Significant interactions were demonstrated between Toll-like Receptor 4 (TLR4) methylation and human alcohol consumption patterns, such that greater methylation was associated with decreased positive and negative self-reported arousal during an alcohol infusion among light-to-moderate drinkers, but increased self-reported positive arousal and physiological arousal (i.e. systolic blood pressure) among heavy drinkers.
Abstract
Aims
Converging evidence has implicated perturbed inflammatory signaling in alcohol use disorders (AUDs), and both animal and human studies suggest that alcohol-induced inflammatory signaling is mediated by Toll-Like Receptor 4 (TLR4). We previously demonstrated that TLR4 is hypermethylated in subjects with AUD compared to control individuals. Examining the relationship between TLR4 methylation and subjective alcohol responses could shed light on the role of TLR4 in promoting AUDs, thereby highlighting its potential as a treatment target.
Short summary
Significant interactions were demonstrated between Toll-like Receptor 4 (TLR4) methylation and human alcohol consumption patterns, such that greater methylation was associated with decreased positive and negative self-reported arousal during an alcohol infusion among light-to-moderate drinkers, but increased self-reported positive arousal and physiological arousal (i.e. systolic blood pressure) among heavy drinkers.
Methods
Latent growth models were used to examine the relationship between TLR4 methylation and subjective responses and physiological measures of arousal during an alcohol infusion across 222 drinkers.
Results
We observed significant interactions of TLR4 methylation and alcohol use (drinks per week) on intercepts for self-report and physiological arousal measures. Specifically, light-to-moderate drinkers had positive associations between methylation and stimulation and tension (r's = 0.21–0.24), and heavy drinkers had negative associations (r's = −0.15 to −0.21). There were also significant interaction effects on changes in tension (β = 0.31, P < 0.01), systolic blood pressure (β = 0.74, P < 0.01) and marginal effects on stimulation (β = 0.15, P = 0.07) during the infusion, such that methylation was associated with decreased arousal among light-to-moderate drinkers (r's = −0.12 to −0.25) but stable or increased arousal among heavy drinkers (r's = 0.05–0.19).
Conclusions
Findings suggest that the relationship between TLR4 methylation and subjective and physiological arousal during acute alcohol intoxication depends upon on self-reported alcohol use. These data demonstrate the influence of TLR4 on subjective responses to alcohol, thereby supporting the need for further research on its potential as a pharmacological treatment target.
INTRODUCTION
Alcohol use disorders (AUD) cause significant public health consequences, including morbidity, disability and mortality (Lozano et al., 2012; Murray et al., 2012). Epidemiologic research estimates the lifetime prevalence of AUD in the USA at 29.1%, of which only 20% receive treatment (Grant et al., 2015). Among individuals who access treatment, current interventions yield modest one-year post-treatment abstention rates for pharmacological (20%) and psychosocial therapies (35%) (e.g. Miller et al., 2001; Anton et al., 2006; Zindel and Kranzler, 2014). The combination of high burden and disappointing treatment efficacy for AUD highlights the need for improved and innovative treatments.
Converging human and animal evidence suggests that the immune system may be an important AUD intervention target (Leclercq et al., 2012; Mayfield et al., 2013). Alcohol binds to pattern-recognition receptors located on immune cells, which activates signaling cascades that produce pro-inflammatory cytokines and other inflammatory mediators (Fernandez-Lizarbe et al., 2009). This inflammatory cascade induces pro-inflammatory effects throughout the peripheral and central nervous systems, suggesting a plausible pathway to affecting the brain and behavior.
Toll-like Receptor 4 (TLR4) is a specific pattern-recognition receptor that mediates normal immune functioning and plays an important role in the immune response to alcohol. In the brain, alcohol and its metabolites bind directly to TLR4 (Alfonso-Loeches et al., 2010). Further, an alcohol binge in humans acutely increases serum levels of the endotoxin lipopolysaccharide (LPS), which also binds TLR4 and initiates pro-inflammatory signaling (Bala et al., 2014). Alcohol and LPS activate TLR4, which initiates signaling cascades and results in aberrant production of inflammatory mediators that may lead to neurodegeneration via oxidative stress (Fernandez-Lizarbe et al., 2009).
TLR4-mediated, alcohol-induced immune signaling may also influence subjective responses to alcohol. TLR4 activity is broadly associated with alcohol-induced cognitive and behavioral changes in rodents (Pascual et al., 2011). Specifically, rodent models have demonstrated that TLR4 mediates the intoxicating effects of alcohol, as TLR4 knockout mice show a reduced duration of alcohol-induced sedation and motor incoordination (Blednov et al., 2017). Further, increased circulating LPS and increased inflammatory signaling follow an alcohol binge in humans (Bala et al., 2014), and activation of inflammatory signaling by LPS can increase alcohol intake in mice (Blednov et al., 2011). Thus, TLR4 activation may promote alcohol consumption within a drinking episode, perhaps by influencing subjective responses to alcohol. Clarifying the role of TLR4 in the context of alcohol use could elucidate the potential influence of inflammatory signaling on these subjective responses, and may thus shed light on mechanism(s) through which AUD treatments can target the immune system. These questions have not yet been directly examined, but the subjective effects of alcohol are known to be an AUD risk factor (Newlin and Thomson, 1990; King et al., 2011, 2014). The role of genetic variation in influencing this relationship is an area of current interest and debate.
