TLR4 has a limited role in CRF’s activation of the CeA under basal conditions, but interacts with the CRF system to regulate GABAergic synapse function in animals that experience repeated psychological stress.
Abstract
Aims
Stress induces neuroimmune responses via Toll-like receptor 4 (TLR4) activation. Here, we investigated the role of TLR4 in the effects of the stress peptide corticotropin-releasing factor (CRF) on GABAergic transmission in the central nucleus of the amygdala (CeA) following restraint stress.
Methods
Tlr4 knock out (KO) and wild-type rats were exposed to no stress (naïve), a single restraint stress (1 h) or repeated restraint stress (1 h per day for 3 consecutive days). After 1 h recovery from the final stress session, whole-cell patch-clamp electrophysiology was used to investigate the effects of CRF (200 nM) on CeA GABAA-mediated spontaneous inhibitory postsynaptic currents (sIPSCs).
Results
TLR4 does not regulate baseline GABAergic transmission in the CeA of naive and stress-treated animals. However, CRF significantly increased the mean sIPSC frequencies (indicating enhanced GABA release) across all genotypes and stress treatments, except for the Tlr4 KO rats that experienced repeated restraint stress.
Conclusions
Overall, our results suggest a limited role for TLR4 in CRF’s modulation of CeA GABAergic synapses in naïve and single stress rats, though TLR4-deficient rats that experienced repeated psychological stress exhibit a blunted CRF cellular response.
Short Summary
TLR4 has a limited role in CRF’s activation of the CeA under basal conditions, but interacts with the CRF system to regulate GABAergic synapse function in animals that experience repeated psychological stress.
INTRODUCTION
The neuroimmune system plays an important role in the homeostatic regulation of brain physiology (Pribiag and Stellwagen, 2014; Becher et al., 2017), and its inflammatory responses have been implicated in numerous psychiatric disorders (major depressive disorder, post-traumatic stress disorder (PTSD), schizophrenia, substance use disorders) (Liu et al., 2014). Many of these disorders involve stress-related pathologies (Zorn et al., 2017), and a growing body of evidence indicates that acute stressors can induce a submaximal activation of the neuroimmune system (Calcia et al., 2016; Wohleb and Delpech, 2017), which can lead to long-term sensitization of the brain’s stress response to subsequent challenges (Frank et al., 2016). Stress also plays an important role in alcohol use disorders, particularly in alcohol drinking. Alcohol has anxiolytic properties and is often used as ‘self-medication’ to cope with stress in non-dependent individuals (Leeies et al., 2010), while stress is a primary trigger for alcohol craving and relapse in alcoholic patients (Blaine and Sinha, 2017).
There are several common mechanisms mediating the cellular and behavioral effects of alcohol and stress in the central nervous system (CNS), including neuropeptides (e.g. corticotropin-releasing factor (CRF), neuropeptide Y), neurotransmitters (e.g. γ-aminobutyric acid (GABA), norepinephrine) and the neuroimmune system (e.g. interleukin-1β (IL-1β), Toll-like receptor 4 (TLR4)) (Akira and Takeda, 2004; Palsson-Mcdermott and O’neill, 2004). Moreover, several studies have shown that pharmacological interventions or transgenic manipulations that target the immune system can ameliorate stress-induced (Breese et al., 2008; Caso et al., 2008; Garate et al., 2013) and alcohol-related (Alfonso-Loeches et al., 2010; Wu et al., 2011, 2012; Bajo et al., 2015; Blednov et al., 2015, 2017a, 2017b; Marshall et al., 2016) changes at the molecular, cellular and behavioral levels.
Importantly, the stress-induced immune response is initiated by TLR4 activation (Liu et al., 2014). Specifically, TLR4 activates the innate immune system, both peripherally and within the CNS, in response to endogenous danger-associated molecular patterns (DAMPs, such as high mobility group box 1 (HMGB1), S100, heat shock proteins (HSP)) and exogenous microbe-associated molecular patterns (MAMPs, such as lipopolysaccharide (LPS)) (Akira and Takeda, 2004). TLR4 activation also triggers the production of several neuroimmune mediators, including type I interferons and cytokines (e.g. tumor necrosis factor α (TNFα), IL-1β, IL-6) (Akira and Takeda, 2004), and as such, plays a critical role in the regulation of the brain’s responses to both stress and inflammatory stimuli (Caso et al., 2008). Moreover, stress increases brain expression of several TLRs, including TLR4 (Garate et al., 2013; Tang et al., 2017), and both TLR4 and TLR2 mediate stress-induced priming of the neuroimmune system to subsequent challenge (Caso et al., 2008; Weber et al., 2013).
