmed that only the N-terminal region was detectable in the p.(Lys91*) mutant (figure 2B). The expression levels of the N-terminal peptides/protein of the wt and p.(Lys91*) mutant A20 were highly comparable (figure 2B).
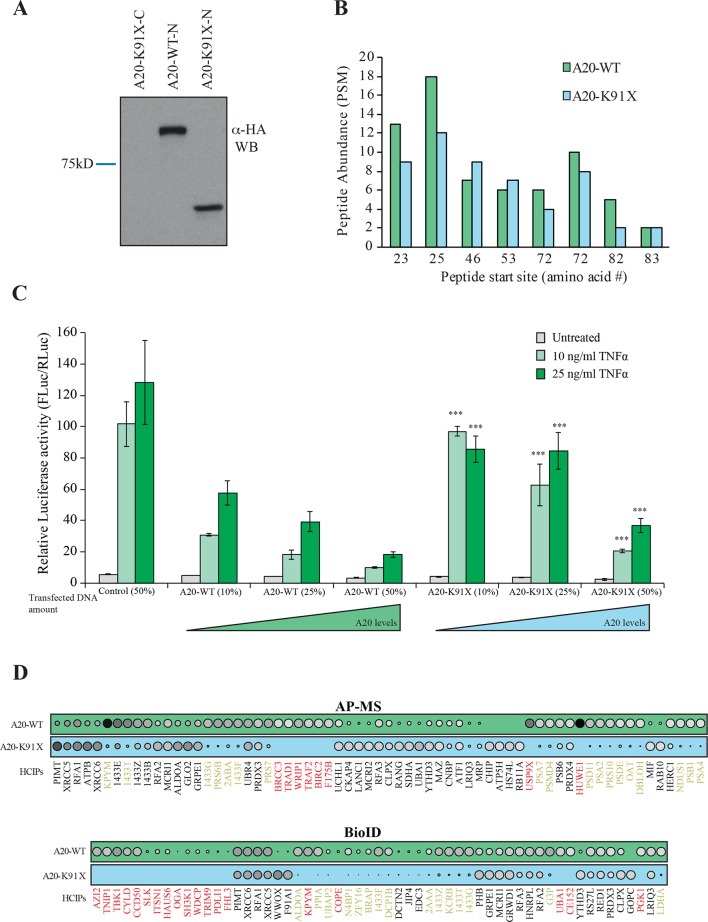
Figure 2.
The p.(Lys91*) mutant A20 fragment is expressed but shows severe impairment of protein–protein interactions and of inhibitory activity on nuclear factor (NF)-κB signaling. (A) Expression profile analysis of wild type (wt) A20 and the p.(Lys91*) mutant in HEK293 cells. The wt protein is detected with both as N- and C-terminally tagged constructs, whereas the p.(Lys91*) mutant is only detected as N-terminally tagged illustrating that no alternative start site is used and only short N-terminal fragment is produced. (B) Quantitative mass spectrometry-based proteomics analysis shows that the N-terminal part of the wt A20 and p.(Lys91*) mutant are expressed in similar levels as shown by the quantitative abundance of the peptides derived from the N-termini. (C) wt A20 dose-dependently inhibits the NF-κB signaling induced by tumour necrosis factor alpha (TNF-α) (light green=10 ng/mL and dark green=25 ng/mL), whereas the p.(Lys91*) mutant shows clearly diminished inhibitory activity suggesting haploinsufficiency. The asterisks represent the significance values; *<0.05, ** <0.01, *** <0.001. (D) Affinity purification mass spectrometry (AP-MS) (upper panel) and BioID (lower panel) analysis of wt and p.(Lys91*) mutant A20 show clear differences in their stable protein–protein interactions and BioID analysis displays differential molecular context of the p.(Lys91*) mutant compared with the wt A20. (The size and the colour gradient shows the relative abundance of the interacting prey protein detected in association with A20, red typeface designates the complete loss of the corresponding interactions and yellow >50% decrease compared with the wt A20).