Relatedly, epigenetic effects may contribute to the relationship between alcohol and TLR4-mediated inflammatory signaling. Epigenetics refers to the biochemical changes that influence the final product of a genetic locus without altering the DNA sequence. DNA methylation is an epigenetic modification that exerts downstream effects on gene transcription and expression. DNA methylation tends to occur at cytosine–guanine (CpG) dinucleotides, often clustered in gene promoter regions (Goldberg et al., 2007). The relationship between methylation and its downstream effects is highly nuanced and can differ across genes and regions (Jones, 2012). Emerging research suggests that TLR4 methylation may be associated with decreased gene expression (i.e. suppressed TLR4), which results in decreased immune responses and blunted inflammatory signaling (e.g. Takahashi et al., 2009). We demonstrated that TLR4 is hypermethylated in AUD compared to control subjects (Hagerty et al., 2016), suggesting that TLR4 methylation may promote, or be caused by, heavy drinking.
The existing literature underscores the potential importance of TLR4 methylation in the context of AUDs, but how TLR4 methylation is related to AUD remains unclear. For the present analysis, we examined whether TLR4 methylation is related to subjective effects of alcohol during intravenous (i.v.) alcohol infusion. Self-report data from these same subjects have demonstrated that alcohol-craving is associated with the subjective effects (i.e. stimulation and sedation) of alcohol, and that this association depends on level of alcohol dependence (Bujarski et al., 2017). Thus, given the link between subjective effects and alcohol problems, evidence that inflammation affects alcohol consumption following acute administration (Blednov et al., 2011), and the potential mediating role of TLR4 methylation on alcohol-induced inflammatory signaling (e.g. Alfonso-Loeches et al., 2010), we examined the relationship between TLR4 methylation and subjective responses during an alcohol infusion. In addition, given that heavier drinkers tend to experience more positive (e.g. stimulating) effects from alcohol compared to lighter drinkers, and because TLR4 methylation may mediate the intoxicating effects of alcohol, we examined whether the relationship between TLR4 methylation and subjective responses varies by level of alcohol use. If TLR4 methylation is associated with increased stimulation in heavier drinkers, relative to lighter drinkers, this would suggest that TLR4-mediated inflammatory signaling is altered in AUD, potentially underlying increased sensitivity to the positive effects of alcohol, thereby underscoring the utility of TLR4 as a potential treatment target.
METHOD
Participants
This study was approved by the Human Research Review Committee at the University of New Mexico. Data collection took place over the course of three years from August 2008 to August 2011. Termination of data collection was planned for once the desired sample size was achieved—no other rules were set about the termination of data collection prior to beginning data collection. Two-hundred and forty non-treatment seeking drinkers were recruited from a large city in the Southwestern USA using flyers and newspaper advertisements targeting drinkers ages 21–40. To decrease the likelihood of participants experiencing adverse events during the alcohol administration, participants were required to be regular drinkers reporting at least three or more drinks (two for women) at least twice per week and to report drinking alcohol within the 7 days prior to their appointment. For the present analyses, all eligible participants were classified as either light-to-moderate drinkers or heavy drinkers based on their self-reported drinking habits on a 60-day Timeline Follow-back conducted at the baseline session (see below). Heavy drinkers were defined based on NIAAA’s heavy drinking criteria of 14+ drinks/week for men, 7+ drinks/week for women. All other participants were considered light-to-moderate drinkers. Thus, participants covered a broad range of alcohol use behaviors. A total of 222 individuals had complete data and were included in the present analysis. Exclusion criteria were a history of depression with suicidal ideation or lifetime psychotic disorder. Additional description of these methods can be found in prior publications from this study (Bujarski et al., 2015; Bujarski et al., 2017). No other experimental manipulations were carried out in this study that are not being reported on here or elsewhere.
Screening and baseline session
Participants completed initial screening via telephone, and eligible participants were scheduled for a laboratory session. At the beginning of this session, participants were breathalyzed to ensure that they had not been drinking, and only individuals with a breath alcohol concentration (BrAC) of 0.000 were allowed to participate. Subjects received informed consent from a trained research assistant and were told that they would receive alcohol during the experiment but remained blinded to the target dose and their BrAC throughout the experiment. Participants completed a urine toxicology screen to ensure no recent illicit drug use (other than cannabis), and women received a urine pregnancy test. Subjects were instructed to abstain from alcohol for 24 h before coming to their appointment. No explicit instructions were given regarding cigarette smoking.
Participants provided a saliva sample (for DNA collection), were administered the SCID and completed a battery of measures related to personality, drinking history, drinking problems and family history of alcohol problems. Subjects also completed several cognitive tasks that were not included in the present analyses. This in-person assessment visit took approximately 2 h, after which participants traveled with the experimenter to a university-based hospital for the alcohol administration session.
Alcohol administration
Alcohol was administered via i.v. infusion, at the University General Medical Research Center (GCRC) (O’Connor et al., 1998). Participants were seated in a reclining chair and administered alcohol through an i.v. placed in their non-dominant arm. Following an infusion protocol (Ray et al., 2013), participants were infused at a rate of 0.166 ml/min × body weight in kilograms (0.126 ml/min × weight for women). For safety reasons, participants were infused at half this rate for the first 5 min, and at the full rate for the remainder of the infusion. Every 3–5 min, breath alcohol concentration (BrAC) was measured by the research assistant using a breathalyzer. Physiological and self-report measures were assessed at BrACs of 0.02, 0.04 and 0.06 g/dl, which occurred 15, 30, and 45 min into the infusion, respectively. After reaching each target BrAC, infusion rates were reduced by half to maintain BrAC while subjects completed self-report assessments. This procedure resulted in highly controlled BrAC levels at each assessment (mean BrACs (SD): 0.020 (0.002), 0.040 (0.002) and 0.060 (0.002), respectively). Infusion appointment times varied during normal business hours (e.g. between 10 a.m. and 4 p.m.) based on participant availability.