TLR4 activation has been hypothesized to promote excessive alcohol drinking, as TLR4 levels are increased in the brains of both human alcoholics and ethanol-dependent rats (Crews et al., 2013). In humans, TLR4 brain expression is also correlated with the age of drinking onset and lifetime alcohol consumption (Crews and Vetreno, 2015), while LPS and cytokine serum concentrations are correlated with the cravings of alcohol-dependent patients (Leclercq et al., 2012, 2014). In rodents, TLR4 activation (via LPS injection) increased the ethanol intake of mice (Blednov et al., 2011). However, recent findings do not fully support a critical role for TLR4 in ethanol drinking (Alfonso-Loeches et al., 2010; Harris et al., 2017; Blednov et al., 2017b). Mice lacking functional TLR4 or systemically administered (+)-naltrexone, a TLR4 inhibitor, did not alter their ethanol intake (Alfonso-Loeches et al., 2010; Harris et al., 2017; Blednov et al., 2017b). In addition, although systemic administration of another TLR4 antagonist, T5342126, decreased the ethanol consumption and preference for ethanol in mice, these changes were likely due to nonspecific effects, as evidenced by T5342126-related reductions in motor activity, saccharin intake (a more general reward-related behavior), and body core temperature (Bajo et al., 2016). Nonetheless, brain region-selective knockdown of TLR4 in the central nucleus of the amygdala (CeA) and paraventricular nucleus (PVN), but not the ventral tegmental area (VTA), of alcohol-preferring (P) rats decreased their binge drinking via a GABAA receptor mechanism (Liu et al., 2011; June et al., 2015). Notably, alcohol consumption in these same P rats increased CeA expression of the stress peptide CRF, which in turn increased local TLR4 expression (June et al., 2015).
Given these recent findings and the prominence of the CeA CRF system in stress and anxiety (Gilpin et al., 2015), we hypothesized that TLR4 signaling plays a role in CRF’s modulation of GABA transmission in the CeA after psychological stress. The CeA is primarily GABAergic (>95% of cell bodies) (Alheid, 2003), and we have previously reported that CRF enhances GABA release to a similar extent in the CeA of naive rats and rats that have undergone restraint stress, although restraint stress also reduces the baseline expression of type 1 CRF receptors (CRF1) (Ciccocioppo et al., 2014). In addition, we have shown that TLR4 activation enhances CeA GABA transmission (Bajo et al., 2014), while TLR4 deletion (i.e. TLR4-deficient rats) or antagonism slightly reduces it or has no effect, respectively (Harris et al., 2017). In the present study, TLR4-deficient rats were exposed to single or repeated restraint stress sessions, and CRF-induced CeA cellular responses were assessed. Our results suggest a limited role for TLR4 in CRF’s modulation of CeA GABAergic transmission in naïve rats and rats exposed to a single stress session; however, TLR4-deficient rats that experienced repeated stress sessions exhibited a blunted CRF cellular response.
MATERIALS AND METHODS
Animals
We used 3–7 months old adult male TLR4-deficient rats (Tlr4 KO) and their littermate wild-type (WT) rats (weight: Tlr4 KO: 522.3 ± 20.6 g; WT: 543.2 ± 26.6 g). The Tlr4 KO rat line is on a Wistar background harboring a nonfunctional Tlr4 gene, as described previously (Ferguson et al., 2013; Harris et al., 2017). Heterozygous (HET) breeding pairs were produced at the University of Pittsburgh and shipped to The Scripps Research Institute for breeding. In this study, we used WT control (n = 12), and homozygous KO (n = 13) littermates produced from HET pairs. Offspring were weaned at 21–28 days of age and genotyped by the Genotyping Center of America (Ellsworth, ME). The rats were housed in a temperature- and humidity-controlled room (6 am–6 pm lights on) with food and water available ad libitum. All care procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the Institutional Animal Care and Use Committee policies of The Scripps Research Institute.
RESTRAINT STRESS
We exposed the Tlr4 KO and WT rats to restraint stress for a single session (1 h) or for three repeated sessions (1 h per day for 3 consecutive days). For each restraint session, the rats were placed in a vented Plexiglas tube fitted with a tail slot to prevent unnatural body posture. The rats were transferred back to their home cages after each stress session for recovery. After the final stress session, the rats recovered for 1 h in their home cage and were then sacrificed for the electrophysiological studies.
WHOLE-CELL RECORDINGS
We anesthetized the rats with 3–5% isofluorane and placed the isolated brains quickly into ice-cold oxygenated high-sucrose cutting solution (composition in mM: sucrose, 206; KCl, 2.5; CaCl2, 0.5; MgCl2, 7; NaH2PO4, 1.2; NaHCO3, 26; glucose, 5; HEPES, 5) gassed with 95% O2 and 5% CO2. We cut coronal slices (300 μm) containing the CeA using a Leica 1200S vibratome cutter (Leica Microsystems, Buffalo Grove, IL) and incubated them in artificial cerebrospinal fluid (ACSF; composition in mM: NaCl, 130; KCl, 3.5; NaH2PO4, 1.25; MgSO4·7H2O, 1.5; CaCl2, 2.0; NaHCO3, 24; glucose, 10) at 37°C for 30 min. The slices were then incubated at room temperature for a minimum of 30 min prior to their use.
