Abstract
Electronic cigarettes (e-cigs) deliver nicotine in an aerosol to the user that simulates the smoke of traditional cigarettes purportedly without the pathology of inhaling tobacco smoke due to the absence of combustion. Advanced versions of e-cigs enable the user to potentially moderate the concentration of drug in the aerosol by selecting from a range of voltages on the power supply. A method was developed to trap the aerosol produced by a KangerTech AeroTank, 1.8 Ω preassembled atomizer in order to analyze the concentration of nicotine and to evaluate the constituents of the aerosol at various voltages on the power supply. A 12-mg/mL formulation of nicotine in 50:50 propylene glycol (PG):vegetable glycerin (VG) was used to produce aerosol at 3.9, 4.3 and 4.7 V. The aerosol was trapped in a simple glass assemblage and analyzed by a 3200 Q Trap HPLC–MS-MS. The dose of nicotine delivered in the aerosol at 3.9, 4.3 and 4.7 V was determined to be 88 ± 12 μg, 91 ± 15 μg and 125 ± 22 μg. The average recovery of nicotine in the trap across the voltages was 99.8%. The glass trap system was an effective device for collecting the aerosol for analysis and an increase in drug yield was observed with increasing voltage from the power supply on the e-cig. The glass trap system was also used in combination with a 100-μm solid-phase microextraction fiber to capture the aerosol and analyze it via DART–MS and GC–MS. Four commercial e-liquids labeled to contain nicotine were aerosolized at 4.3 V. The pharmacologically active ingredient, nicotine, as well as PG, VG and a number of flavoring agents found in these formulations were identified.
Introduction
Early versions of electronic cigarettes (e-cigs) were patented in 1930 to “hold and heat medicine” and in 1965 to provide a healthier alternative to inhale nicotine. The modern e-cig was patented in 2004 in China as a safer alternative to smoking after the death of the inventor’s father from lung cancer (1–5). Although these e-cigs were originally created as a nicotine delivery system, Drugs Other Than Nicotine (DOTNs) are readily available in commercial liquid formulations (e-liquids) (6–9). E-cigs became readily available in the USA between 2006 and 2007 and have since evolved. Early generations consisted of closed system that resembled a traditional cigarette and were known as “ciga-likes”. This system is a simple design containing a battery at a single-low voltage, a coil and an e-liquid which produces the aerosol. These systems are not refillable. The second-generation, mid-size pen-like e-cigarettes are customizable and have rechargeable batteries and refillable tanks. The third generation, advanced personal vaporizers or clearomizers (10–12), have customizable coil and wick configurations, batteries with adjustable settings and refillable tanks.
The components of e-cigs can be purchased separately from a myriad of web-based stores and easily assembled. The second and third generations of e-cigs have seen dramatic changes in the past few years, resulting in the fourth generation of e-cigs, the “Mod”. These devices allow for a variety of customizations to moderate drug dosage, including adjustable power supplies to change voltage, variable coil configurations at the posts to impact heat production and adjustable slits to manage airflow. A culture has arisen around just the craftsmanship involved in the wrapping of coils into “hives” (13, 14). These modifications have been anecdotally reported by users to improve the vaping experience by controlling the amount of aerosol that is produced and, thereby, controlling the amount of drug delivered.
The e-liquids can be purchased from commercial vendors or can be made by the user. E-liquids are typically comprised of propylene glycol (PG) and/or glycerin, nicotine and/or other pharmacological active ingredients and flavor additives (15). Vegetable glycerin (VG) and PG are hygroscopic and, once vaporized, will condense with the water in the atmosphere to produce an aerosol containing the pharmacologically active ingredients and flavor additives. Users choose a ratio of VG to PG based on their personal preferences. VG is used to enhance the appearance the aerosol, while PG adds body to the e-liquids and is believed to enrich the e-liquid flavors (15).
Previous studies have attempted to understand and describe nicotine delivery from e-cigs. First-generation e-cigs have been reported to have relatively low nicotine delivery, and some studies later established that a “learning curve” was associated with the use of second and third generation e-cigs. The users of the second and third generation e-cigs had increased plasma nicotine concentrations compared to those of first-generation e-cig users (16–18). Talih et al. observed an increase in nicotine yield as voltage of the e-cigs increased, but described the nicotine yield as complex because users can adjust puff duration, velocity and voltage output (19). Gillman et al. reported the mass of aerosol and the production of aldehydes, formaldehyde, acetaldehyde and acrolein changed depending on the e-cig and voltage used (20). Further, studies have highlighted that the nicotine concentration in commercial e-liquids do not always match the concentration advertised on the label (21–26). The difference in advertised vs. measured concentration is needed to determine an accurate nicotine aerosol concentration (21).