A20 p.(Lys91*) mutant fails to suppress NF-κB activation and shows severely impaired protein–protein interactome
To analyse the possible functional consequences of the p.(Lys91*) mutation and the loss of OTU domain on the A20 functions, we monitored the NF-κB pathway activity using a luciferase-based reporter assay. The wt A20 dose-dependently inhibited TNF-α induced NF-κB pathway activation, whereas the p.(Lys91*) mutant failed to do so, clearly suggesting a haploinsufficiency (figure 2C).
The physical effects of the p.(Lys91*) mutation were further analysed by proteome-wide affinity purification mass spectrometry (AP-MS) (see online supplementary figure 1 for details). These analyses revealed that the p.(Lys91*) mutant completely loses physical interactions with BRCC3, TRAD1, WRIP1, TRAF2, BIRC2, F175B, USP9X and HUWE1, with several additional weakened interactions (figure 2D). To obtain more insight into the molecular mechanisms of A20 and especially on the p.(Lys91*) mutation, we further quantitatively analysed the functional and close-proximity interactions using BioID MS24 (see online supplementary figure 1 for details). This analysis revealed complete loss of interactions with AZI2, TNIP1, TBK1, CYLD, CCD50, SLK, ITSN1, HAUS6, OGA, SH3K1, WDCP, TRIM9, PDLI1, FHL3, KPYM, COPE, UBA1, CE152 and PGK1. Many more interactions were severely diminished (figure 2D). Majority of the detected interactions of wt A20 were novel (figure 3). Functional annotation revealed extensive interactions with ubiquitinylation-related, proteasome-related and NF-κB-related proteins, as well as with proteins involved, for example, in cell fate decisions (14-3-3 proteins), DNA repair (53BP1 complex) and glycolysis (figure 3). Of these, the interactions with ubiquitinylation-related and NF-κB-related proteins were most severely affected in the A20 p.(Lys91*) mutant.
Figure 3.
Interactome analysis reveals known and novel interactions for A20. Affinity purification mass spectrometry and BioID analysis of A20 identified 98 high-confidence protein–protein interactions (known interactions are shown with green lines, novel interaction detected on this study with blue and known prey–prey interactions with a dashed line). The interacting proteins are grouped based on their molecular functions/complexes. Interactions, which are lost with the p.(Lys91*) mutation, are illustrated with red node fill colour.
rmdopen-2018-000740supp001.pdf (522.8KB, pdf)
Blood immune cells of TNFAIP3 p.(Lys91*) carriers display strongly increased secretion of inflammasome-controlled cytokines IL-1β and IL-18
The NF-κB pathway and A20 have been shown to play regulatory roles in the activation of NLRP3 inflammasome, a pathway triggering caspase-1-mediated proteolytic maturation and secretion of proinflammatory cytokines IL-1β and IL-18.15 31 32 Excessive NLRP3-dependent cytokine secretion was demonstrated in HA20,11 yet the mechanism(s) linking reduced A20 to NLRP3 inflammasome activation in patient cells were not elucidated. We stimulated PBMCs of the HA20 patients with Toll-like receptor (TLR) 4 agonist LPS, with or without a subsequent ATP pulse; both stimuli trigger the NLRP3 inflammasome in monocytes via distinct mechanisms and with different kinetics.31 We found elevated secretion of IL-1β and IL-18 in PBMCs of the TNFAIP3 p.(Lys91*) carriers in response to LPS +ATP-induced ‘canonical’ (figure 4A,B; left panels) as well as LPS-induced ‘alternative’ pathway of NLRP3 inflammasome activation (figure 4A,B; right panels). To control for potential confounding factors in FBS preparations, PBMC cytokine secretion was analysed both in commercial serum-free monocyte-macrophage medium (figure 4) and in RPMI containing 10% FBS (online supplementary figure 2), yielding similar results. Also, TNF-α secretion in response to LPS, Pam3Cys and poly(I:C) was increased in the patients (figure 4C, online supplementary figure 2C. We further studied the levels of IL-1β and IL-18 in patients’ plasma by ELISA and found increased levels of circulating IL-18 in TNFAIP3 p.(Lys91*) carriers compared with the age-matched and sex-matched controls (online supplementary figure 3A), whereas IL-1β was not detectable.
