Abstract
Cocaine continues to be one of the most widespread abused illicit drugs in the USA. Rapid methods are needed for the identification and quantitation of cocaine and its metabolites, benzoylecgonine (BE), ecgonine methyl ester (EME) and cocaethylene (CE), in biological specimens by clinical and forensic toxicology laboratories. Presented is a differential ion mobility spectrometry–tandem mass spectrometry (DMS–MS-MS) method for the analysis of cocaine and its major metabolites in human serum that requires minimal sample preparation and no column chromatography. A Shimadzu Nexera X2 ultra-high performance liquid chromatography system was used to infuse the samples into the DMS cell at a rate of 30 μL/min. Separation of cocaine and its metabolites were performed in a SelexION DMS component from Sciex coupled to a QTRAP 6500 with an IonDrive Turbo V source for TurbolonSpray® using acetonitrile as a chemical modifier. Analysis consisted of ramping the CoV from −35 V to −6 V while monitoring the multiple reaction monitoring (MRM) transitions of each analyte. The assay was evaluated for linearity, bias, precision, carryover, interferences and stability. Calibration curves ranged from 10 to 1,000 ng/mL with linear regression correlation coefficients (r2) of 0.9912 or greater for each analyte. The limit of quantitation was set at 10 ng/mL. Intra-day precision (%CV) ranged from 0% to 15% for cocaine, 1% to 19% for BE, 1% to 17% for EME and 0% to 18% for CE. Inter-day precision ranged from 9% to 14% for cocaine, 2% to 17% for BE, 5% to 11% for EME and 5% to 15% for CE. No carryover or interferences were detected. Bland–Altman analysis of previously analyzed specimens by UPLC–MS-MS showed variability of 30% or less. The method demonstrates the applicability of DMS–MS-MS for high throughout analysis of drugs and their metabolites in clinical and forensic toxicology laboratories.
Introduction
Cocaine is a potent central nervous system stimulant that blocks the reuptake of the neurotransmitters dopamine, serotonin and norepinephrine and results in a state of euphoria and increased alertness (1, 2). Cocaine continues to be one of the most abused illicit drugs in the USA with a 1.6-fold increase in the total number of cocaine overdose deaths since 2010 (3). It has a short half-life of approximately 0.7–1.5 h and is rapidly metabolized by plasma and liver cholinesterases and carboxylesterases to form the major metabolites benzoylecgonine (BE) and ecgonine methyl ester (EME) (1, 2). The co-ingestion of cocaine and ethanol results in the hepatic formation of the active metabolite cocaethylene (CE), which has a longer half-life and appears to be more euphorigenic than cocaine (4).
Rapid methods are needed for the identification and quantitation of cocaine and its metabolites, BE, EME and CE, in biological specimens by clinical and forensic toxicology laboratories. Several liquid chromatography combined with mass spectrometry (LC–MS-MS) methods have been reported for simultaneous determination of cocaine and its metabolites in biological specimens (1, 2, 5–7). Though LC–MS-MS techniques are well-established and effective methods for these analyses, chromatographic separation methods are often time-consuming and can result in matrix effects, decreased sensitivity and lack of separation of isobaric compounds. The development of a robust and rapid separation technique with quantitation capabilities without the use of column chromatography has emerged over the past few decades.