Solid-phase microextraction (SPME) has been shown to successfully extract nicotine from traditional cigarette smoke. Free-base nicotine from mainstream cigarette smoke and volatile organic compounds associated with traditional cigarette smoke have been successfully extracted by SPME for analysis (27–30). Consequently, SPME was used as an extraction method in the present study for the components of the e-cig generated aerosol. SPME is a one-step extraction technique that provides rapid sample preparation with little solvent use or sample preparation, resulting in a decrease in analysis time (31).
In order to assess the concentration of a drug in the aerosol from an in-house produced e-liquid at various voltage settings on an e-cig, a trap was developed to capture the aerosol produced by a mechanical puff from the e-cig and allow for the sampling of the aerosol by SPME. An e-liquid fortified with nicotine in-house and four commercially available nicotine containing e-liquids were assessed. A second-generation e-cig with easily modifiable components was chosen for this study. Modifiable components included the coil and wick configuration, a tank able to be refilled with the user’s choice of e-liquid, and a variable voltage power supply, which allowed for a range of voltages to ostensibly moderate aerosol production. The confirmation and quantitation of the nicotine in the captured aerosol was performed using high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS-MS). The analysis of the SPME captured constituents of the aerosol was performed using a DART ionization source coupled to a time-of-flight mass spectrometer (DART–MS) as well as by gas chromatography–mass spectrometry (GC–MS)
Experimental
Reagents and supplies
Free-base nicotine, nicotine and nicotine-d4 reference standards were purchased from Sigma-Aldrich (St. Louis, MO). USP grade PG and VG were purchased from Wizard Labs (Orlando, FL). The KangerTech AeroTank e-cig was purchased from 101vape.com (Carlsbad, CA). The 1.8 Ω KangerProTank & Evod Coil Replacement atomizers were purchased from Discount Vapers (Orlando, FL). Glassware, Tygon tubing with ¼-inch ID and fritted gas dispersion tubes were purchased from Colonial Scientific (Richmond, VA). The flow meter was purchased from Cole Parmer (Vernon Hills, IL). An in-house vacuum system was used for the aerosol generations. HPLC-grade methanol was purchased from Pharmco-Aaper (Brookfield, CT) and used for all dilutions and preparations of stock and working solutions. The HPLC-grade methanol, HPLC-grade water and ammonium formate comprising the HPLC mobile phase were purchased from Fisher Scientific (Pittsburgh, PA). Polyethylene glycol (PEG) with an average molecular mass of 600 Da was used for DART–MS calibration and obtained from ULTRA Inc. (North Kingstown, Rhode Island). The commercial e-liquids, purchased from various retail sources, were VapeWell Cheery, STLVapor Spearmint, Supreme Nicotine 258 Rally Squirrel and Fennet High Janty. The SPME fibers were purchased from Supelco (Bellefont, PA).