Figure 4.
Inflammasome-dependent and inflammasome-independent proinflammatory cytokine secretion is elevated in peripheral blood mononuclear cells (PBMCs) of TNFAIP3 p.(Lys91*) carriers. PBMCs from TNFAIP3 p.(Lys91*) mutation carriers, patients 1 (II-1, woman, 30 years) and 2 (III-1, female, 8 years), were compared with sex-matched and age-matched controls 1 and 2, respectively. (A–C, left panels) The cells were primed with LPS for 6 hours, followed by treatment with ATP for 45 min. (A–C, right panels) The cells were treated with TLR agonists for 16 hours. Inhibitors necrostatin-1 (nec), Z-YVAD-FMK (yvad) and Z-IETD-FMK (ietd), or solvent control dimethyl sulfoxide (dmso) were added as indicated and cytokines were detected from PBMC culture supernatants by ELISAs. IL, interleukin; TNF, tumour necrosis factor; LPS, lipopolysaccharide.
rmdopen-2018-000740supp002.pdf (229.4KB, pdf)
rmdopen-2018-000740supp004.pdf (309.7KB, pdf)
The increased NLRP3 inflammasome response in TNFAIP3 p.(Lys91*) carriers is dependent on enhanced pro-IL-1β transcription and caspase-8 activity, but not on RIPK1
To elucidate the mechanism of altered NLRP3 inflammasome activation in the HA20 patients’ PBMCs, we analysed the mRNA expression of inflammasome pathway components and cytokines and the effect of inflammasome-related inhibitors on cytokine secretion. PBMCs of patient III-1 displayed strongly increased baseline expression of pro-IL-1β and NLRP3 receptor, whereas patient II-1 cells showed only moderate changes (online supplementary figure 3B). After LPS stimulation, PBMCs from both patients showed slightly elevated levels of pro-IL-1β mRNA and NLRP3 receptor expression remained moderately elevated only in patient III-1 (figure 5A). LPS-induced expression of NF-κB target cytokines IL-6, TNF-α and IL-8 was elevated in patient III-1, and IL-6 also in patient II-1 (figure 5B). Receptor interacting serine/threonine-protein kinase 1 (RIPK1) inhibitor necrostatin-1 did not affect IL-1β or IL-18 secretion in the patients’ PBMCs (figure 4A,B), although it normalised the excessive TNF-α response to 16-hour LPS treatment (figure 4C, right panel). As expected, both canonical and alternative NLRP3 inflammasome responses were efficiently blocked by the caspase-1 inhibitor Z-YVAD-FMK (figure 4A,B). However, levels of active caspase-1 in monocytes after whole blood inflammasome stimulation were increased only in patient II-1 (figure 5C), supporting a more indirect enhancement of inflammasome complex function. Remarkably, caspase-8 inhibitor Z-IETD-FMK efficiently suppressed IL-1β and IL-18 secretion in PBMCs of the TNFAIP3 p.(Lys91*)-positive patients, particularly after canonical NLRP3 inflammasome activation (figure 4A,B).
Figure 5.
Expression and activation of the NLRP3 inflammasome is altered in peripheral blood mononuclear cells (PBMCs) of TNFAIP3 p.(Lys91*) carriers. Samples from TNFAIP3 p.(Lys91*) mutation carriers, patients 1 (II-1, woman, 30 years) and 2 (III-1, female, 8 years), were compared with sex-matched and age-matched controls 1 and 2, respectively. Relative expression of (A) NLRP3 inflammasome components and target cytokines and (B) inflammasome-independent proinflammatory cytokines was analysed in PBMCs by quantitative PCR and normalised against housekeeping gene expression (arbitrary units). (C) Whole blood was stimulated with LPS and ATP, followed by the detection of NLRP3 inflammasome-triggered caspase-1 activity in monocytes using a fluorescent FLICA probe; the data are presented as median fluorescence intensity (MFI).