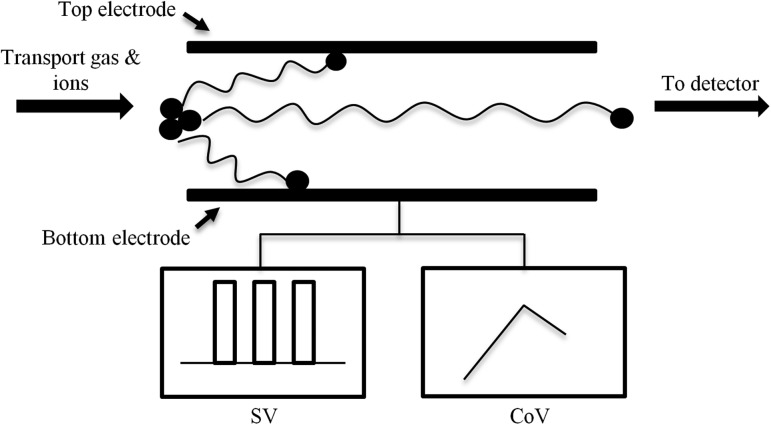
The origins of the separation of ions without column chromatography date back to the early 1980s when the Soviet Union created the “Gas Analyzer of Ions” device, which separated ions based on their mobility in an electric field (8). This principle is the basis for ion mobility spectrometry (IMS) which is an analytical technique that separates ions through a drift tube while applying a weak direct current electric field (9). The degree of separation depends on the ions’ respective mobilities in the drift tube, which is a function of their m/z. Differential ion mobility spectrometry (DMS) is a form of IMS that operates at atmospheric pressure and separates ions based on the difference between ion mobility in low and high electric fields (10). The DMS cell consists of two planar electrodes in which a high-voltage radio frequency asymmetric waveform is applied between the two electrodes. This voltage is called the separation voltage (SV), and it causes ions to oscillate toward one electrode or the other depending on the difference in the ion’s mobility during the high- and low-field portions of the waveform. A direct current voltage, called the compensation voltage (CoV), is applied and deflects ions away from the electrodes and toward the mass detector (9) (Figure 1). Separation power can be increased by the addition of a polar modifier to the gas transporting the ions into the DMS cell. The modifier enhances the formation of clusters between the analyte ions and the neutral molecules in the modifier, thereby increasing the difference between the high- and the low-field mobility, resulting in improved separation (9).
Figure 1.
Concept of differential mobility spectrometry (DMS); SV, separation voltage, CoV, compensation voltage.
DMS-based separation methods combined with mass spectrometry are effective at the rapid separation of ions at atmospheric pressure and have been shown to improve the limitations of chromatography-based methods such as a reduction in chemical noise from contaminants or diluents in drug evidence (10–15) and resolution of isobaric and isomeric compounds (16–18). Previous applications of the rapid separation and quantitation achieved by DMS include several drugs of abuse in postmortem tissue samples (18) and urine (19), and alcohols in human saliva (20).
Presented is a differential ion mobility spectrometry–tandem mass spectrometry (DMS–MS-MS) method for the analysis of cocaine and its major metabolites, BE, EME and CE, in human serum that neither requires chromatographic separation nor extensive sample preparation. A comparison study of previously analyzed specimens by LC–MS-MS was also performed and evaluated for variability.
Materials and Methods
Reagents
Cocaine (COC), BE, EME, CE and their deuterated internal standards (ISTD) cocaine-d3 (COC-d3), benzoylecgonine-d8 (BE-d8), ecgonine methyl ester-d3 (EME-d3) and cocaethylene-d8 (CE-d8) were purchased from Cerilliant (Round Rock, TX). Ammonium acetate, ammonium formate, acetonitrile and water were purchased from Fisher Scientific (Hanover Park, IL). In-house certified drug-free serum provided the matrix for all prepared calibrators, and quality control (QC) specimens. Bio-Rad Liquicheck™ Therapeutic Drug Monitoring Quality Control and Liquid Assay Multiqual were used for interference studies (Hercules, CA).
Sample preparation
Appropriate volumes of each drug working solution prepared in acetonitrile were added to serum to obtain a seven-point calibration curve with a range of 10–1,000 ng/mL. The following QC serum specimens were prepared and analyzed with the test specimens: limit of quantitation (LOQ) quality control, target concentration of 10 ng/mL; low control (LQC), target concentration of 30 ng/mL; medium control (MQC), target concentration of 300 ng/mL; and a high control (HQC), target concentration of 750 ng/mL. A drug-free control (negative control) that did not contain any drug but, did contain the ISTDs, and a double negative control that did not contain analytes or ISTDs were also run with each sample batch. All QC samples were stored at −20°C until analysis. Sample preparation was based on a previous method (21). In brief, 200 μL of acetonitrile was added to 100 μL of sample. Samples were vortexed for 30 s followed by centrifugation at 3,500 rpm for 5 min. Hundred microliter of the supernatant was aliquoted into autosampler vials and capped.