Measures
Baseline
At baseline, participants completed a demographics questionnaire containing information on age, sex, height, weight, marital status, SES, occupation, income, education and race/ethnicity. Alcohol, cannabis and cigarette use were assessed using the timeline follow-back (TLFB; Sobell and Sobell, 1992) and past-year and lifetime alcohol-related problems were assessed with the alcohol use disorders identification test (AUDIT) (Saunders et al., 1993), which demonstrated adequate reliability in this sample (10 items, α = 0.77).
Physiological
To obtain objective measures of stimulation in response to the infusion, physiological measurements were taken throughout the infusion. Specifically, heart rate was measured because prior research suggests that alcohol-induced HR during the rising limb of the blood alcohol curve is may be an index of alcohol-related stimulation (Conrod et al., 2001), and blood pressure was measured given that laboratory studies have demonstrated that acute alcohol administration is associated with increased blood pressure (Pierucci-Lagha et al., 2005). Participants’ heart rate and blood pressure were measured by study staff using standard equipment four times on the ascending limb (BrAC’s = 0.00, 0.02, 0.04, 0.06).
Self-report
During the infusion, self-report scales assessed changes in subjective effects, craving and mood. The Alcohol Urge Questionnaire (AUQ), which consists of eight items related to urge to drink, and rated on a seven-point Likert scale anchored by ‘Strongly Disagree’ and ‘Strongly Agree,’ was used to assess craving. The AUQ has demonstrated adequate internal consistency in studies of acute alcohol-craving (Bohn et al., 1995) and was found to be highly reliable in the present sample (α = 0.81–0.91 across all time points). The Biphasic Alcohol Effects Scale (BAES), which has stimulation and sedation subscales, was used to collect information on changes in self-reported stimulation and sedation after alcohol administration. The BAES has demonstrated good internal consistency with alphas between 0.82 and 0.94 (Martin et al., 1993). In the present study, both subscales demonstrated adequate reliability across all time points (stimulation, which we examined as a measure of positive arousal, seven items α = 0.89–0.93; sedation, seven items, α = 0.80–0.87). The Subjective High Assessment Scale (SHAS; Schuckit, 1984), which consists of brief descriptors of alcohol effects, was used to assess subjective feelings of alcohol intoxication. This measure was highly reliable across all time points during and after the infusion (α = 0.88–0.90). Finally, the Profile of Mood States (POMS) was used to measure mood before, during and after alcohol administration. The POMS is a reliable and valid measure of momentary affect (McNair et al., 1992), and has demonstrated validity in the context of alcohol administration (Ray et al., 2009). The POMS tension (examined as a measure of negative subjective arousal; 10 items, α = 0.68–0.75), depression (10 items, α = 0.61–0.70) and happiness (10 items, α = 0.87–0.88) subscales were all examined in the present study. The POMS vigor subscale demonstrated unacceptable α at baseline, and thus was not included in these analyses.
DNA collection and methylation assay
Participants provided 5 ml of saliva in a sterile 50 ml conical centrifuge tube and stored in a refrigerator until the DNA was extracted. Once extracted, DNA was quantified using Invitrogen’s Qubit™ dsDNA BR Assay Kit (Cat. no. Q-32853) and cryogenically stored at −80°C. The average yield of DNA was 40 ± 5 μg. To prepare samples for pyrosequencing analysis, we pulled the original DNA that was extracted from the freezer and re-quantified and concentrated it to 20 ng/μl (1μg of DNA in 50μl of solution).
To determine the methylation of CpG sites near the TLR4 promotor, pyrosequencing was performed at EpigenDX (Worcester, MA). Pyrosequencing quantitatively monitors the real-time incorporation of nucleotides through the enzymatic conversion of released pyrophosphate into a proportional light signal (Tost and Gut, 2007). The assay covered 4 CG dinucleotides in the first exon after the 5′ untranslated transcription start site, ranging from +27 to +54 in reference to the translational start site (see Fig. 1). Site analysis was based on the ability to generate primers located around CpG islands and that meet the requirements required for accurate pyrosequencing. All primers are owned by EpigenDx. DNA methylation procedures have been described in depth in our previous work (Karoly et al., 2017). The methylation status of each locus in TLR4 was analyzed individually as a T/C SNP using QCpG software (Pyrosequencing, Qiagen) (England and Pettersson, 2005). Analyzed DNA was presented as percent methylation at each of the four TLR4 CpGs. To reduce the number of tests conducted, and given that methylation at each of the four sites was significantly correlated (all rs > 0.9), an average percent methylation at each CpG was calculated to form the average methylation score used in analyses. No other corrections were applied to the methylation data.
Fig. 1.
Location of TLR4 CPGs. Four CpGs located within the first exon of TLR4 were assayed. CpGs were chosen based on their proximity to the transcription start site (TSS) and location within an important regulatory region of the TLR4 gene. This figure was original published in Karoly et al.. (2017) and is under copyright of Alcohol Research Documentation, Inc., publisher of the Journal of Studies on Alcohol and Drugs. The figure is used with permission.