Electronic cigarette and puff topography
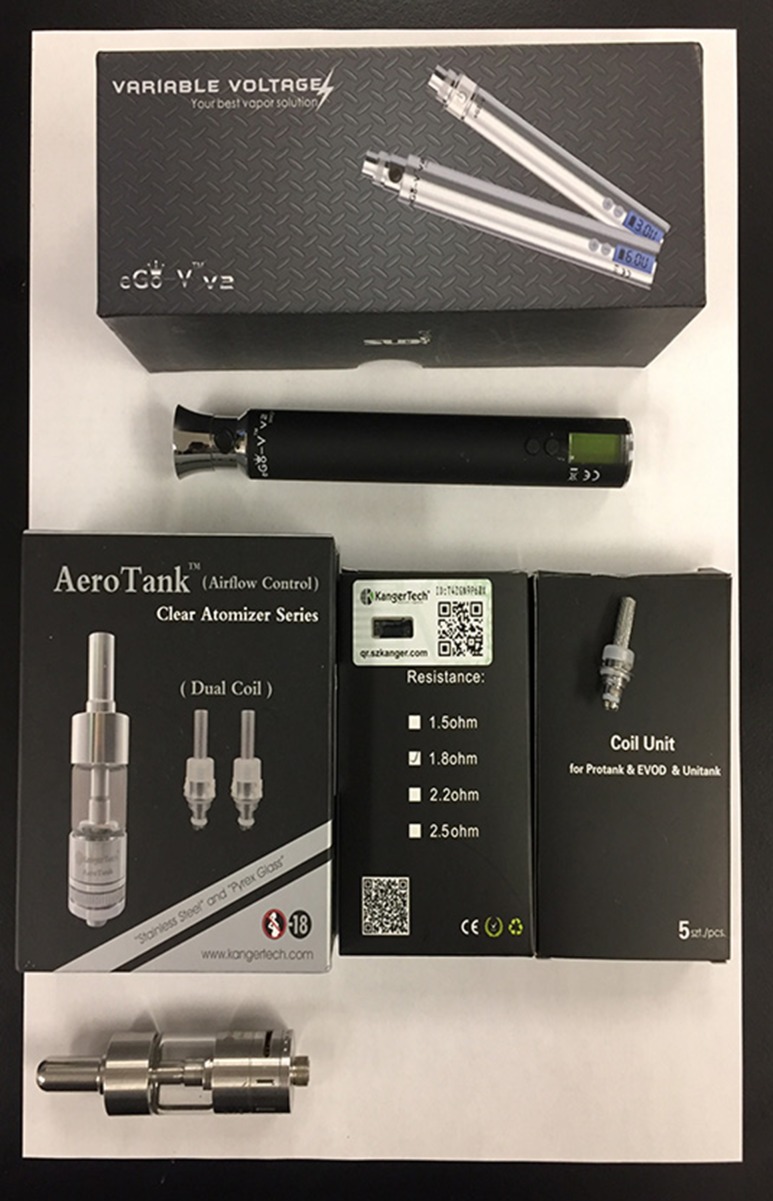
Based on recommendations from users and vendors for a popular, dependable and easily modifiable “mid-range” option to serve as an appropriate model for studies, the e-cig selected for this study was the KangerTech AeroTank with an eGo-V2 variable voltage power supply. This e-cigarette was considered “modifiable” in the fact that unit items available for purchase came with different options that the users could interchange with the device to modify the e-cigarette to their preferences (Figure 1). The KangerProtank atomizer contained a single coil that was wrapped in non-contact configuration with 34-gauge Nichrome wire to 1.8 Ω, and a 2-mm diameter silica string was used as a wick. The e-cig has a tank that allows the user to refill and reuse the atomizer. For this study, the tank was filled with 50:50 PG:VG with 12 mg/mL nicotine, which was formulated in-house. The “puff” duration (or time the activator button was depressed) was 4 s, and the interval between “puffs” was 20 s to allow for the atomizer to cool and the wick to re-saturate. The flow rate on the vacuum to pull the aerosol out of the atomizer was 2.3 L/min.
Figure 1.
The commercial products in the KangerTech series that can be used to modify the e-cigarette to the users preferences.
Aerosol trap
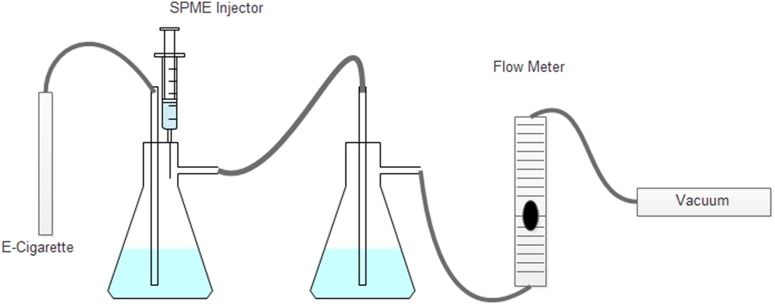
The aerosol trap consisted of tubing placed snugly over the mouthpiece of the atomizer and connected to two filtering flasks that were connected in tandem followed by a flow meter. Glass wool was packed between the two traps as a filter. Deionized (DI) water (150 mL) was placed in the reservoir of each trap and a gas dispersion tube bubbled the aerosol through the system at a flow rate of 2.3 L/min. The flow rate was set to 2.3 L/min as stated in a previously published method (22). This flow rate allowed the capture of the aerosol in the first trap without carryover into the second trap and no non-aerosolized e-liquid to be pulled out of the e-cigarette prior to aerosolization. A small inlet in the top of the first trap allowed for insertion of a SPME fiber (Figure 2). The trap was started 20 s before the e-cig was activated and the e-cig was used to aerosolize the e-liquid for 4 s at 3.5, 4.3 and 4.7 V to account for potential variability of nicotine concentration at all voltages. The four second puff duration is meant to mimic the puff duration of an average, experienced e-cig user (19). The e-cigarette tank was weighed before and after each activation of the e-cig in order to calculate recovery. Tubing, flask, dispersion tube and glass wool were rinsed with 100 mL of DI water. An aliquot of the water was collected for analysis, and the traps were emptied and washed for the next collection. The process was repeated with an e-cig filled with the nicotine enriched 50:50 PG:VG and 50:50 PG:VG, 0 mg/L nicotine to serve as a drug-free analysis.
Figure 2.
Diagram of trapping system to capture aerosol generated electronic cigarette.