Differential mobility spectrometry–mass spectrometry
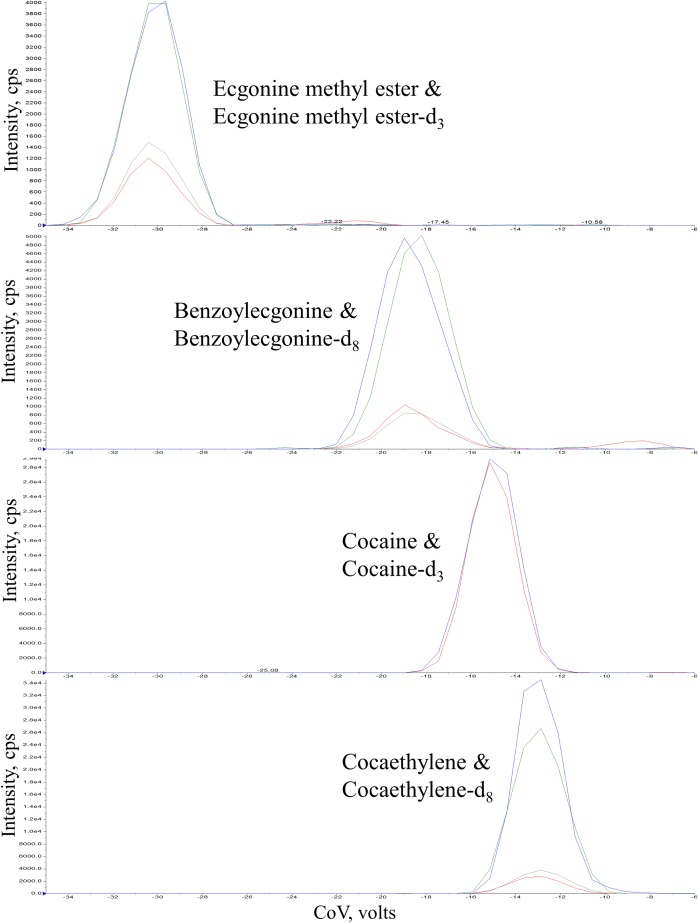
Separation of cocaine and its metabolites in serum were performed in a SelexION® differential ion mobility device from Sciex (Ontario, Canada) (Figure 2). The DMS cell was coupled to a QTRAP 6500+ with an IonDrive Turbo V source for TurbolonSpray® with a Valco Diverter Valve (Ontario, Canada). DMS operation conditions were cell temperature “Low”, offset of −3 V, and SV of 3,900 V. Acetonitrile was used as the chemical modifier. The analysis consisted of ramping the CoV value from −35 V to −6 V over 1 min while monitoring the MRM transitions of each analyte. The transitions monitored and CoV for each analyte are presented in Table I. The mass spectrometer was operated in positive ionization mode under the following conditions: ion-spray voltage 5,500 V, temperature 300 °C, Gas 1 and Gas 2 at 15 and 30 psi, respectively, with the collision gas at medium. A Shimadzu Nexera X2 ultra-high performance liquid chromatography system (UPLC) (Kyoto, Japan) was used to directly infuse 30 μL each of the samples at 1 μL/min into the DMS cell being carried along with water containing 1 g/L ammonium acetate with a flow rate 30 μL/min. No analytical column was used for chromatographic separation by the UPLC system. The Valco Diverter Valve was initially set to waste and the DMS–MS-MS was not in operation. At 0.8 min, the Valco Diverter Valve was changed to allow infusion of the sample in as the DMS–MS-MS turned on to allow for the dead volume of the injector system. The DMS–MS-MS was operated for 1 min and then shut off. The injector valve was internally washed during the analysis using a rinse solvent consisting of 40:40:20 methanol:acetonitrile:isopropanol to eliminate any carryover from the injector valve. At 2.0 min, the Valco Diverter Valve was switched back to waste. At 2.10 min, the solvent in the liquid chromatography system was switched to acetonitrile and flow rate was increased to 0.4 mL/min for 0.8 min to remove any carryover within the injector pumps and pumping system. At 2.9 min, the system was returned to the initial conditions of water with 1 g/L ammonium acetate with a flow rate 30 μL/min. The total runtime was 3.0 min.
Figure 2.
Separation of cocaine and it metabolites using differential mobility spectrometry (DMS) in 100 ng/mL calibrator. CoV was ramped from −35 V to −6 V in 1 min.
Table I.