Discussion
HA20 is a recently described familial autoinflammatory disease originally reported to manifest as an early-onset Behçet-like disease,11 yet the range of known clinical manifestations is rapidly expanding.16–21 Here we studied patients with HA20 caused by a novel heterozygous TNFAIP3 p.(Lys91*) mutation resulting in severe truncation and production of an N-terminal protein fragment with highly limited functional capacity. The patients exhibit an unusual combination of early-onset autoimmune diseases, autoinflammatory symptoms and immunodeficiency, reflecting the widespread functions of A20 in controlling inflammation. To gain further insight into the molecular mechanism underlying the development of this complex disease, we focused on scrutinising two pathways strongly linked with A20 function; the NF-κB pathway and the NLRP3 inflammasome.
Mutations causing HA20 were previously reported to increase TNF-α-induced IKKα/IKKβ and MAPK phosphorylation and activation of NF-κB via defective removal of K63-linked ubiquitin from RIPK1, TNF receptor-associated factor 6 and NEMO.11 18 19 As expected, also the A20 p.(Lys91*) mutant was defective in suppressing TNF-α-induced NF-κB activation. We employed state-of-the-art proteomics tools to comprehensively map global changes in A20 protein–protein interactions caused by the p.(Lys91*) mutation. In agreement with A20 ubiquitin-editing functions, the results revealed loss of physical interactions with ubiquitin ligases (BIRC2, TRAF2, HUWE1), deubiquitinases (BRCC3, USP9X) and other ubiquitinylation modulators (WRIP1 and F175B/ABRX2). BIRC2/3 and TRAF2/3 form a regulatory complex that suppresses apoptotic functions of caspase-8 to enforce canonical (inflammatory) NF-κB signalling, while blocking non-canonical NF-κB activation.33 34 A20 modulates this balance by inducing TRAF2 degradation to suppress canonical NF-κB activation, and by binding c-IAP1/2 to support their caspase-8-inhibiting function while blocking their noncanonical NF-κB-inhibiting function.35–37 Thus, the loss of A20 p.(Lys91*) interaction with BIRC2 and TRAF2 is compatible with enhancement of caspase-8 activity and canonical NF-κB signalling. In turn, BRCC3, F175B/ABRX2, WRIP1 and HUWE1 have specific functions in DNA damage responses,38–41 which could be a significant finding regarding the association of reduced A20 levels with systemic lupus characterised by autoantibodies against nuclear antigens.14 Moreover, BRCC3 and F175B/ABRX2 are also components of the BRISC deubiquitinase complex that cleaves K63-linked ubiquitin and regulates mitotic spindle assembly,42 type I interferon signalling43 and NLRP3 inflammasome activation.44 Also, functional close-proximity protein–protein interactions of the A20 p.(Lys91*) mutant, particularly those related to ubiquitinylation and NF-κB signalling, were severely impaired, whereas interactions with proteasome, 53BP1-containing DNA repair complex and 14-3-3 regulatory proteins remained partially functional.
The roles of A20 in regulation of the NLRP3 inflammasome, both NF-κB-dependent and NF-κB-independent, are just beginning to be uncovered.15 32 Mice deficient in myeloid A20 develop erosive polyarthritis specifically due to poor control of NLRP3 inflammasome activation by A20.15 Moreover, increased NLRP3-dependent secretion of IL-1β and IL-18 was reported in HA20 patients,11 17 yet the upstream mechanism(s) linking this effect to A20 in patient immune cells remain unknown. We found increased IL-1β and IL-18 secretion in PBMCs of TNFAIP3 p.(Lys91*)-positive patients in response to both canonical and alternative NLRP3-activating stimuli. In mouse macrophages, A20 suppresses the NF-κB-induced mRNA expression of NLRP3 receptor and pro-IL-1β, but also blocks RIPK1/3-dependent pro-IL-1β ubiquitination that supports the inflammasome-mediated processing into mature IL-1β.15 32 Our patients’ PBMCs showed elevated LPS-induced transcription of pro-IL-1β, but NLRP3 receptor expression was not consistently increased. The RIPK1 inhibitor necrostatin-1 that reduced aberrant IL-1β secretion in Tnfaip3-deficient mouse macrophages32 had no effect on IL-1β nor IL-18 secretion in the patients’ PBMCs, which was, instead, strongly suppressed by caspase-8 inhibition. This suggests a novel caspase-8-dependent mechanism linking reduced A20 function to enhanced NLRP3 inflammasome activation. The finding is in line with the increased caspase-8 activity reported in Tnfaip3-deficient mouse cells32 45 and with the caspase-8-suppressing functions of A20,37 45 and further supported by the loss of physical interaction between A20 p.