Analysis of nicotine yields
The e-cig was activated for 4 s to mimic the “puff” topography of a user. The power supply was set at 3.9, 4.3 and 4.7 V, and the flow in the sample trap was set to 2.3 L/min. A different e-cig was committed to each voltage with same wick and coil setup. The initial weight of the e-cig tank was recorded before and after each aerosol generation. The differences in weight were used to determine the theoretical dose per puff. Each sample trap and its components were washed with DI water and an aliquot from the trap was collected for HPLC analysis. The experiment was repeated for a total of five aerosol generations at each voltage in triplicate on separate days. The significance was assessed by ANOVA-Kruskal–Wallis test with a significance value of 0.05. A P-value less than this indicated a significant difference between the identified groups.
Identification and quantitation of nicotine was performed using a 3200 Q Trap (Applied Biosystems, Foster City CA) attached to a SCL HPLC system (Shimadzu, Kyoto Japan). Chromatographic separation was performed on a Hypersil® Gold 3 × 50 mm, 5 μm column (Thermo Scientific, Waltham MA). The injection volume was 10 μL with a flow rate of 0.5 mL/min, with an isocratic mobile phase consisting of 90:10 Methanol: 10 mmol ammonium formate in water. The ion spray voltage was set to 5,000 V with a declustering potential of 35 eV and the source temperature was 600°C with 30 mL/min curtain gas flow, with the ion source gas 1 at 50 mL/min and ion source Gas 2 at 30 mL/min. Total runtime for this method was 2 min, and the instrument was operated in multiple reaction monitoring mode monitoring the following m/z transitions: nicotine, 163 > 130 and 163 > 117; and nicotine-d4, 167 > 134. A seven-point calibration curve was constructed with nicotine concentrations of 10, 25, 50, 100, 250, 500 and 1,000 ng/mL with 250 ng/mL of nicotine-d4 as internal standard. Aliquots were fortified with internal standard post-collection from the aerosol trap. A linear regression was generated using the peak area ratio of nicotine to internal standard versus nicotine concentration and r2 > 0.9985 for all curves. The limit of quantitation was administratively set at 10 ng/mL and signal-to-noise ratio was greater than 10 times the baseline. All determined sample concentrations were bracketed within the calibration range 10–1,000 ng/mL. Six controls were included with each analytical batch: a blank, a double blank, limit of quantitation quality control (10 ng/mL), low-quality control (30 ng/mL), mid-quality control (300 ng/mL) and high-quality control (900 ng/mL). Intra-day (within-run) accuracy and precision were determined by taking the largest percent coefficient of variation (%CV) and most extreme accuracies for each control concentration out of each of the three runs (n = 6). Carryover on the instrument was assessed by running a nicotine-free negative control immediately following the highest concentration calibrator (1,000 ng/mL).
Analysis of the solid-phase microextraction sampling
A 100-μm polydimethylsiloxane (PDMS) SPME fiber was used to sample the aerosol produced using the e-cig of four commercial e-liquids. The fiber was introduced to the first aerosol trap simultaneously as the e-cig was activated for 4 s at 4.3 V. The fiber was removed after 5 min and either introduced to helium stream of the DART–MS or into the inlet of the GC–MS. Following manufacture specifications, each fiber was thermally cleaned between analysis to ensure no carryover occurred.
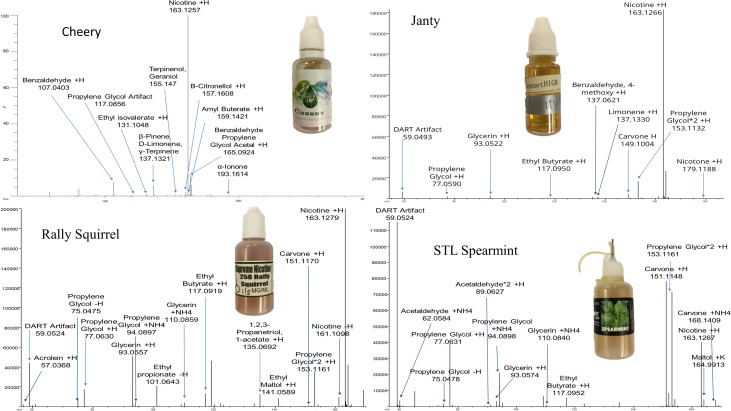
The DART–MS analysis was performed using a modified method on a JEOL (Peabody, MA) JMS T100LC Accu-TOFTM DART–MS (32, 33). The instrument was operated in positive-ion mode with a helium stream of 2.0 L/min at 300°C. The discharge electrode needle was set to 150 V and the ion guide peak voltage to 400 V. Orifice 2 was set to 5 V, and the ring lens was set to 3 V. Orifice 1 was operated at 20 V. The mass range was measured from 40 to 1,100 Da and the exact mass was determined within 5 mDa of the calculated monoisotopic mass (M + H)+. The data was analyzed using the averaged, background subtracted and centroid mass instrument calibrator PEG 600. PG, VG and nicotine reference materials were analyzed for comparison with the commercial products, and all analytes were presumptively identified based upon their monoisotopic mass of the [M + H]+ ion and the associated isotopic ratios and were compared to the NIST 11.0 Mass Spectral Library (Figure 3).