MRM conditions and compensation voltages for cocaine and its metabolites
| Compound | Transition ions (m/z) | DP (V) | CE (V) | CoV (V) | Elution time (Min) |
|---|---|---|---|---|---|
| COC | 304.1 > 182.2 | 38 | 28 | −15.5 | 0.73 |
| COC-d3 | 307.1 > 185.2 | 38 | 28 | −15.5 | 0.73 |
| BE | 290.1 > 168.1 | 37 | 28 | −19.8 | 0.60 |
| 290.1 > 105.1 | 37 | 35 | −19.8 | 0.60 | |
| BE-d8 | 298.1 > 171.1 | 37 | 28 | −19.8 | 0.60 |
| 398.1 > 110.1 | 37 | 35 | −19.8 | 0.60 | |
| EME | 200.1 > 182.2 | 35 | 26 | −31.4 | 0.18 |
| 200.1 > 82.1 | 35 | 39 | −31.4 | 0.18 | |
| EME-d3 | 203.1 > 185.2 | 35 | 26 | −31.4 | 0.18 |
| 203.1 > 85.1 | 35 | 39 | −31.4 | 0.18 | |
| CE | 318.1 > 196.2 | 45 | 25 | −13.2 | 0.81 |
| 318.1 > 82.2 | 45 | 50 | −13.2 | 0.81 | |
| CE-d8 | 326.1 > 204.1 | 45 | 25 | −13.2 | 0.81 |
| 326.1 > 85.2 | 45 | 50 | −13.2 | 0.81 |
DP, deprotonation energy; CE, collision energy CoV, compensation voltage.
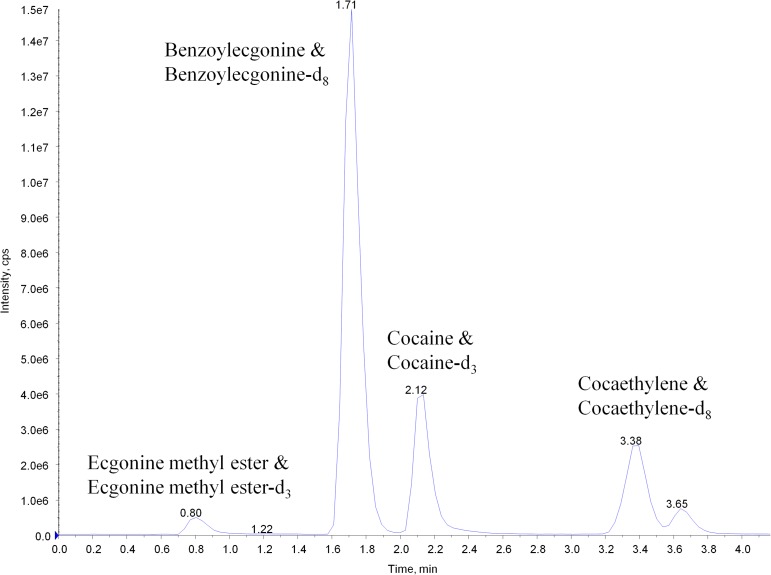
Ultra-high performance chromatography spectrometry–mass spectrometry
The initial analysis of samples used in the comparison study was analyzed by a UPLC–MS-MS method routinely employed by our laboratory for analysis. This analysis was performed separately and not part of an in-line system with the DMS–MS-MS. In brief, samples were prepared as stated above and analyzed on the QTRAP 6500+ with an IonDrive Turbo V source for TurbolonSpray® attached to the Shimadzu Nexera X2 ultra-high performance liquid chromatography system. Chromatographic separation of cocaine, its metabolites and the ISTDs was performed using a Thermo Hypersil Gold column, 50 × 2.1 mm, 3 micron (Thermofisher Scientific, USA) (Figure 3). The mobile phase was water/methanol (40:60, v/v) with 0.1 mM ammonium formate and was delivered at a flow rate of 1 mL/min. The source temperature was set at 600 °C, and curtain gas had a flow rate of 30 mL/min. The ion-spray voltage was 5,500 V, with the ion source gases 1 and 2 having flow rates of 60 and 45 mL/min, respectively. The acquisition mode used was MRM. The transition ions and collision energies (CE) monitored were the same as those in Table I. The total runtime was 4.25 min.
Figure 3.
UPLC–MS-MS total ion chromatograph of 100 ng/mL calibrator.