Data analytic procedures
Analyses were conducted in Mplus Version 7.4 (Muthén and Muthén, 1998). Latent growth models (LGMs) examined change in physiological and subjective states during the infusion. Analyses were conducted in two steps: (1) exploratory LGMs identified the best-fitting growth structure for the data (Grimm et al., 2013; Wood et al., 2015); and (2) confirmatory LGMs, incorporating the best-fitting growth structures, estimated associations between TLR4 methylation and the intercept and change factors. Covariates were included in step 2. Specifically, physiological outcome measures (e.g. blood pressure) have demonstrated associations with age (e.g. Franklin et al., 1997), sex and body mass index (Wilsgaard et al., 2000). Thus, age, sex, height and weight have been included as covariates in models with physiological outcome variables. Cannabis use, cigarette use and age were included in TLR4 methylation models, given associations between age and methylation (Bell et al., 2012), cigarette smoking and methylation (Lee and Pausova, 2013) and the finding that cannabinoids directly mediate TLR4-induced pro-inflammatory signaling (e.g. Rajan et al., 2016; Fitzpatrick and Downer, 2017). Given the sex differences in AUDs (e.g. Erol et al., 2017), sex was also included as a covariate in models of subjective effects.
In step one, an atheoretical approach used exploratory LGMs to model change during alcohol infusion. This analytic approach consists of two accommodations to determine the best fit of growth to the data (Grimm et al., 2013). First, the combination of intercept and growth factors that provided the best fit was determined for each measure. A sequence of models within an exploratory structural equation modeling (ESEM) framework was conducted on four-time points during the ascending limb of the infusion: (1) a tau-equivalent model which consisted of an intercept only (i.e. loadings fixed to one for all waves); (2) a 1-factor model (i.e. loadings freely estimated for each wave) and (3) a 1-factor+intercept model (i.e. loadings freely estimated on one factor, fixed to one on the intercept). The best-fitting model was chosen based on the Comparative Fit Index (CFI) and root mean square error of approximation (RMSEA). After determining the best-fitting model, indicator loadings were consulted to choose how to model change.
In step two, a LGM was chosen and incorporated into a final confirmatory model to assess the relationship between changes during infusion, TLR4 methylation, and alcohol use. In confirmatory models of subjective self-reported outcomes, growth factors were regressed on sex as a covariate. For physiological outcomes, covariates were sex, age, height, weight, cigarette use (number of cigarettes in past 60 days) and cannabis use (number of days of cannabis use in the past 60 days). Further, TLR4 methylation was regressed on age, cigarette use and cannabis use. The regression coefficients of all covariates on growth factors and TLR4 methylation are provided in supplemental tables (see Supplementary Tables S1–S3). Of primary interest, we assessed main and interaction effects of TLR4 methylation and alcohol use (drinks per week) on the intercept and change factors for each measure.
RESULTS
Participant demographic characteristics
The present analyses included 222 subjects (36.9% female, mean age = 25.5 years [SD = 4.2]). One-hundred twenty-five met criteria for the heavy drinking group and 97 met criteria for the light-moderate drinking group. Racial and ethnic composition of the sample was reflective of the geographical region of the USA in which the study took place (47.5% White, 1.8% Black, 1.8% Asian, 22.2% Latino/a, 4.1% Native and 22.6% Mixed Race). In addition, 38% of participants identified as Hispanic. Participants consumed an average of 15.12 drinks per week (SD = 11.94), 4.96 drinks per drinking day (SD = 2.62) and reported a mean AUDIT total score of 12 (SD = 5.3). 58% of participants reported being regular cigarette smokers, with average FTND total = 4.57 (SD = 3.45), average number of smoking days on the 60-day TLFB = 22 (SD = 70.4) and average number of cigarettes per smoking day = 3.17 (SD = 5.64). About 20% of participants reported daily cigarette smoking over the past 60 days. About 49% of participants reported any cannabis use in the past 60 days, with average number of cannabis use days on the TLFB = 12.19 (SD = 20.84).
Exploratory latent growth models
ESEM determined that a model with an intercept and linear change across the ascending limb assessments best fit the data for all measures (Table 1). Estimating the final time point (at minute 45) provided optimal fit for most self-report measures (AUQ, BAES scales, SHAS and POMS scales), and fixing the final time point to index linear change in BAC (BrAC = 0.06) provided optimal fit for physiological measures (heart rate, blood pressure). These models provided excellent fit for the AUQ, POMS depression, and POMS happiness (RMSEA < 0.05; CFI > 0.98); adequate fit for diastolic blood pressure, systolic blood pressure, heart rate, BAES stimulation, and POMS tensions (RMSEA < 0.08, CFI > 0.95); and inadequate fit for BAES sedation and SHAS. Thus, findings for the BAES sedation and SHAS scales should be interpreted with caution.
Table 1.