We recorded spontaneous inhibitory postsynaptic currents (sIPSCs) mediated by GABAA receptors in the medial subdivision of the CeA using whole-cell voltage-clamp electrophysiology, as described previously (Ciccocioppo et al., 2014). Briefly, we visualized CeA neurons using infrared/DIC optics followed by digitization and image enhancement via an upright, fixed-stage Olympus microscope (Olympus Scientific Solutions Americas Corp, Waltham, MA) and a CCD camera (EXi Aqua, QImaging, Surrey, BC, Canada). For the recordings, we used borosilicate glass micropipettes (Warner Instruments, Hamden, CT and King Precision, Claremont, CA) filled with an internal solution containing (in mM): 145 KCl, 10 HEPES, 2 MgCl2, 0.5 EGTA, 2 ATP and 0.2 GTP (the latter two added fresh on the day of recording), pH 7.2–7.4, osmolarity 290–305 mOsm and with input resistances of 2.5–5 MΩ (access resistance <20 MΩ, compensated 60–80%). GABAA-sIPSCs were pharmacologically isolated by adding glutamatergic (20 μM DNQX, 30 μM DL-AP5) and GABAB (1 μM CGP 55845A) receptor blockers to the bath. We applied a maximal effective concentration of CRF (200 nM; Tocris, Ellisville, MO) (Roberto et al., 2010; Ciccocioppo et al., 2014) by adding a known concentration of a stock solution directly to the bath and we took all the measures before (baseline) and during 15 min of CRF superfusion. For data acquisition, we used the Multiclamp 700B and pClamp 10.2 software (Molecular Devices, Sunnyvale, CA).
DATA ANALYSIS AND STATISTICS
To analyze the data we used MiniAnalysis 5.1 software (Synaptosoft, Leonia, NJ). For statistical analyses, we used GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) and applied the one-sample t-test, two-tailed unpaired t-test, or two-way ANOVA, with statistical significance accepted at P < 0.05.
RESULTS
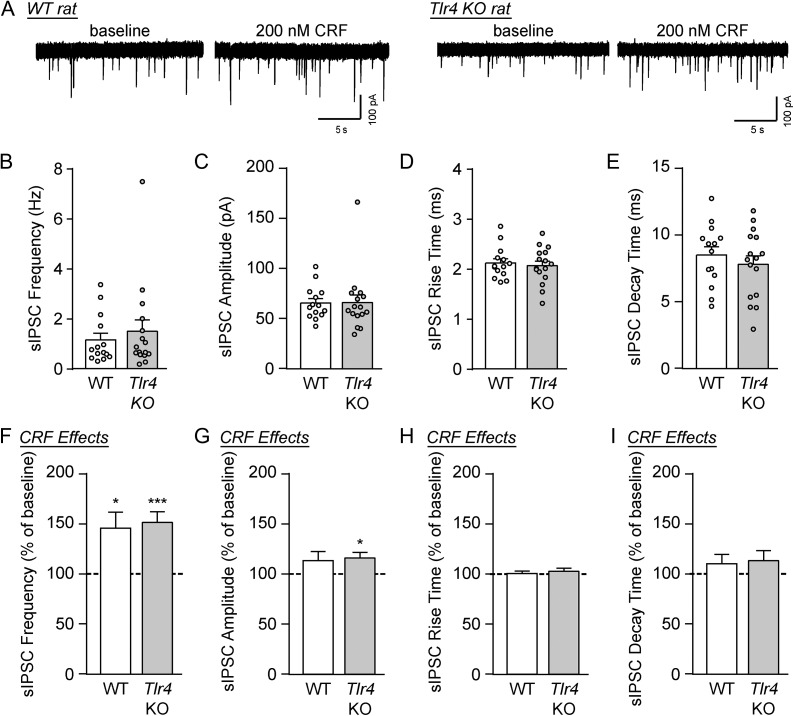
We recorded GABAA-mediated sIPSCs in the medial CeA of naïve WT and Tlr4 KO rats. There were no significant differences in baseline sIPSC frequencies, amplitudes, rise times and decay times of CeA neurons from naive WT and KO rats (unpaired t-test; Fig. 1A–E and Table 1), indicating that TLR4 does not regulate basal GABAergic transmission in this region.
Fig. 1.
CRF increased GABA transmission in the CeA of WT and Tlr4 KO rats. (A) Representative sIPSC traces from CeA neurons of WT and Tlr4 KO rats at baseline and during CRF (200 nM) superfusion. (B–E) Baseline sIPSC frequencies (B), amplitudes (C), rise times (D) and decay times (E) were similar across both genotypes (WT: 14 cells from three rats; Tlr4 KO: 16 cells from five rats). (F) CRF significantly increased the sIPSC frequencies in WT rats (12 cells from three rats; one-sample t-test, t(11) = 2.86, *P < 0.05) and Tlr4 KO rats (14 cells from five rats; t(13) = 4.84, ***P < 0.001). There was no difference in the magnitude of drug effects between groups (unpaired t-test, t(24) = 0.31, P = 0.76). (G) The mean sIPSC amplitude was increased by CRF in Tlr4 KO rats (one-sample t-test, t(13) = 3.01, *P < 0.05), though the drug’s effects were not significantly different across genotypes (unpaired t-test, t(24) = 0.27, P = 0.79). (H–I) CRF had no effect on sIPSC kinetics across both genotypes. All data are presented as mean ± SEM.
Table 1.