Figure 3.
DART–MS spectra of the commercial e-liquids.
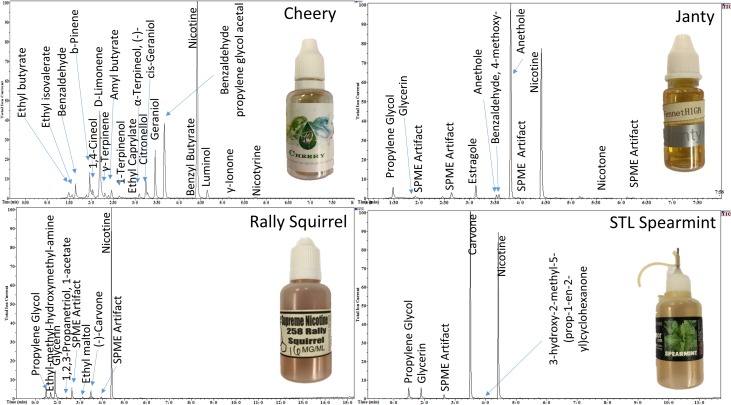
The SPME-GC–MS analysis was performed using an Agilent (Santa Clara, CA) 6890 N Gas Chromatograph with a 5973 Mass Selective Detector (MSD). Chromatographic separation was a performed on a HP-5MS 30 m × 0.25 mm id × 0.25 μm column using helium as the carrier gas. The carrier gas had a linear velocity of 35 cm/s. The inlet temperature was set at 275°C in splitless mode and the SPME fiber was allowed to desorb for 15 min. The oven temperature program had an initial temperature of 120°C with a rate of 10°C/min to a final temperature of 300°C. The final hold time was 12 min for a total runtime of 30 min. The MSD was run in scan mode with a mass range of 40–550 m/z. Analytes were presumptively identified by retention time, full scan mass spectral match to the NIST 11.0 Mass Spectral Library, and greater than a 90% confidence level. Nicotine, PG and glycerin were compared to primary reference materials (Figure 4).
Figure 4.
GC/MS total ion chromatograph of the commercial e-liquids.
Results
The simple glass assemblage was effective in trapping the aerosol produced by an e-cig to analyze the drug in the aerosol. At 3.9, 4.3 and 4.7 V, the first trap was effective in trapping nicotine produced in the aerosol. No nicotine was detected in the second glass trap. No nicotine was detected in the trap from the drug-free PG:VG samples generated between nicotine fortified PG:VG samples at any voltages, indicating no carryover contamination.
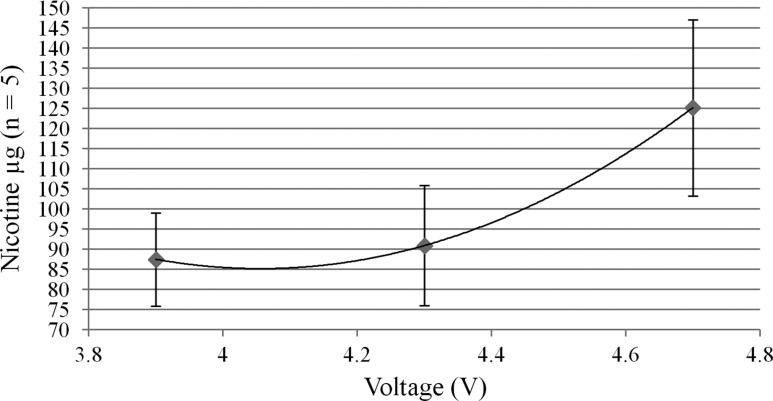
The expected nicotine concentration was calculated based on the change in weight of the AeroTank pre- and post-aerosolization and was compared to the nicotine concentration measured by HPLC–MS-MS quantitation. The recovery as mean ± SD from the trap (N = 5) for 3.9, 4.3 and 4.7 V, was 84 ± 10%, 87 ± 15% and 93 ± 11%, respectively. Accuracy was determined to be between 98% and 107% and inter-day precision was determined to be between 1% and 10% for all quality control samples. The intra-day bias was determined to be 95–109% with a precision of 7–16% for all quality control samples. Injection of the LQC (30 ng/mL) followed injection of the HQC (900 ng/mL) with a lack of bias in the LQC showed no carryover of the assay. The LOQ (n = 18) was 9.5 ± 0.9 ng/mL. Overall, a total of 18 samples were analyzed on three different days, 14 had CV < 20% and four had CVs between 27% and 38%. The dose of nicotine delivered in the aerosol at 3.9, 4.3 and 4.7 V was 88 ± 12 μg, 91 ± 15 μg and 125 ± 22 μg, respectively, as measured by HPLC–MS-MS quantitation (Figure 5). The dose of nicotine per puff was only significantly different between 3.9 and 4.7 V (P < 0.05).