Fit statistics for latent growth models of affect during alcohol infusion
| Measure | Model | CFI | RMSEA |
|---|---|---|---|
| AUQ Total | Intercept Only | 0.91 | 0.26 |
| Intercept + Linear Change | 0.97 | 0.15 | |
| Intercept + Free Change* | 1.00 | 0.00 | |
| BAES Sedation | Intercept Only | 0.82 | 0.32 |
| Intercept + Linear Change | 0.93 | 0.23 | |
| Intercept + Free Change* | 0.95 | 0.21 | |
| BAES Stimulation | Intercept Only | 0.90 | 0.28 |
| Intercept + Linear Change | 0.99 | 0.08 | |
| Intercept + Free Change* | 1.00 | 0.00 | |
| SHAS Total | Intercept Only | 0.82 | 0.33 |
| Intercept + Linear Change | 0.89 | 0.30 | |
| Intercept + Free Change* | 0.96 | 0.21 | |
| POMS Depression | Intercept Only | 0.99 | 0.08 |
| Intercept + Linear Change | 1.00 | 0.00 | |
| Intercept + Free Change* | 1.00 | 0.00 | |
| POMS Happiness | Intercept Only | 0.98 | 0.14 |
| Intercept + Linear Change | 1.00 | 0.04 | |
| Intercept + Free Change* | 1.00 | 0.00 | |
| POMS Tension | Intercept Only | 0.97 | 0.13 |
| Intercept + Linear Change | 0.99 | 0.08 | |
| Intercept + Free Change* | 1.00 | 0.06 | |
| Heart Rate | Intercept Only | 0.97 | 0.15 |
| Intercept + Linear Change* | 0.99 | 0.07 | |
| Intercept + Free Change | 0.99 | 0.11 | |
| Diastolic Blood Pressure | Intercept Only | 0.99 | 0.06 |
| Intercept + Linear Change* | 0.99 | 0.07 | |
| Intercept + Free Change | 0.98 | 0.09 | |
| Systolic Blood Pressure | Intercept Only | 0.99 | 0.08 |
| Intercept + Linear Change* | 0.99 | 0.08 | |
| Intercept + Free Change | 0.99 | 0.09 |
Note. Estimates are rounded to the nearest hundredth. The linear change model fixed all four-time points to estimate linear change. The free change model fixed the first three time points to estimate linear change and freely estimated the final time point. AUQ, Alcohol Urge Questionnaire; BAES, Biphasic Alcohol Effects Scale; SHAS, Subjective High Assessment Scale; POMS, Profile of Mood States. *p < .05.
Confirmatory latent growth models
The fit was adequate for all confirmatory LGMs (CFIs = 0.96–1.00, RMSEAs = 0.001–0.076 [90% CIs = 0.00, 0.10]), except the SHAS, for which results should be interpreted with caution. Table 2 displays main and interaction effects of TLR4 methylation and alcohol use. TLR4 methylation was associated with increased happiness (β = 0.19 SE = 0.09) and decreased subjective high (β = −0.14 SE = 0.07) during infusion. Opposing effects such as this (i.e. feeling both happier and less subjective intoxication) can suggest underlying moderation effects; however, it should be noted that statistical significance was weak (ps = 0.04) and was absent when covariates were excluded.
Table 2.
Main and interaction effects of alcohol use and TLR4 methylation on intercept and change factors in subjective and physiological response during alcohol infusion
| Outcome | Growth factor | Drinks/week | TLR4 Methylation | Drinking x TLR4 |
|---|---|---|---|---|
| Subjective effects | ||||
| AUQ | Intercept | 0.33 (0.07)*** | −0.06 (0.07) | 0.00 (0.07) |
| AUQ | Slope | 0.04 (0.08) | −0.10 (0.08) | −0.05 (0.08) |
| BAES Sedation | Intercept | 0.15 (0.07)* | 0.12 (0.07) | −0.03 (0.07) |
| BAES Sedation | Slope | −0.20 (0.07)** | −0.11 (0.07) | 0.05 (0.07) |
| BAES Stimulation | Intercept | −0.08 (0.08) | 0.03 (0.08) | −0.23 (0.07)** |
| BAES Stimulation | Slope | 0.01 (0.09) | −0.03 (0.08) | 0.15 (0.08) |
| SHAS Total | Intercept | NA | NA | NA |
| SHAS Total | Slope | −0.20 (0.07)** | −0.14 (0.07)* | −0.05 (0.07) |
| POMS Depression | Intercept | 0.11 (0.08) | 0.03 (0.07) | −0.14 (0.07) |
| POMS Depression | Slope | −0.02 (0.09) | −0.13 (0.10) | 0.15 (0.10) |
| POMS Happiness | Intercept | −0.08 (0.08) | −0.04 (0.07) | −0.13 (0.07) |
| POMS Happiness | Slope | 0.21 (0.10)* | 0.19 (0.09)* | 0.07 (0.09) |
| POMS Tension | Intercept | −0.05 (0.08) | −0.04 (0.07) | −0.18 (0.08)* |
| POMS Tension | Slope | 0.19 (0.11) | −0.09 (0.11) | 0.31 (0.12)** |
| Physiological effects | ||||
| Heart Rate | Intercept | 0.09 (0.07) | 0.05 (0.07) | 0.10 (0.07) |
| Heart Rate | Slope | 0.05 (0.10) | 0.04 (0.10) | −0.05 (0.09) |
| Diastolic BP | Intercept | 0.17 (0.08)* | 0.01 (0.08) | −0.03 (0.08) |
| Diastolic BP | Slope | 0.03 (0.35) | 0.20 (0.47) | −0.10 (0.37) |
| Systolic BP | Intercept | 0.08 (0.07) | −0.02 (0.07) | −0.10 (0.07) |
| Systolic BP | Slope | 0.24 (0.33) | 0.00 (0.33) | 0.74 (0.23)** |
AUQ, Alcohol Urge Questionnaire; BAES, Biphasic Alcohol Effects Scale; SHAS, Subjective High Assessment Scale; POMS, Profile of Mood States.