Method validation procedure
The evaluation of the assay was conducted over 5 days. Sample batches were analyzed as recommended for biomedical assay validation for linearity, lower limit of quantitation (LOQ), accuracy/bias, precision, carryover, interferences and stability. Validation sample batches contained calibrators, compound free controls (negative control) with ISTD added and a double negative control neither containing drugs, drug metabolites, nor ISTDs. Aliquots of QC specimens were analyzed in triplicate on each day. QC specimens were prepared with the following target values: limit of quantitation quality control (LOQC), 10 ng/mL; low control (LQC), 30 ng/mL; medium control (MQC), 300 ng/mL; high control (HQC), 750 ng/mL. All QC specimens were stored at −20°C until testing.
Linearity, limit of quantitation
Linearity of the assay was verified from seven-point calibration curves with the following concentrations: 10, 20, 50, 100, 200, 500, 1,000 ng/mL. A linear regression of the ratio of the peak area counts of the compound to that of the ISTDs versus corresponding concentration ratios was used to construct the calibration curves. The lower limit of quantitation (LOQ) was administratively set at 10 ng/mL. LOQC samples were used to verify that the LOQ was within ±20% of the target value and had a response at least ten times greater than the signal to noise ratio of the blank.
Bias and precision
Bias and precision were determined from the prepared QC samples (LOQ, MQC, HQC). QC samples were analyzed in triplicate each run over five different analytical runs. Acceptable bias did not exceed ±20% at each concentration. Two types of precision were assessed during the validation: intra-day and inter-day. The coefficient of variation (% CV) was calculated and ensured it did not exceed 20% at each concentration.
Carryover
Sample carryover of cocaine and its metabolites was evaluated in each of the five validation batches using two different procedures. First, immediately following the injection of the highest calibrator (1,000 ng/mL) a negative control (drug-free) was injected. Second, an injection of HQC (750 ng/mL) was immediately followed by injection of the LQC (10 ng/mL). This procedure was routinely applied each time the high calibrator, HQC and LQC samples were analyzed.
Interference studies
Matrix interferences and stable-isotope ISTD interferences were evaluated using 10 different lots of cocaine and metabolites-free expired serum obtained from the Virginia Commonwealth University Health System Department of Pathology. Each individual lot was analyzed with and without ISTD to ensure that endogenous serum components or the stable-isotope ISTD did not interfere with the assay. Cocaine and metabolite-free serum specimens were also fortified with general chemistry analytes and common therapeutic drugs (Bio-Rad controls).
Stability
Stability of cocaine, BE, EME and CE in serum was determined under several specific conditions and time intervals. The experiments were performed using two of the control specimens: LQC and HQC. All studies included three replicate analyses of each QC specimen. Serum specimens were stored frozen and thawed for re-analysis. QC specimens stored at −20°C were put through three freeze/thaw cycles with the last freeze cycle lasting 24 h. They were then prepared and quantitated against freshly prepared calibrators. The “bench-top” stability of cocaine and its metabolites was assessed to evaluate the possible effects of specimen transportation and processing in the laboratory by having the QC specimens sit at room temperature for 72 h. They were then prepared and quantitated against freshly prepared calibrators. The “post-preparative” stability of the analytes was evaluated by having extracts sit in the UPLC–MS-MS’s autosampler. A batch of the extracted LQC, MQC and HQC were quantitated against a freshly prepared calibration curve. The extracted controls were then allowed to sit in the autosampler for 72 h at 4°C after which they were re-injected and quantitated from the initial calibration. The results of the initial analysis were compared to those of the re-injected samples.