(Lys91*) mutant and BIRC2, which is required for caspase-8 inhibition by A20.37 Previous studies suggest that alongside its apoptotic functions caspase-8 facilitates the priming and activation of NLRP3 inflammasome via multiple mechanisms.46 In addition, the loss of interaction between A20 p.(Lys91*) mutant and the BRCC3-containing BRISC complex suggests a further novel NLRP3-regulating function of A20 that is lost in haploinsufficient cells, as BRCC3 deubiquitinates the NLRP3 receptor directly promoting its activation.44
In summary, we report here a TNFAIP3 p.(Lys91*) loss-of-function mutation leading to HA20 and autoimmune/inflammatory symptoms. We use this mutation as a model to uncover novel molecular mechanisms related to the role of A20 in disease development. We defined, for the first time, the global physical and functional interactome of wt A20 and found severely diminished interactions in the p.(Lys91*) mutant. Moreover, we discovered a novel caspase-8-dependent mechanism linking HA20 to hyperactivation of the NLRP3 inflammasome in patient immune cells. Collectively, these data greatly deepen our understanding of the molecular mechanisms underlying the clinical disease phenotype of HA20 and other diseases associated with reduced A20 expression or function. We believe that defining the physical and functional interaction changes in (m)any diseases is highly usable for understanding these diseases better on the molecular level and believe such studies will be seen in immense quantity in the near future.
Acknowledgments
Sini Miettinen is thanked for expert mass spectrometry skills, and Xiaonan Liu for help with Chimera.
Footnotes
KR and SK contributed equally.
TH, KKE and MV contributed equally.
Contributors: KR and SK designed and performed the experiments, analysed data, prepared the figures and wrote the manuscript. TH identified the index patient. LT and JS performed variant identification. VG organised clinical samples. MS, OK, PV, AV, PK, RK, AJ, NH, PJ, DCN and TH provided medical care and sampling. KKE and MV participated in designing experiments, data analysis, figure preparation, and manuscript writing and submission.
Funding: The study was supported by Finska Läkaresällskapet (KKE, DN), The Canadian Institutes of Health Research (THC 135230; KKE), the Stockmann foundation (KKE) and the Paulo foundation (KR). Molecular graphics and analyses were performed with the UCSF Chimera package. Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103311). Exome sequencing of the index patient was conducted in the Institute for Molecular Medicine Finland (FIMM) Technology Centre.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Ethical Committee for Clinical Science of Oulu University Hospital, and Coordinating Ethics Committee of The Hospital District of Helsinki and Uusimaa.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc Natl Acad Sci U S A 1996;93:6721–5. 10.1073/pnas.93.13.6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Lett 1999;442 147–50. 10.1016/S0014-5793(98)01645-7 [DOI] [PubMed] [Google Scholar]
- 3. Wertz IE, O'Rourke KM, Zhou H, et al. . De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 2004;430:694–9. 10.1038/nature02794 [DOI] [PubMed] [Google Scholar]
- 4. Boone DL, Turer EE, Lee EG, et al. . The ubiquitin-modifying enzyme A20 is required for termination of toll-like receptor responses. Nat Immunol 2004;5:1052–60. 10.1038/ni1110 [DOI] [PubMed] [Google Scholar]
- 5. Düwel M, Welteke V, Oeckinghaus A, et al. . A20 negatively regulates T cell receptor signaling to NF-kappaB by cleaving malt1 ubiquitin chains. J Immunol 2009;182:7718–28. 10.4049/jimmunol.0803313 [DOI] [PubMed] [Google Scholar]
- 6. Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 2010;327:1135–9. 10.1126/science.1182364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wertz IE, Newton K, Seshasayee D, et al. . Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature 2015;528:370–5. 10.1038/nature16165 [DOI] [PubMed] [Google Scholar]