Figure 5.
Nicotine yield as a function of voltage of the KangerTech AeroTank, 1.8 Ω.
Compounds were identified with a mass tolerance of ±5 mmu for SPME-DART–MS analysis, and a comparison of retention times and the electron ionization spectra for the SPME-GC–MS analysis (Figures 3 and 4). Nicotine was detected in all four commercially available e-liquids. PG and/or VG were also readily identified. Various flavoring agents were also presumptively identified (Table I). Principle flavorants were limonene (citrus), benzaldehyde (almond or cherry) and benzaldehyde PG acetal in VapeWell Cheery; carvone (spearmint) in STLVapor Spearmint; limonene (citrus) and ethyl maltol in Supreme Nicotine 258 Rally Squirrel and anethole (licorice) and limonene (citrus) in Fennet High Janty.
Table I.
Presumptive Compounds Identified in the aerosol of four commercial e-liquids
| VapeWell Cheery | STLVapor Spearmint | Supreme Nicotine 258 Rally Squirrel | Fennet High Janty | |
|---|---|---|---|---|
| Nicotine | X | X | X | X |
| Propylene Glycol | X | X | X | X |
| Glycerin | X | X | X | |
| 1, 2, 3, Propanetriol, 1-acetate | X | |||
| 3-Hydroxy-2-methyl-5(prop-1-en-2yl) | X | |||
| Acetaldehyde | X | |||
| Acrolein | X | |||
| Amyl butyrate | X | |||
| Anethole | X | |||
| Benzaldehyde | X | |||
| Benzaldehyde, 4-methoxy | X | |||
| Benzaldehyde propylene glycol acetal | X | |||
| Carvone | X | X | X | |
| Citronellol | X | |||
| Estragole | X | |||
| Ethyl Butyrate | X | X | X | X |
| Ethyl Isovalerate | X | |||
| Ethyl Maltol | X | |||
| Ethyl-methyl-hydroxymethyl-amine | X | |||
| Ethyl Propionate | X | |||
| Geraniol | X | |||
| Limonene | X | X | ||
| Maltol | X | |||
| Nicotone | X | |||
| Pinene | X | |||
| α-Terpineol | X | |||
| γ-Terpinene | X |
Discussion
As e-cigs become more pervasive, the methods to characterize and understand how the aerosol is produced by the e-cigs are needed. The progression of these modifiable devices has led to an increase in the ability for these devices to deliver DOTNs and illicit substances. Therefore, the presented methods were developed to efficiently analyze the aerosol produced by e-cig and to understand if and how the yield of drug increases as the power output of the device is increased.
A baseline understanding of the device is critical for future studies, particularly for those studies intended to evaluate the evolution of the devices in construction and use. To this end, studies need to fully describe the e-cig device used in investigations, particularly the constituent parts that can be modified as e-cig users try to improve drug delivery. The KangerTech AeroTank e-cig selected for this study was chosen to develop a model because it had several components typically modified by users: the coil, the wick, a variable power supply and a refillable tank. These components come as commercially available products that can be exchanged by the user at will in order to improve their overall experience. With limited regulations in this industry and reports of deviations of labeled versus actual contents in manufactured e-liquid, this research group found that 18 of 27 e-liquids purchased in the USA deviated more than 10% from the labeled concentration, with nine being greater than 20% (21). This study of e-liquid nicotine concentration has been corroborated by several studies of products purchased world-wide (26–30). In order to control variability from purchased e-liquids and to develop a model for comparisons, a formulation of 50:50 PG:VG with 12 mg/mL nicotine was used at all voltages in this study with the single e-cig model.
The presented data support claims that the yield of nicotine per puff increases as voltage increases. The measured concentration of nicotine and the dose of nicotine per puff were significantly different between 3.9 and 4.7 V. Conceivably, the temperature change of the coil was only significant enough from 3.9 to 4.9 V to aerosolize more nicotine in a puff. These results are similar to the previous findings by Farsalinos, Gillman, Talih and Kosmider. Farsalinos et al. attributing a higher plasma concentration of nicotine in subjects using a second-generation e-cig with higher power outputs as opposed to a first-generation ciga-like e-cig (18). While the increased nicotine dose at higher voltages is statistically significant, the practical significance remains questionable given the inherent variability of a single device, as demonstrated by wide standard deviations and, as Talih concluded (19), the variability by which the user can moderate dosage with puff duration, puff volume, puff velocity and inhalation practice.