Application of the method
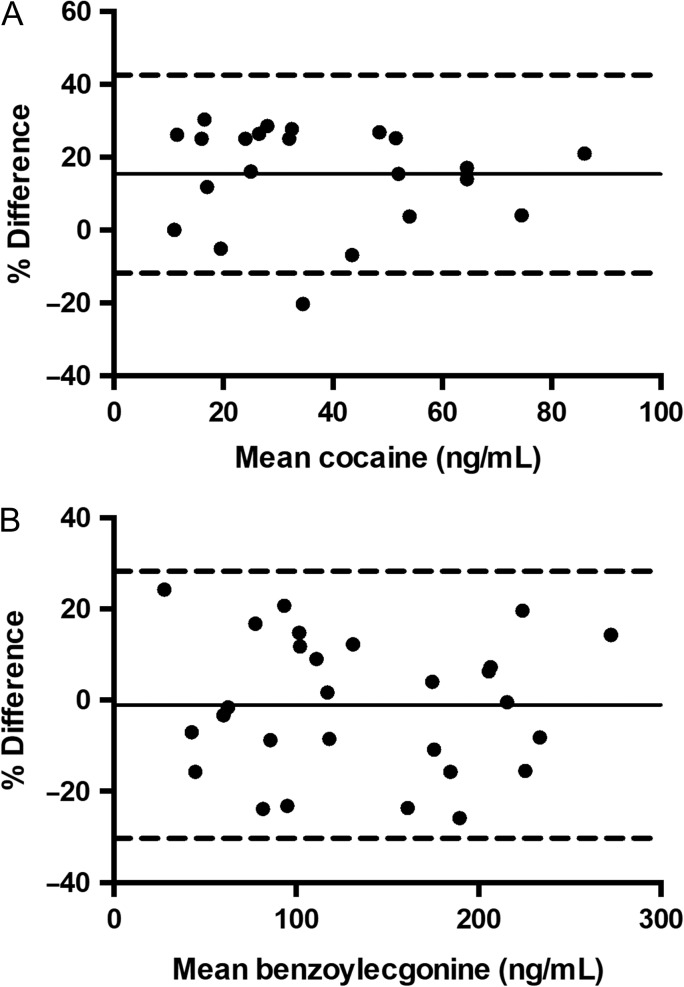
A total of 33 cocaine and/or BE positive serum samples were collected and analyzed as part of the Virginia Commonwealth University institutional review board approved study number HM20003768. Samples were analyzed by the presented method and the described routinely used UPLC–MS-MS method. These serum samples had concentrations ranging from not detected (ND) to ≤100 ng/mL for cocaine, 10 ng/mL to ≤300 ng/mL for BE, ≤10 ng/mL for EME and ND for CE. Quantitative comparison was performed on cocaine and BE concentrations determined by DMS–MS-MS and UPLC–MS-MS using the Bland–Altman analysis.
Results
The validation yielded linear regression correlation coefficients (r2) for the all the calibration curves in the five batches ranged from 0.9939 to 0.9997 for cocaine, 0.9942 to 0.9998 for BE, 0.9912 to 0.9991 for EME and 0.9960 to 0.9999 for CE. The LOQC samples (10 ng/mL) were within ±20% of the target value and had a response at least ten times greater than the signal to noise ratio of drug-free serum. Bias for cocaine and its metabolites did not exceed ±20% at each concentration. Intra-day precision (%CV) ranged from 0% to 15% for cocaine, 1% to 19% for BE, 1% to 17% for EME and 0% to 18% for CE. Intday precision ranged from 9% to 14% for cocaine, 2% to 17% for BE, 5% to 11% for EME and 5% to 15% for CE. Ten different lots of cocaine and metabolite-free serum were analyzed with and without ISTDs to assess possible interferences. No peaks were detected that co-eluted with cocaine, BE, EME, CE or with the ISTDs. Cocaine and metabolite-free serum specimens were also fortified with common therapeutic drugs and general chemistry analytes. None of the compounds tested were found to interfere with the assay. Lack of carryover was confirmed as cocaine, BE, EME or CE were not detected in the negative control after analysis of the highest calibrator. Lack of carryover was also confirmed for cocaine and its metabolites as the analyses of the LQC samples did not demonstrate a significant quantitated bias of more than 20% after analyses of the HQC. The stability of cocaine and its metabolites were evaluated at low (30 ng/mL) and high (750 ng/mL) concentrations. The results of these stability tests demonstrated that cocaine, BE, EME or CE in serum were stable under the conditions of the freeze-thaw and post-preparative studies. In these studies, cocaine and its metabolites’ concentrations were within ±20% of the target values. Cocaine and its metabolites were determined to be unstable at bench-top conditions, which was consistent with previous findings (5). The presented DMS–MS-MS method was used to quantify cocaine and its metabolites in human serum samples that were previously analyzed by the described UPLC–MS-MS method and Bland–Altman analysis was performed. The agreement between the two methods was plotted as % difference as a function of mean concentration. % Difference was calculated as (100*(A−B)/average), where A is DMS–MS-MS determined concentrations and B is UPLC–MS-MS determined concentrations (Figure 4). Variability between the two methods was 30% or less for cocaine (Figure 4A) and 25% or less for BE (Figure 4B). Bland–Altman analysis showed a mean bias between DMS–MS-MS and UPLC–MS-MS of 15.3 ng/mL for cocaine and −1.0 ng/mL for BE. EME was found in concentrations ≤10 ng/mL, and CE was not detected in any of the samples. No positive CE samples were available for validation of this DMS–MS-MS method.