- 8. Skaug B, Chen J, Du F, et al. . Direct, noncatalytic mechanism of IKK inhibition by A20. Mol Cell 2011;44:559–71. 10.1016/j.molcel.2011.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verhelst K, Carpentier I, Kreike M, et al. . A20 inhibits LUBAC-mediated NF-κB activation by binding linear polyubiquitin chains via its zinc finger 7. Embo J 2012;31:3845–55. 10.1038/emboj.2012.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krikos A, Laherty CD, Dixit VM. Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J Biol Chem 1992;267:17971–6. [PubMed] [Google Scholar]
- 11. Zhou Q, Wang H, Schwartz DM, et al. . Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet 2016;48:67–73. 10.1038/ng.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee EG, Boone DL, Chai S, et al. . Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 2000;289:2350–4. 10.1126/science.289.5488.2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol 2012;12:774–85. 10.1038/nri3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Das T, Chen Z, Hendriks RW, et al. . A20/Tumor necrosis factor α-induced protein 3 in immune cells controls development of autoinflammation and autoimmunity: lessons from mouse models. Front Immunol 2018;9:104 10.3389/fimmu.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vande Walle L, Van Opdenbosch N, Jacques P, et al. . Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 2014;512:69–73. 10.1038/nature13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aeschlimann FA, Batu ED, Canna SW, et al. . A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease. Ann Rheum Dis 2018;77:728–35. 10.1136/annrheumdis-2017-212403 [DOI] [PubMed] [Google Scholar]
- 17. Kadowaki T, Ohnishi H, Kawamoto N, et al. . Haploinsufficiency of A20 causes autoinflammatory and autoimmune disorders. J Allergy Clin Immunol 2018;141:1485–8. 10.1016/j.jaci.2017.10.039 [DOI] [PubMed] [Google Scholar]
- 18. Takagi M, Ogata S, Ueno H, et al. . Haploinsufficiency of TNFAIP3 (A20) by germline mutation is involved in autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol 2017;139:1914–22. 10.1016/j.jaci.2016.09.038 [DOI] [PubMed] [Google Scholar]
- 19. Duncan CJA, Dinnigan E, Theobald R, et al. . Early-onset autoimmune disease due to a heterozygous loss-of-function mutation in TNFAIP3 (A20). Ann Rheum Dis 2018;77:783–6. 10.1136/annrheumdis-2016-210944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohnishi H, Kawamoto N, Seishima M, et al. . A Japanese family case with juvenile onset Behçet's disease caused by TNFAIP3 mutation. Allergol Int 2017;66:146–8. 10.1016/j.alit.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 21. Shigemura T, Kaneko N, Kobayashi N, et al. . Novel heterozygous C243Y A20/TNFAIP3 gene mutation is responsible for chronic inflammation in autosomal-dominant Behçet's disease. RMD Open 2016;2:e000223 10.1136/rmdopen-2015-000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trotta L, Hautala T, Hämäläinen S, et al. . Enrichment of rare variants in population isolates: single AICDA mutation responsible for hyper-IgM syndrome type 2 in Finland. Eur J Hum Genet 2016;24:1473–8. 10.1038/ejhg.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trotta L, Martelius T, Siitonen T, et al. . ADA2 deficiency: Clonal lymphoproliferation in a subset of patients. J Allergy Clin Immunol 2018;141:1534–7. 10.1016/j.jaci.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 24. Liu X, Salokas K, Tamene F, et al. . An AP-MS- and BioID-compatible MAC-tag enables comprehensive mapping of protein interactions and subcellular localizations. Nat Commun 2018;9:1188 10.1038/s41467-018-03523-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turunen M, Spaeth JM, Keskitalo S, et al. . Uterine leiomyoma-linked MED12 mutations disrupt mediator-associated CDK activity. Cell Rep 2014;7:654–60. 10.1016/j.celrep.2014.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heikkinen T, Kämpjärvi K, Keskitalo S, et al. . Somatic MED12 nonsense mutation escapes mRNA decay and reveals a motif required for nuclear entry. Hum Mutat 2017;38:269–74. 10.1002/humu.23157 [DOI] [PubMed] [Google Scholar]