The changes in voltage also did not impact the recovery of nicotine. This may indicate that the nature of the aerosol may not be changing as it leaves the mouthpiece of this atomizer. The voltage change may not have been large enough in this study to impact the particle size of the aerosol. Research to define the nature and composition of the e-cig aerosol, particularly as parameters on the e-cigs are modified and constituents of the refill formulations are varied, is imperative. These variable can potentially alter the composition of the aerosol, specifically the particle size distribution, thereby impacting drug absorption.
Based on the success of SPME with extracting components of traditional cigarette smoke, 100 μm PDMS fibers were selected for the analysis, as they are made to sample compounds that are volatile or semi-volatile non-polar compounds with a molecular weight range of MW 40–275. Since nicotine falls within this range, this fiber type was selected for sampling the aerosol from the e-liquid and subsequently analyzed by SPME-DART–MS and SPME-GC–MS. These techniques were fast and effective methods for the analysis of the e-cigarette aerosol to identify the pharmacologically active ingredient, nicotine, in four commercial available e-liquids. The methods also identified the PG and VG found in these formulations as well as a number of flavorings agents.
Conclusion
The simple glass trap system was an effective method to capture aerosol from the e-cig for drug quantitation and confirmed that the increase in voltage on a second-generation e-cig could increase the nicotine yield. The trap system used in combination with SPME-DART–MS and/or SPME-GC–MS was effective in the qualitative analysis of components found in the generated aerosol.
Funding
This project was supported by Award No. 2014-R2-CX-K010, awarded by the National Institute of Justice, Office of Justice Programs, US Department of Justice and the National Institutes of Health Award No P30DA033934. The opinions, findings and conclusions or recommendations expressed in this publication/program/exhibition are those of the author(s) and do not necessarily reflect those of the Department of Justice.
References
- 1. Robinson J., inventor. Electronic Vaporizer. 1930. US1775947.
- 2. Gilbert H.A., inventor. Smokeless Non-Tobacco Cigarette. 1965. US3200819.
- 3.(2004) Lik H., inventor. Flameless Atomizing Electronic Cigarette. WO2004080216. World Intellectual Property Organization. http://patentscope.wipo.int/search/en/WO2004080216 (accessed March 13, 2016).
- 4. Flouris A.D., Oikonomou D.N. (2010) Electronic cigarettes: miracle or menace? British Medical Journal, 340, 215. [DOI] [PubMed] [Google Scholar]
- 5.(2011). E-cigarette history. The Consumer Advocates for Smoke-free Alternatives Association. http://casaa.org/E-cigarette_history.html (accessed 27 February 2014).
- 6. Peace M.R., Stone J.W., Poklis J.L., Turner J.B.M., Poklis A. (2016) Analysis of commercial Marijuana e-cigarette formulation. Journal Analytical Toxicology, 40, 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peace M.R., Butler K.E., Wolf C.E., Poklis J.K., Poklis A. (2016) Evaluation of two commercially available cannabidiol formulations for use in electronic cigarettes. Frontiers in Pharmacology, 7, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peace M.R., Krakowiak R.I., Wolf C.E., Poklis A., Poklis J.L. (2017) Identification of MDMB-FUBINACA in Commercially available e-liquid formulations for use in electronic cigarettes. Forensic Science International, 272, 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poklis J.L., Mulder H.A., Halquist H.S., Wolf C.E., Poklis A., Peace M.R. (2017) The Blue Lotus Flower (Nymphea caerulea) resin used in a new type of electronic cigarette, the rebuildable dripper atomizer. Journal of Psychoactive Drugs, 49, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(2015). The 4 Generations of Electronic Cigarettes. Ecigclopedia. http://ecigclopedia.com/the-4-generations-of-electronic-cigarettes/ (accessed 13 March 2016).
- 11. Breland A., Soule E., Lopez A., Ramoa C., El-Hellani A., Eissenberg T. (2016) Electronic cigarettes: what are they and what do they do? Annals of the New York Academy of Sciences, 1394, 5–30. 10.1111/nyas.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farsalinos K.E., Romagna G., Voudris V., Spyrou A., Tsimopoulou K., Stefopoulos C. (2014) Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Scientific Reports, 4 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(2015). Wire Porn Thread. Vaping Underground. http://vapingunderground.com/threads/wire-porn-thread.177096/ (accessed 13 March 2016).