Note. ***P < 0.001, **P < 0.01, *P < 0.05, P < 0.10. Estimates are standardized path coefficients, with standard errors in parentheses. Models fitted were the best-fitting models identified by exploratory structural equation models (see Table 1). NA, intercept effects were not tested for subjective effects of alcohol as there was not enough variability in subjective high at baseline (prior to alcohol infusion) to test this effect.
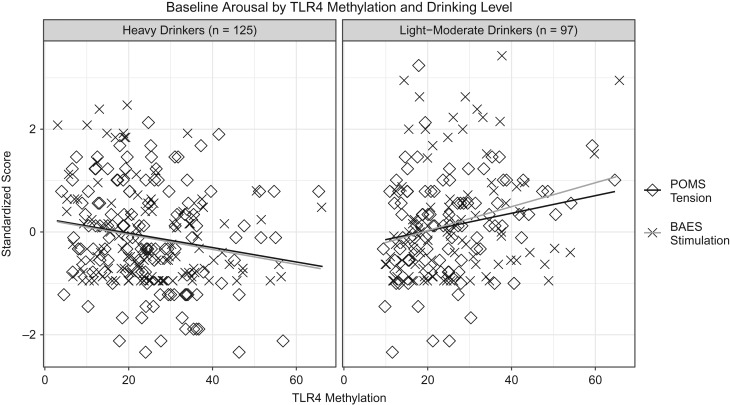
Interaction effects were significant for TLR4 methylation and alcohol use on measures of self-reported arousal and physiological arousal. TLR4 methylation and alcohol use were modeled as continuous, but effects displayed with participants grouped into heavy drinkers (based on NIAAA’s heavy drinking criteria of 14+ drinks/week for men, 7+ drinks/week for women) and light-moderate drinkers. There were similar interaction effects on the intercepts for BAES stimulation (β = −0.23 [SE = 0.07], P < 0.01) and POMS tension (β = −0.18 [SE = 0.08], P = 0.02). The intercept is an index of the variance shared by all time points. Thus, light-moderate drinkers had positive associations between TLR4 methylation and measures of stimulation (r = 0.24) and tension (r = 0.21), whereas heavy drinkers had negative associations (r's = −0.15 to −0.21; see Fig. 2). There were also significant interaction effects on the change over the course of the infusion for tension (β = 0.31 [SE = 0.12], P < 0.01) and systolic blood pressure (β = 0.74 [SE = 0.23], P < 0.01), and a marginal effect for stimulation (β = 0.15 [SE = 0.08], P = 0.07). Though the effect for stimulation does not meet statistical significance, it was statistically significant prior to modeling covariates and is consistent with the effects observed for tension and systolic blood pressure. Nevertheless, less weight should be given to interpreting the effect of change in stimulation, relative to tension and systolic blood pressure. Interaction effects were such that light-to-moderate drinkers had decreased positive and negative self-reported arousal with increasing methylation (stimulation r = −0.24, P = 0.02; tension r = −0.23, P = 0.03), and heavy drinkers had increased positive arousal with increasing methylation (stimulation r = 0.19, P = 0.03) as well as increased physiological arousal with increasing methylation (systolic BP r = 0.19, P = 0.03) (Fig. 3).
Fig. 2.
Associations between TLR4 methylation and arousal (intercepts) among heavy and light-moderate drinkers. Among heavy drinkers, TLR4 methylation was associated with decreased tension and stimulation, and among light-to-moderate drinkers TLR4 methylation was associated with greater tension and stimulation.
Fig. 3.
Associations between TLR4 methylation and standardized change in arousal among heavy and light-to-moderate drinkers. Among heavy drinkers, TLR4 methylation was associated with significant increases in stimulation and systolic blood pressure from baseline (BAC = 0.00) to Time 3 (BAC = 0.06) and among light-to-moderate drinkers TLR4 methylation was associated with significant decreases in tension and stimulation from baseline to Time 3.
DISCUSSION
TLR4 methylation was associated with changes in subjective arousal and affective states during acute alcohol intoxication, depending upon level of regular drinking. Among heavy drinkers, greater methylation was associated with increases in positive self-reported arousal (stimulation only) and physiological arousal (systolic BP) during the infusion. For light-to-moderate drinkers, methylation was associated with decreased positive and negative self-reported arousal (stimulation and tension) during the infusion.
Given the role of TLR4 in mediating alcohol-induced inflammatory signaling (Alfonso-Loeches et al., 2010), evidence that TLR4 methylation may be associated with reduced expression of TLR4 (e.g. Takahashi et al., 2009), and prior work demonstrating that chronic inflammatory signaling is associated with negative arousal in mice (Bercik et al., 2010), we hypothesize that greater TLR4 methylation may inhibit inflammatory signaling, thereby decreasing immune-mediated effects of alcohol, and altering subjective arousal (the valence of which may vary by expectancies and level of alcohol consumption). Specifically, light-to-moderate drinkers may show a negative relationship between TLR4 methylation and arousal (both positive and negative) during the infusion because lighter drinkers tend to report less stimulation and more sedation on the ascending limb, compared to heavy drinkers (Morean and Corbin, 2009). Blunted inflammatory signaling via TLR4 methylation may make this difference even more pronounced. In contrast, among heavy drinkers, who tend to experience more positive subjective effects from alcohol compared to lighter drinkers, the blunted inflammatory signaling associated with TLR4 methylation may be associated with fewer subjectively aversive acute effects, thereby making the rewarding effects (e.g. stimulation) even more salient. Further, heavy drinkers tend to have more positive alcohol expectancies (Reich et al., 2012), thereby promoting positive interpretations of elevated physiological arousal (systolic BP). In summary, among heavy drinkers, higher levels of TLR4 methylation (and presumably reduced inflammatory signaling) could ultimately promote further heavy drinking by increasing the positive (stimulating) effects of alcohol (King et al., 2011). Future research should explore TLR4 methylation and inflammation as potential risk factors for heavy drinking or relapse.