Figure 4.
Bland–Altman plots of cocaine (A) and benzoylecgonine (B) determined by DMS–MS-MS and UPLC–MS-MS. Dashed lines, upper and lower 95% limits of agreement (average % difference ± 1.96 SD of the % difference); Solid line, mean bias.
Discussion
Rapid methods are needed for the identification and quantitation of cocaine and its metabolites, BE, EME and CE, in biological specimens by clinical and forensic toxicology laboratories. The presented method provided rapid separation of cocaine and its metabolites with minimal sample preparation and no column chromatography. Previously published methods implementing DMS for its separation power use more time-consuming sample preparation and present limited validation data. To the authors’ knowledge, this is the first reported validated method using minimal sample preparation and DMS–MS-MS to quantitate cocaine and its metabolites in human serum. Samples were prepared by a simple acetonitrile precipitation, and cocaine and its metabolites were separated using DMS in a period of 1 min by ramping the CoV while monitoring MRM transitions for each compound. Only one transition was used to monitor cocaine due to interferences of other transition ions. Use of a different infusion system would allow for shorter overall run times as the DMS–MS-MS analysis time was only 1 min. Even with the additional time due to the injection system, the method was shown to be an improved alternative to traditional LC–MS-MS methods as no analytical column was needed, minimal solvent was used per injection and the DMS–MS-MS analysis time was only 1 min.
Conclusion
A DMS–MS-MS separation and quantitation method was developed for cocaine and its metabolites BE, EME and CE in human serum. The method provided rapid determination of the analytes without extensive sample preparation or column chromatography. The method demonstrates that DMS-based separation methods combined with mass spectrometry for quantitation can provide a viable platform for high-throughput analysis for a variety of drugs of abuse and their metabolites commonly analyzed by clinical and forensic toxicology laboratories.
Funding
This work was supported by National Institutes of Health (NIH) grants P30DA033934, U54DA03899, and National Institute on Drug Abuse (NIDA) grant T32DA007027-42.
References
- 1. Langman L.J., Bjergum M.W., Williamson C.L., Crow F.W. (2009) Sensitive method for detection of cocaine and associated analytes by liquid chromatography-tandem mass spectrometry in urine. Journal of Analytical Toxicology, 33, 447–455. [DOI] [PubMed] [Google Scholar]
- 2. Jagerdeo E., Montgomery M.A., Lebeau M.A., Sibum M. (2008) An automated SPE/LC/MS/MS method for the analysis of cocaine and metabolites in whole blood. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 874, 15–20. [DOI] [PubMed] [Google Scholar]
- 3.(2017) Overdose Death Rates. National Institute on Drug Abuse. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
- 4. Wilson L.D., Jeromin J., Garvey L., Dorbandt A. (2001) Cocaine, ethanol, and cocaethylene cardiotoxity in an animal model of cocaine and ethanol abuse. Academic Emergency Medicine, 8, 211–222. [DOI] [PubMed] [Google Scholar]
- 5. Klingmann A., Skopp G., Aderjan R. (2001) Analysis of cocaine, benzoylecgonine, ecogonine methyl ester, and ecgonine by high-pressure liquid chromatography-API mass spectrometry and application to a short-term degradation study of cocaine in plasma. Journal of Analytical Toxicology, 25, 425–430. [DOI] [PubMed] [Google Scholar]
- 6. Lin S.N., Moody D.E., Bigelow G.E., Foltz R.L. (2001) A validated liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry method for quantitation of cocaine and benzoylecgonine in human plasma. Journal of Analytical Toxicology, 25, 497–503. [DOI] [PubMed] [Google Scholar]