Basal GABAA-mediated sIPSC parameters in the CeA of WT and Tlr4 KO rats
| Genotype (# cells) | Frequency (Hz) | Amplitude (pA) | Rise time (ms) | Decay time (ms) | |
|---|---|---|---|---|---|
| Naive | WT (n = 14) | 1.15 ± 0.26 | 65.84 ± 4.44 | 2.12 ± 0.09 | 8.53 ± 0.62 |
| Tlr4 KO (n = 16) | 1.50 ± 0.45 | 66.18 ± 7.50 | 2.07 ± 0.09 | 7.83 ± 0.65 | |
| Single stress | WT (n = 12) | 1.37 ± 0.33 | 60.11 ± 4.74 | 2.33 ± 0.12 | 6.37 ± 0.61 |
| Tlr4 KO (n = 13) | 1.15 ± 0.20 | 78.03 ± 10.41 | 2.30 ± 0.13 | 7.70 ± 0.80 | |
| Repeated stress | WT (n = 15) | 1.14 ± 0.18 | 74.72 ± 12.98 | 2.35 ± 0.11 | 7.81 ± 0.73 |
| Tlr4 KO (n = 12) | 0.98 ± 0.10 | 72.63 ± 6.60 | 2.08 ± 0.12 | 9.36 ± 1.02 |
There were no significant differences in baseline sIPSC frequencies, amplitudes, rise times and decay times of CeA neurons from naïve and restraint stress WT and KO rats.
To investigate the potential role of the TLR4 system in CRF signaling in the CeA, we exogenously applied a maximally effective concentration (200 nM) of CRF (Roberto et al., 2010; Ciccocioppo et al., 2014) onto the recording cells for 15 min. CRF significantly increased the sIPSC frequency in naïve WT rats (to 146.1 ± 16.1% of baseline; one-sample t-test, t(11) = 2.86, P < 0.05) and KO rats (to 151.9 ± 10.7% of baseline; one-sample t-test, t(13) = 4.84, P < 0.001) to the same extent in both groups (unpaired t-test, t(24) = 0.31, P = 0.76; Fig. 1A and F). The mean sIPSC amplitude was significantly increased by CRF in Tlr4 KO rats compared to the pre-drug baseline (to 116.5 ± 5.5% of baseline; one-sample t-test, t(13) = 3.01, P < 0.05), but not in WT rats (113.8 ± 9.1%, P = 0.16); however, CRF’s effects were not significantly different between the two genotypes (unpaired t-test, t(24) = 0.27, P = 0.79; Fig. 1G). Finally, CRF had no effect on sIPSC kinetics (Fig. 1H–I). For these experiments, increases in sIPSC frequencies reflect increases in GABA release probabilities, while altered amplitudes and kinetics denote changed GABAA receptor function (Otis et al., 1994). Therefore, TLR4 does not mediate CRF’s facilitation of basal GABA release at CeA synapses.
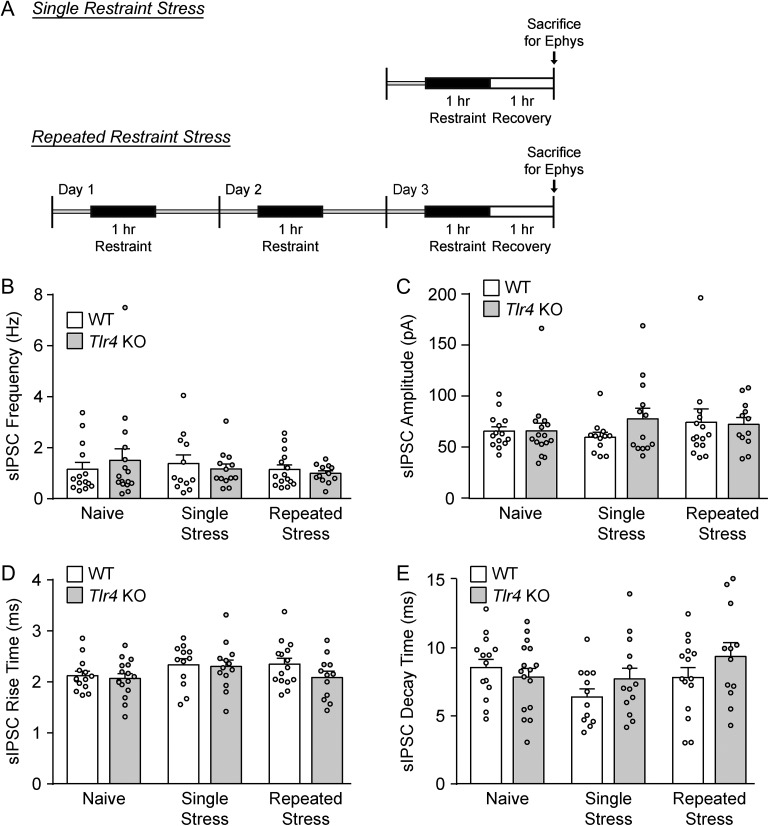
As TLR4 regulates stress-induced immune responses (Liu et al., 2014; Cheng et al., 2016) and can interact with CRF signaling in the CeA after alcohol exposure (June et al., 2015), we then examined the effects of CRF on CeA activity in Tlr4 KO and WT rats exposed to either single (1 h) or repeated (1 h per day for 3 consecutive days) restraint stress and a final 1 h recovery period (Fig. 2A). Restraint stress did not alter the baseline sIPSC properties of WT and KO rats compared to their naïve counterparts, as measured by two-way ANOVA (Fig. 2B–E and Table 1), indicating that these paradigms do not impact baseline CeA GABAergic transmission.