Contrary to hypotheses, TLR4 methylation was unrelated to craving (i.e. AUQ) during the infusion. This contrasts prior work linking craving to subjective alcohol responses (Bujarski et al., 2017) and inflammation (Leclercq et al., 2012). In light of these findings, we speculate that inflammation may not directly drive inter-episode desire to drink, but it may be related to the subjective effects of alcohol and promote drinking after BAC has come down (Blednov et al., 2011). Future research should measure inflammation during an acute drinking episode and assess subsequent alcohol use to determine whether inflammatory responses during an acute bout of drinking predict future drinking.
Limitations and future directions
This work has several limitations that should be considered. First, although we selected TLR4 CpGs due to their location within a regulatory region of TLR4, it is possible that methylation elsewhere in TLR4 would confer different effects. In addition, the relationship between TLR4 methylation and gene expression was not directly measured. We hypothesized that TLR4 methylation may decrease gene expression, thereby blunting inflammatory signaling, as TLR4 methylation has been associated with TLR4 silencing in several cell types (Takahashi et al., 2009). However, it should be noted that the present study measured TLR4 methylation only in saliva, and prior work has demonstrated increased TLR4 expression in post-mortem alcoholic human brain tissue (e.g. Crews et al., 2013). Given our prior work linking AUD to increased TLR4 methylation in brain tissue (Hagerty et al., 2016), it seems plausible that the relationship between TLR4 methylation and expression may differ across tissue types (e.g. increased methylation may be associated with increased expression in brain tissue and decreased expression in peripheral tissues). Although emerging work has demonstrated associations between buccal and brain tissue methylation (Smith et al., 2015), and we previously found that methylation of numerous CpGs (including CpGs in TLR4) differed significantly between AUD and control subjects and was consistent across brain and buccal cells (Hagerty et al., 2016), further studies are needed to directly measure the relationships between TLR4 methylation and TLR4 expression in human brain and peripheral tissue—and between alcohol and immune signaling more generally—across tissue types. For example, a recent study used positron emission tomography (PET) imaging to measure brain levels of 18-kDa translocator protein (TSPO), a marker of microglial activation and neuroinflammation, in alcohol-dependent and control subjects, and demonstrated that alcohol dependence was associated with decreased TSPO and blunted peripheral pro-inflammatory responses (Hillmer et al., 2017). These findings are in contrast to prior work (e.g. Crews et al., 2013), and underscore the importance of continued exploration in this area.
It should also be noted that given that the alcohol infusion only explored BrAC levels up to 0.06, BrAC levels typically reached by heavy drinkers were likely not examined. Differences in subjective experiences may emerge at higher BrAC levels for heavy drinkers, and those differences may be related to TLR4 and inflammation. Specifically, heavy drinkers are typically more sensitive to alcohol’s rewarding and stimulating effects, and this effect may become more pronounced at higher BrAC levels, when alcohol’s effects become more aversive for lighter drinkers. Such effects may be largely explained by alcohol expectancies and family history, but inflammatory response may also modulate alcohol’s rewarding and aversive effects. Although future studies could shed light on these questions, there are substantial ethical considerations when conducting alcohol challenge studies.
Another minor limitation is that the heavy drinkers in the present study were not required to have a diagnosed AUD. This limits comparisons between the present results and our previous work (e.g. Hagerty et al., 2016), given that the prior study examined individuals with alcohol dependence. Finally, data were collected at a single assessment, and thus do not address the causal nature of the relations observed. Longitudinal data are needed to draw causal conclusions about the relationship between TLR4 methylation and alcohol-related stimulation, in which alcohol use and TLR4 methylation are observed over time, to examine whether the relationship between TLR4 methylation and subjective responses changes at the within-subject level based upon level of alcohol consumption.
CONCLUSIONS
These findings suggest that TLR4-mediated inflammatory signaling may broadly underlie physiological arousal during acute intoxication, but not specifically relate to craving. However, given the link between subjective responses and craving (Bujarski et al., 2017), immune-mediating treatments could indirectly promote drinking behavior by modulating subjective responses such as arousal. Overall, results support a role for TLR4-mediated signaling in influencing acute responses to alcohol, and implicate physiological arousal as subjective responses that could be linked to inflammation. The TLR4-mediated signaling cascade may thus be an important possible treatment target. However, because of potential differences in alcohol-induced immune responses in central and peripheral tissues, explicating potential treatment implications will require animal and post-mortem human brain studies to examine relationships between alcohol consumption and TLR4 methylation and expression, as well as concordance between TLR4 methylation and expression across tissue types. Further, human research is needed to examine the effects of alcohol-induced inflammation in the brain (e.g. using PET imaging of TSPO) and periphery on acute responses to alcohol and long-term drinking patterns.