- 7. Chen X., Zheng X., Ding K., Zhou Z., Zhan C.G., Zheng F. (2017) A quantitative LC-MS/MS method for simultaneous determination of cocaine and its metabolites in whole blood. Journnal of Pharmaceutical Biomedical Analysis, 134, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buryakov I.A. (2011) Russian publications (1991–2010) devoted to ion mobility spectrometry. Journal of Analytical Chemistry, 66, 1095–1102. [Google Scholar]
- 9. Schneider B.B., Nazarov E.G., Londry F., Vouros P., Covey T.R. (2016) Differential mobility spectrometry/mass spectrometry history, theory, design optimization, simulations, and applications. Mass Spectrometry Reviews, 35, 687–737. [DOI] [PubMed] [Google Scholar]
- 10. Hall A.B., Coy S.L., Nazarov E.G., Vouros P. (2012) Rapid separation and characterization of cocaine and cocaine cutting agents by differential mobility spectrometry-mass spectrometry. Journal of Forensic Science, 57, 750–756. [DOI] [PubMed] [Google Scholar]
- 11. Schneider B.B., Covey T.R., Coy S.L., Krylov E.V., Nazarov E.G. (2010) Planar differential mobility spectrometer as a pre-filter for atmospheric pressure ionization mass spectrometry. International Journal of Mass Spectrometry, 298, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varesio E., Le Blanc J.C., Hopfgartner G. (2012) Real-time 2D separation by LC x differential ion mobility hyphenated to mass spectrometry. Analytical and Bioanalytical Chemistry, 402, 2555–2564. [DOI] [PubMed] [Google Scholar]
- 13. Purves R.W. (2013) Enhancement of biological mass spectrometry by using separations based on changes in ion mobility (FAIMS and DMS). Analytical and Bioanalytical Chemistry, 405, 35–42. [DOI] [PubMed] [Google Scholar]
- 14. Ayodeji I., Vazquez T., Bailey R., Evans-Nguyen T. (2017) Rapid pre-filtering of amphetamine and derivatives by direct analysis in real time (DART)-differential mobility spectrometry (DMS). Analytical Methods, 9, 5044–5051. [Google Scholar]
- 15. Hall A.B., Coy S.L., Kafle A., Glick J., Nazarov E., Vouros P. (2013) Extending the dynamic range of the ion trap by differential mobility filtration. Journal of the American Society for Mass Spectrometry, 24, 1428–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parson W.B., Schneider B.B., Kertesz V., Corr J.J., Covey T.R., Van Berkel G.J. (2011) Rapid analysis of isomeric exogenous metabolites by differential mobility spectrometry-mass spectrometry. Rapid Communications in Mass Spectrometry, 25, 3382–3386. [DOI] [PubMed] [Google Scholar]
- 17. Li H., Giles K., Bendiak B., Kaplan K., Siems W.F., Hill H.H. Jr. (2012) Resolving structural isomers of monosaccharide methyl glycosides using drift tube and traveling wave ion mobility mass spectrometry. Analytical Chemistry, 84, 3231–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Porta T., Varesio E., Hopfgartner G. (2013) Gas-phase separation of drugs and metabolites using modifier-assisted differential ion mobility spectrometry hyphenated to liquid extraction surface analysis and mass spectrometry. Analytical Chemistry, 85, 11771–11779. [DOI] [PubMed] [Google Scholar]
- 19. Hall A.B., Coy S.L., Nazarov E., Vouros P. (2012) Development of rapid methodologies for the isolation and quantitation of drug metabolites by differential mobility spectrometry–mass spectrometry. International Journal for Ion Mobililty Spectrometry, 15, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bocos-Bintintan V., Moll V.H., Flanagan R.J., Thomas C.L.P. (2010) Rapid determination of alcohols in human saliva by gas chromatography differential mobility spectrometry following selective membrane extraction. International Journal for Ion Mobililty Spectrometry, 13, 55–63. [Google Scholar]
- 21. Singh G., Arora V., Fenn P.T., Mets B., Blair I.A. (1999) A validated stable isotope dilution liquid chromatography tandem mass spectrometry assay for the trace analysis of cocaine and its major metabolites in plasma. Analytical Chemistry, 71, 2021–2027. [DOI] [PubMed] [Google Scholar]