Fig. 2.
Restraint stress does not alter basal GABAergic transmission in the CeA of WT and Tlr4 KO rats. (A) Diagrams illustrating the single and repeated restraint stress paradigms. (B) The basal sIPSC frequency was not affected by restraint stress. Two-way ANOVA showed no significant main effects of genotype (F(1,76) < 0.001, P = 0.98) and restraint stress treatment (F(2,76) = 0.45, P = 0.64) on the basal sIPSC frequencies in the CeA of WT and Tlr4 KO rats, as well as no significant interaction between these factors (F(2,76) = 0.56, P = 0.57). (C) Neither genotype (F(1,76) = 0.57, P = 0.45) nor stress treatment (F(2,76) = 0.41, P = 0.67) had significant main effects on sIPSC amplitudes, and there was no significant interaction (F(2,76) = 0.75, P = 0.48). (D–E) There were no significant main effects of genotype (rise time: F(1,76) = 1.72, P = 0.19 and decay time: F(1,76) = 1.46, P = 0.23) or stress treatment (rise time: F(2,76) = 2.10, P = 0.13 and decay time: F(2,76) = 2.23, P = 0.11) on the sIPSC kinetics, and no significant interactions (rise time: F(2,76) = 0.66, P = 0.52 and decay time: F(2,76) = 1.48, P = 0.24). For all data presented in this figure, the WT/single stress group comprised 12 cells from four rats, Tlr4 KO/single stress group of 13 cells from four rats, WT/repeated stress group of 15 cells from five rats, Tlr4 KO/repeated stress group of 12 cells from four rats, and the naïve data were taken from Fig. 1B–E. All data are presented as mean ± SEM.
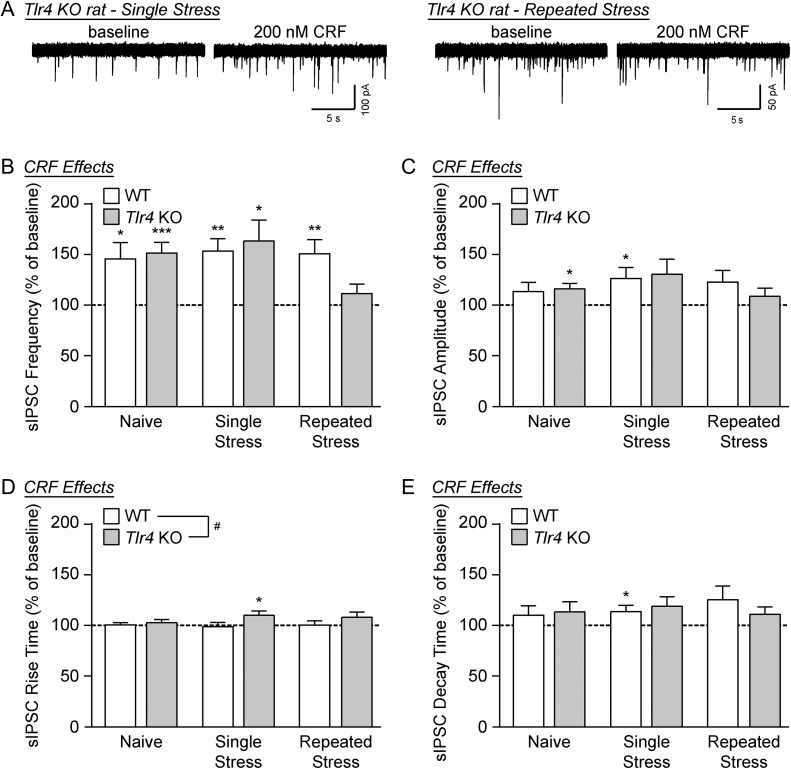
Bath application of CRF significantly increased the mean sIPSC frequencies compared to baseline across all genotype and treatment groups by one-sample t-test (WT/single restraint: t(11) = 4.40, P < 0.01; KO/single restraint: t(10) = 3.09; P < 0.05; WT/repeated restraint: t(14) = 3.70; P < 0.01), except for the Tlr4 KO rats that experienced repeated restraint stress (t(11) = 1.27, P = 0.23; Fig. 3A and B). Despite this within-group difference, a two-way ANOVA revealed no significant main effects of genotype or restraint stress on CRF’s facilitation of the mean sIPSC frequency, and no significant interaction. CRF also increased the sIPSC amplitude compared to baseline only in WT rats that experienced a single restraint session (one-sample t-test, t(11) = 2.49, P < 0.05), but two-way ANOVA revealed no significant effects of CRF on sIPSC amplitudes across all animal and treatment groups (Fig. 3C). Similarly, CRF increased the sIPSC rise and decay time in the CeA of single stress Tlr4 KO rats (one-sample t-test, t(10) = 2.38, P < 0.05; Fig. 3D) and single stress WT rats (one-sample t-test, t(11) = 2.25, P < 0.05; Fig. 3E), respectively. Comparison by two-way ANOVA revealed a significant main effect of genotype on CRF-induced sIPSC rise times (F(1, 70) = 4.59, P < 0.05), but no other significant main effects or interactions with regard to CRF’s effects on sIPSC kinetics. Overall these data suggest a limited role for TLR4 in CRF’s modulation of CeA GABA synapses, mainly on the postsynaptic side of transmission, in naïve rats and rats exposed to a single stress session; however, the TLR4-deficient rats that experienced repeated stress sessions exhibited a blunted CRF facilitation of GABA release.