Supplementary Material
FUNDING
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [grant number R01AA015968 to K.E.H.]
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, et al. (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 30:8285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, et al. COMBINE Study Research Group (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–17. [DOI] [PubMed] [Google Scholar]
- Bala S, Marcos M, Gattu A, et al. (2014) Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One 9:e96864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JT, Tsai PC, Yang TP, et al. (2012) Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet 8:e1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, et al. (2010) Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139:2102–12. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, et al. (2011) Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun 25:S92–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Benavidez JM, et al. (2017) Sedative and motor incoordination effects of ethanol in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res 41:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR (1995) The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol 56:423–32. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Prause N, et al. (2017) Functional significance of subjective response to alcohol across levels of alcohol exposure. Addict Biol 22:235–45. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Roche DJ, et al. (2015) Factor structure of subjective responses to alcohol in light and heavy drinkers. Alcohol Clin Exp Res 39:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO (2001) Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology (Berl) 157:20–30. [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, et al. (2013) High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry 73:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England R, Pettersson M (2005) Pyro Q-CpGTM: quantitative analysis of methylation in multiple CpG sites by Pyrosequencing®. Nat Methods Appl Notes 2:798. [Google Scholar]
- Erol A, Ho AMC, Winham SJ, et al. (2017) Sex hormones in alcohol consumption: a systematic review of evidence. Addict Biol. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C (2009) Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol 183:4733–44. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JMK, Downer EJ (2017) Toll-like receptor signalling as a cannabinoid target in multiple sclerosis. Neuropharmacology 113:618–26. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Gustin W, Wong ND, et al. (1997) Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation 96:308–15. [DOI] [PubMed] [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E (2007) Epigenetics: a landscape takes shape. Cell 128:635–8. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, et al. (2015) Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA. Psychiatry 72:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm KJ, Steele JS, Ram N, et al. (2013) Exploratory latent growth models in the structural equation modeling framework. Struct Equ Modelling 20:568–91. [Google Scholar]
- Hagerty SL, Bidwell L, Harlaar N, et al. (2016) An exploratory association study of alcohol use disorder and DNA methylation. Alcohol Clin Exp Res 40:1633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer AT, Sandiego CM, Hannestad J, et al. (2017) In vivo imaging of translocator protein, a marker of activated microglia, in alcohol dependence. Mol Psychiatry 22:1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA. (2012) Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat Rev Genet 13:484–92. [DOI] [PubMed] [Google Scholar]
- Karoly HC, Thayer RE, Hagerty SL, et al. (2017) TLR4 methylation moderates the relationship between alcohol use severity and gray matter loss. J Stud Alcohol Drugs 78:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, De Wit H, McNamara PJ, et al. (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68:389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, et al. (2014) Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry 75:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, et al. (2012) Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun 26:911–8. [DOI] [PubMed] [Google Scholar]
- Lee KW, Pausova Z (2013) Cigarette smoking and DNA methylation. Front Genet 4:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, et al. (1993) Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res 17:140–6. [DOI] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L, Harris RA (2013) Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol 23:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF (1992) Profile of mood states. San Diego, CA: Educational and industrial testing service. [Google Scholar]
- Miller WR, Walters ST, Bennett ME (2001) How effective is alcoholism treatment in the United States? J Stud Alcohol 62:211–20. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR (2010) Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res 34:385–395. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO (1998) Mplus User's Guide, 7th edn Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Newlin DB, Thomson JB (1990) Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull 108:383–402. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, et al. (1998) Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res 22:202–10. [PubMed] [Google Scholar]
- Pascual M, Baliño P, Alfonso-Loeches S, et al. (2011) Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun 25:S80–91. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, et al. (2005) GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology 30:1193–1203. [DOI] [PubMed] [Google Scholar]
- Rajan TS, Giacoppo S, Iori R, et al. (2016) Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 112:104–15. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, MacKillop J, et al. (2013) Subjective response to alcohol among alcohol-dependent individuals: effects of the mu-opioid receptor (OPRM1) gene and alcoholism severity. Alcohol Clin Exp Res 37:E116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, et al. (2009) Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res 33:2154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich RR, Ariel I, Darkes J, et al. (2012) What do you mean ‘drunk’? Convergent validation of multiple methods of mapping alcohol expectancy memory networks. Psychol Addict Behav 26:406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, et al. (1993) Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. (1984) Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry 41:879–84. [DOI] [PubMed] [Google Scholar]
- Smith AK, Kilaru V, Klengel T, et al. (2015) DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am J Med Genet B Neuropsychiatr Genet 168:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992). Timeline follow-back. In Measuring Alcohol Consumption (pp. 41–72). Totowa, NJ: Humana Press. [Google Scholar]
- Takahashi K, Sugi Y, Hosono A, et al. (2009) Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol 183:6522–9. [DOI] [PubMed] [Google Scholar]
- Tost J, Gut IG (2007) DNA methylation analysis by pyrosequencing. Nat Protoc 2:2265–75. [DOI] [PubMed] [Google Scholar]
- Wilsgaard T, Schirmer H, Arnesen E (2000) Impact of body weight on blood pressure with a focus on sex differences: the Tromsø Study, 1986–1995. Arch Intern Med 160:2847–53. [DOI] [PubMed] [Google Scholar]
- Wood PK, Steinley D, Jackson KM (2015) Right-sizing statistical models for longitudinal data. Psychol Methods 20:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindel LR, Kranzler HR (2014) Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J Stud Alcohol Drugs Suppl 75:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.