- 27. Richards S, Aziz N, Bale S, et al. . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet Med 2015;17:405–23. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rose PW, Prlić A, Altunkaya A, et al. . The RCSB protein data bank: integrative view of protein, gene and 3D structural information. Nucleic Acids Res 2017;45 D271–81. 10.1093/nar/gkw1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mevissen TET, Kulathu Y, Mulder MPC, et al. . Molecular basis of Lys11-polyubiquitin specificity in the deubiquitinase cezanne. Nature 2016;538:402–5. 10.1038/nature19836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pettersen EF, Goddard TD, Huang CC, et al. . UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 2004;25:1605–12. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 31. He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 2016;41:1012–21. 10.1016/j.tibs.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duong BH, Onizawa M, Oses-Prieto JA, et al. . A20 restricts ubiquitination of pro-interleukin-1β protein complexes and suppresses NLRP3 inflammasome activity. Immunity 2015;42:55–67. 10.1016/j.immuni.2014.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang CY, Mayo MW, Korneluk RG, et al. . NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998;281:1680–3. 10.1126/science.281.5383.1680 [DOI] [PubMed] [Google Scholar]
- 34. Zarnegar BJ, Wang Y, Mahoney DJ, et al. . Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol 2008;9:1371–8. 10.1038/ni.1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hymowitz SG, Wertz IE. A20: from ubiquitin editing to tumour suppression. Nat Rev Cancer 2010;10:332–41. 10.1038/nrc2775 [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi N, Oyama M, Kozuka-Hata H, et al. . Involvement of A20 in the molecular switch that activates the non-canonical NF-кB pathway. Sci Rep 2013;3:2568 10.1038/srep02568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamaguchi N, Yamaguchi N. The seventh zinc finger motif of A20 is required for the suppression of TNF-α-induced apoptosis. FEBS Lett 2015;589:1369–75. 10.1016/j.febslet.2015.04.022 [DOI] [PubMed] [Google Scholar]
- 38. Chen X, Arciero CA, Wang C, et al. . BRCC36 is essential for ionizing radiation-induced BRCA1 phosphorylation and nuclear foci formation. Cancer Res 2006;66:5039–46. 10.1158/0008-5472.CAN-05-4194 [DOI] [PubMed] [Google Scholar]
- 39. Zhang J, Cao M, Dong J, et al. . ABRO1 suppresses tumourigenesis and regulates the DNA damage response by stabilizing p53. Nat Commun 2014;5:5059 10.1038/ncomms6059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bish RA, Myers MP. Werner helicase-interacting protein 1 binds polyubiquitin via its zinc finger domain. J Biol Chem 2007;282:23184–93. 10.1074/jbc.M701042200 [DOI] [PubMed] [Google Scholar]
- 41. Markkanen E, van Loon B, Ferrari E, et al. . Regulation of oxidative DNA damage repair by DNA polymerase λ and MutYH by cross-talk of phosphorylation and ubiquitination. Proc Natl Acad Sci U S A 2012;109:437–42. 10.1073/pnas.1110449109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yan K, Li L, Wang X, et al. . The deubiquitinating enzyme complex BRISC is required for proper mitotic spindle assembly in mammalian cells. J Cell Biol 2015;210:209–24. 10.1083/jcb.201503039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng H, Gupta V, Patterson-Fortin J, et al. . A BRISC-SHMT complex deubiquitinates IFNAR1 and regulates interferon responses. Cell Rep 2013;5:180–93. 10.1016/j.celrep.2013.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Py BF, Kim MS, Vakifahmetoglu-Norberg H, et al. . Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 2013;49:331–8. 10.1016/j.molcel.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 45. Jin Z, Li Y, Pitti R, et al. . Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 2009;137:721–35. 10.1016/j.cell.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 46. Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol 2016;16:7–21. 10.1038/nri.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2018-000740supp003.docx (37KB, docx)
rmdopen-2018-000740supp001.pdf (522.8KB, pdf)
rmdopen-2018-000740supp002.pdf (229.4KB, pdf)
rmdopen-2018-000740supp004.pdf (309.7KB, pdf)