Figure 3.
CRF facilitation of GABA release is reduced in the CeA of Tlr4 KO rats following repeated restraint stress. (A) Representative sIPSC traces recorded in the CeA of Tlr4 KO rats that experienced either single or repeated restraint stress, at baseline and during CRF (200 nM) superfusion. (B) CRF significantly increased sIPSC frequencies in all restraint stress-treated animals by one-sample t-test (WT/single stress: t(11) = 4.40, **P < 0.01; KO/single stress: t(10) = 3.09; *P < 0.05; WT/repeated stress: t(14) = 3.70; **P < 0.01), except for the repeated stress Tlr4 KO rats (t(11) = 1.27, P = 0.23). Despite this difference, two-way ANOVA across both genotypes (WT and Tlr4 KO) and all three treatment groups (naïve, single stress and repeated stress) showed no significant main effects (genotype: F(1,70) = 0.47, P = 0.50; stress treatment: F(2,70) = 1.94, P = 0.15), and no significant interaction (F(2,70) = 1.94, P = 0.15). (C) The sIPSC amplitude was also increased by CRF in single stress WT rats (one-sample t-test, t(11) = 2.49, *P < 0.05), but two-way ANOVA revealed no significant main effects of genotype (F(1,70) = 0.08, P = 0.78) or stress treatment (F(2,70) = 1.01, P = 0.35), and no interaction (F(2,70) = 0.50, P = 0.61). (D) CRF increased the sIPSC rise time in single stress Tlr4 KO rats (one-sample t-test, t(10) = 2.38, *P < 0.05). In addition, by two-way ANOVA there was a significant main effect of genotype (F(1,70) = 4.59, #P < 0.05) on CRF-induced rise times, which was associated with no main stress treatment effect (F(2,70) = 0.29, P = 0.75) and no stress x genotype interaction (F(2,70) = 0.62, P = 0.54). (E) The sIPSC decay time was also increased by CRF in single stress WT rats (one-sample t-test, t(11) = 2.25, *P < 0.05), but two-way ANOVA revealed no significant main effects of genotype (F(1,70) = 0.05, P = 0.82) or stress treatment (F(2,70) = 0.22, P = 0.80), and no interaction (F(2,70) = 0.58, P = 0.56). For all data presented in this figure, the WT/single stress group comprised 12 cells from four rats, Tlr4 KO/single stress group of 11 cells from four rats, WT/repeated stress group of 15 cells from five rats, Tlr4 KO/repeated stress group of 12 cells from four rats, and the naïve data was taken from Fig. 1F–I. All data are presented as mean ± SEM.
DISCUSSION
A growing body of evidence indicates that submaximal activation of the neuroimmune system, such as that triggered by psychological stress, can sensitize the brain’s response to subsequent challenges (Weber et al., 2013; Frank et al., 2016). Therefore, here we investigated the role of TLR4, a potent regulator of the innate immune system, on CRF-induced changes in GABAergic signaling in the CeA, with a particular focus on whether previous stress exposure primes cellular responses. We found that CRF facilitates CeA GABA release similarly in naïve and stress-treated WT and Tlr4 KO rats, with the exception of the repeated stress-treated Tlr4 KO rats where CRF’s effects were blunted. Thus, TLR4 signaling has a limited postsynaptic role in CRF’s activation of the CeA under basal conditions, but interacts with the CRF system to regulate GABAergic synapse function in animals that experience repeated psychological stress.
There are several lines of evidence implicating TLR4 in stress-induced CRF signaling in the brain. Most notably, the hypothalamic–pituitary–adrenal (HPA) axis comprises the body’s neuroendocrine stress response (Stephens and Wand, 2012), and its stimulation by TLR4 increased adrenal glucocorticoid secretion (Vakharia and Hinson, 2005; Kanczkowski et al., 2013), hypothalamic CRF gene expression (Singh and Jiang, 2004; Loum-Ribot et al., 2006) and serum CRF levels (Goebel et al., 2011). Moreover, early life stress (Tang et al., 2017) or LPS exposure (Mouihate et al., 2010) increased hypothalamic CRF expression in adult rodents and sensitized their pain and stress responses, respectively, indicating that TLR4 has long-term influence over the brain’s CRF signaling. Acute and chronic stressors also upregulate extra-hypothalamic CRF expression in several brain regions, including the CeA (Sterrenburg et al., 2011), where alcohol-induced activation of the CRF system increased local TLR4 expression (June et al., 2015). Surprisingly, here we found that repeated restraint stress did not alter CRF’s facilitation of GABA release in the CeA of WT rats (compared to their naïve counterparts), but blunted it in TLR4-deficient rats (compared to the naïve KO rats and the repeated stress WT rats). While these results in the WT rats (after both single and repeated stress restraint) match our previous findings (Ciccocioppo et al., 2014), the results of our KO studies indicate that repeated psychological stress reveals a novel role for TLR4 signaling in the CeA CRF system.