- 14.(2015). Complicated builds. Are they so much better. E-cigarette Forum. http://vapingunderground.com/threads/wire-porn-thread.177096/ (accessed 13 March 2016).
- 15. Tayyarah R., Long G.A. (2014) Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regulatory Toxicology and Pharmacology, 70, 704–710. [DOI] [PubMed] [Google Scholar]
- 16. Farsalinos K.E., Voudris V., Romagna G., Tsiapras D., Kyrzopoulos S. (2013) Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. International Journal of Environmental Research and Public Health, 10, 2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farasalinos K.E., Yannovits N., Sarri T., Voudris V., Poulas K. (2016) Protocol proposal for, and evaluation of, consistency in nicotine from the liquid to the aerosol of electronic cigarettes atomizers: regulatory implications. Addiction (Abingdon, England), 111, 1069–1076. [DOI] [PubMed] [Google Scholar]
- 18. Farsalinos K.E., Romagna G., Voudris V., Spyrou A., Tsimopoulou K., Stefopoulos C. (2014) Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Scientific Reports, 4 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talih S., Salman R., Baalbaki R., Balhas Z., Karaoghlanian N., Saliba N., et al. (2015) Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine & Tobacco Research, 17, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gillman I.G., Kistler K.A., Stewart E.W., Paolantonio A.R. (2016) Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regulatory Toxicology and Pharmacology, 75, 58–65. [DOI] [PubMed] [Google Scholar]
- 21. Peace M.R., Baird T.R., Smith N., Wold C.E., Poklis J.L., Poklis A. (2016) Concentration of nicotine and glycols in 27 electronic cigarette formulations. Journal of Analytical Toxicology, 40, 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goniewicz M.L., Hajek P., McRobbie H. (2014) Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction (Abingdon, England), 109, 500–507. [DOI] [PubMed] [Google Scholar]
- 23. Goniewicz M.L., Kuma T., Gawron M., Knysak J., Kosmider L. (2013) Nicotine levels in electronic cigarettes. Nicotine & Tobacco Research, 15, 158–166. [DOI] [PubMed] [Google Scholar]
- 24. Kavvalakis M.P., Stivaktakis P.D., Tzatzarakis M.N., Kouretas D., Liesivuori J., Alegakis A.K., et al. (2015) Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. Journal of Analytical Toxicology, 39, 262–269. [DOI] [PubMed] [Google Scholar]
- 25. Etter J.F., Zather E., Svensson S. (2013) Analysis of refill liquids for electronic cigarettes. Addiction (Abingdon, England), 108, 1671–1679. [DOI] [PubMed] [Google Scholar]
- 26. Pagano T., DiFrancesco A.G., Smith S.B., George J., Wink G., Rahman I., et al. (2015) Determination of nicotine content and delivery in disposable electronic cigarettes available in the United States by gas chromatography-mass spectrometry. Nicotine and Tobacco Research, 18, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lisko J.G., Tran H., Stanfill S.B., Blount. B.C., Watson C.H. (2015) Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e-cigarette cartridges and refill solutions. Nicotine & Tobacco Research, 17, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watson C.H., Trommel J.S., Ashley D.L. (2004) Solid-phase microextraction-based approach to determine free-base nicotine in trapped mainstream cigarette smoke total particulate matter. Journal of Agricultural Food Chemistry, 52, 7240–7245. [DOI] [PubMed] [Google Scholar]
- 29. Bao M., Joza P., Ricket W.S., Lauterback J.H. (2010) An improved headspace solid-phase microextraction method for the analysis of free-base nicotine in particulate phase of mainstream cigarette smoke. Analytica Chimica Acta, 663, 49–54. [DOI] [PubMed] [Google Scholar]
- 30. Dane A.J., Cody R.B. Using solid phase microextraction with AccuTOF-DART for Fragrance Analysis. [Internet]. 2009. [cited 2015 April 20]. Available from http://www.chromatographyonline.com/node/227045.
- 31. Yashiki N., Magasawa T., Kojima T., Miyazaki T., Iwasaki Y. (1995) Rapid analysis of nicotine and cotinine in urine using head space solid phase microextraction. Journal of Forensic Toxicology, 13, 17–24. [Google Scholar]
- 32. Poklis J.L., Raso S.A., Alford K.N., Poklis A., Peace M.R. (2015) Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and other dimethoxyphenyl-N-[(2-methoxyphenyl) methyl]ethanamine derivatives on blotter paper. Journal of Analytical Toxicology, 8, 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peace M.R., Stone J.W., Poklis J.L., Turner J.B., Poklis A. (2016) Analysis of commercial marijuana e-cigarette formulation. Journal of Analytical Toxicology, 5, 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]