Notably, a parallel study in our laboratory examined the role of TLR4 in acute ethanol’s facilitation of CeA GABA transmission after LPS injection and found the opposite results; LPS treatment reduced ethanol’s actions in WT rats, but had no effect in the KO rats (Harris et al., 2017). It is, therefore, possible that our current findings in the TLR4-deficient rats reflect long-term compensatory changes in other TLR pathways (e.g. TLR2) or downstream TLR4 signaling (e.g. myeloid differentiation primary response 88 (MyD88), protein kinase B (Akt)). More likely, TLR4 is both temporally and spatially regulated, and our restraint stress paradigms may not have robustly activated it in WT rats (vs. its activation by LPS in our previous work (Harris et al., 2017)). In support of this possibility, Knapp et al. recently observed brain region-specific dynamic changes in TLR4 expression in rats recovering from a single 1 h restraint stress. They reported that cortical TLR4 mRNA levels were elevated 4 h (but not 2 h or 8 h) after the stress, and this increase was similar to that observed in the cortex of ethanol-withdrawn rats; but TLR4 gene expression was unchanged in the amygdala, hippocampus or hypothalamus at the 4 h post-stress time point, as well as after withdrawal (Knapp et al., 2016). Therefore, our overall findings suggest that single (1 h) and repeated restraint stress paradigms (1 h per day for 3 days) may not induce TLR4 signaling and do not produce adaptive changes in CeA GABAergic signaling in WT rats, similar to the effects of a more potent restraint stress paradigm (6 h per day for 10 days) (Reznikov et al., 2009). Thus, our data, in combination with the work of others, suggest that while moderate stress activates the HPA axis (Leggett et al., 2007) and induces brain region-specific gene expression (Wang et al., 2010; Knapp et al., 2016), a more severe stress exposure may be needed to robustly engage the TLR4 system and induce adaptive changes in CeA neurotransmitter (GABA) and neuropeptide (CRF) systems of WT rats.
In conclusion, here we report a role for TLR4 in CRF’s modulation of CeA spontaneous GABAergic transmission in naïve and single stress-treated rats, though TLR4-deficient rats that experienced repeated stress sessions exhibit a blunted CRF cellular response. Given these current findings and our previous observation that TLR4 activation reduces acute ethanol’s actions on CeA GABA signaling (Harris et al., 2017), we speculate that the TLR4 system may mediate a synergistic interaction between chronic alcohol exposure and stress in the CeA. Notably, Breese and colleagues report that in a protocol comprising three acute withdrawals from chronic ethanol exposure, either restraint stress or LPS injection can be substituted for the initial two withdrawal periods to produce an anxiogenic phenotype that is not observed after a single ethanol withdrawal, LPS injection or restraint stress session (Breese et al., 2008; Knapp et al., 2016). Therefore, a systematic determination of how common neuroimmune components, such as TLR4, independently regulate the cellular and behavioral effects of alcohol and stress, is critical to our overall understanding of the role of these neuroimmune factors and their therapeutic potentials in protecting against stress-induced relapse and reducing alcohol-stress disorder comorbidity (e.g. PTSD) in humans.
ACKNOWLEDGEMENTS
We thank Dr. Olivier George for the TLR4 rat colony maintenance. The authors declare no conflict of interest. This is TSRI manuscript number 29603.
FUNDING
Support for this study was provided by National Institute on Alcohol Abuse and Alcoholism (NIH/NIAAA) grants as part of Integrative Neuroscience Initiative on Alcoholism (INIA) Neuroimmune Consortium [U01 AA013498 (M.R.) and U01 AA020889 (G.E.H.)], NIH/NIAAA R01 AA015566 and AA006420 (M.R.), K99 AA025408 (F.P.V.), and T32 AA007456 (R.R.P. and M.Q.S.), as well as the Austrian Science Fund (FWF J-3942 to S.K.) and the Pearson Center for Alcoholism and Addiction Research.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, et al. (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 30:8285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF. (2003) Extended amygdala and basal forebrain. Ann NY Acad Sci 985:185–205. [DOI] [PubMed] [Google Scholar]
- Bajo M, Herman MA, Varodayan FP, et al. (2015) Role of the IL-1 receptor antagonist in ethanol-induced regulation of GABAergic transmission in the central amygdala. Brain Behav Immun 45:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Madamba SG, Roberto M, et al. (2014) Innate immune factors modulate ethanol interaction with GABAergic transmission in mouse central amygdala. Brain Behav Immun 40:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Montgomery SE, Cates LN, et al. (2016) Evaluation of TLR4 inhibitor, T5342126, in modulation of ethanol-drinking behavior in alcohol-dependent mice. Alcohol Alcohol 51:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Spath S, Goverman J (2017) Cytokine networks in neuroinflammation. Nat Rev Immunol 17:49–59. [DOI] [PubMed] [Google Scholar]
- Blaine SK, Sinha R (2017) Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, et al. (2015) Role of interleukin-1 receptor signaling in the behavioral effects of ethanol and benzodiazepines. Neuropharmacology 95:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, et al. (2011) Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun 25:S92–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Benavidez JM, et al. (2017. a) Sedative and motor incoordination effects of ethanol in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res 41:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Chernis J, et al. (2017. b) Ethanol consumption in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res 41:516–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, et al. (2008) Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology 33:867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia MA, Bonsall DR, Bloomfield PS, et al. (2016) Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 233:1637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, et al. (2008) Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and in the worsening of experimental stroke. Stroke 39:1314–20. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Pardo M, Armini RS, et al. (2016) Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun 53:207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, De Guglielmo G, Hansson AC, et al. (2014) Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci 34:363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, et al. (2013) High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry 73:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP (2015) Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl) 233:1543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson C, Mckay M, Harris RA, et al. (2013) Toll-like receptor 4 (Tlr4) knockout rats produced by transcriptional activator-like effector nuclease (TALEN)-mediated gene inactivation. Alcohol 47:595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, et al. (2016) Stress-induced neuroinflammatory priming: a liability factor in the etiology of psychiatric disorders. Neurobiol Stress 4:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garate I, Garcia-Bueno B, Madrigal JL, et al. (2013) Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry 73:32–43. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M (2015) The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77:859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, et al. (2011) Lipopolysaccharide increases plasma levels of corticotropin-releasing hormone in rats. Neuroendocrinology 93:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Bajo M, Bell RL, et al. (2017) Genetic and pharmacologic manipulation of TLR4 has minimal impact on ethanol consumption in rodents. J Neurosci 37:1139–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Liu J, Warnock KT, et al. (2015) CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology 40:1549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanczkowski W, Chatzigeorgiou A, Samus M, et al. (2013) Characterization of the LPS-induced inflammation of the adrenal gland in mice. Mol Cell Endocrinol 371:228–35. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Harper KM, Whitman BA, et al. (2016) Stress and withdrawal from chronic ethanol induce selective changes in neuroimmune mRNAs in differing brain sites. Brain Sci 6:E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, et al. (2012) Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun 26:911–8. [DOI] [PubMed] [Google Scholar]
- Leclercq S, De Saeger C, Delzenne N, et al. (2014) Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry 76:725–33. [DOI] [PubMed] [Google Scholar]
- Leeies M, Pagura J, Sareen J, et al. (2010) The use of alcohol and drugs to self-medicate symptoms of posttraumatic stress disorder. Depress Anxiety 27:731–6. [DOI] [PubMed] [Google Scholar]
- Leggett JD, Jessop DS, Fulford AJ (2007) The nociceptin/orphanin FQ antagonist UFP-101 differentially modulates the glucocorticoid response to restraint stress in rats during the peak and nadir phases of the hypothalamo-pituitary-adrenal axis circadian rhythm. Neuroscience 147:757–64. [DOI] [PubMed] [Google Scholar]
- Liu J, Buisman-Pijlman F, Hutchinson MR (2014) Toll-like receptor 4: innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Front Neurosci 8:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, et al. (2011) Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci USA 108:4465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loum-Ribot E, Lafon P, Chaigniau M, et al. (2006) Glucocorticoids down-regulate lipopolysaccharide-induced de novo production of neurotensin mRNA in the rat hypothalamic, paraventricular, corticotrophin-releasing hormone neurons. Neuroimmunomodulation 13:170–8. [DOI] [PubMed] [Google Scholar]
- Marshall SA, Casachahua JD, Rinker JA, et al. (2016) IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav Immun 51:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouihate A, Galic MA, Ellis SL, et al. (2010) Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J Neurosci 30:7975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I (1994) Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci USA 91:7698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson-Mcdermott EM, O’neill LA (2004) Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribiag H, Stellwagen D (2014) Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology 78:13–22. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR (2009) Effects of acute and repeated restraint stress on GABA efflux in the rat basolateral and central amygdala. Brain Res 1256:61–8. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, et al. (2010) Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry 67:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Jiang Y (2004) How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology 201:197–207. [DOI] [PubMed] [Google Scholar]
- Stephens MA, Wand G (2012) Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34:468–83. [PMC free article] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, et al. (2011) Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One 6:e28128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HL, Zhang G, Ji NN, et al. (2017) Toll-like receptor 4 in paraventricular nucleus mediates visceral hypersensitivity induced by maternal separation. Front Pharmacol 8:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakharia K, Hinson JP (2005) Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology 146:1398–402. [DOI] [PubMed] [Google Scholar]
- Wang K, Xiang XH, He F, et al. (2010) Transcriptome profiling analysis reveals region-distinctive changes of gene expression in the CNS in response to different moderate restraint stress. J Neurochem 113:1436–46. [DOI] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Sobesky JL, et al. (2013) Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain Behav Immun 32:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Delpech JC (2017) Dynamic cross-talk between microglia and peripheral monocytes underlies stress-induced neuroinflammation and behavioral consequences. Prog Neuropsychopharmacol Biol Psychiatry 79:40–8. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, et al. (2012) Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br J Pharmacol 165:1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, et al. (2011) Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun 25:S155–64. [DOI] [PubMed] [Google Scholar]
- Zorn JV, Schur RR, Boks MP, et al. (2017) Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology 77:25–36. [DOI] [PubMed] [Google Scholar]