Abstract
Background
Warfarin is associated with poor outcomes after trauma, an effect correlated with elevations in the international normalized ratio (INR). In contrast, the novel oral anticoagulants (NOAs) have no validated laboratory measure to quantify coagulopathy. We sought to determine if use of NOAs was associated with elevated activated partial thromboplastin time (aPTT) or INR levels among trauma patients or increased clotting times on thromboelastography (TEG).
Methods
This was a post-hoc analysis of a prospective observational study across 16 trauma centers. Patients on dabigatran, rivaroxaban, or apixaban were included. Laboratory data were collected at admission and after reversal. Admission labs were compared between medication groups. Traditional measures of coagulopathy were compared with TEG results using Spearman’s rank coefficient for correlation. Labs before and after reversal were also analyzed between medication groups.
Results
182 patients were enrolled between June 2013 and July 2015: 50 on dabigatran, 123 on rivaroxaban, and 34 apixaban. INR values were mildly elevated among patients on dabigatran (median 1.3, IQR 1.1–1.4) and rivaroxaban (median 1.3, IQR 1.1–1.6) compared with apixaban (median 1.1, IQR 1.0–1.2). Patients on dabigatran had slightly higher than normal aPTT values (median 35, IQR 29.8–46.3), whereas those on rivaroxaban and apixaban did not. Fifty patients had TEG results. The median values for R, alpha, MA and lysis were normal for all groups. Prothrombin time (PT) and aPTT had a high correlation in all groups (dabigatran p=0.0005, rivaroxaban p<0.0001, and apixaban p<0.0001). aPTT correlated with the R value on TEG in patients on dabigatran (p=0.0094) and rivaroxaban (p=0.0028) but not apixaban (p=0.2532). Reversal occurred in 14%, 25%, and 18% of dabigatran, rivaroxaban, and apixaban patients, respectively. Both traditional measures of coagulopathy and TEG remained within normal limits after reversal.
Discussion
Neither traditional measures of coagulation nor TEG were able to detect coagulopathy in patients on NOAs.
Level of evidence
Level IV.
Keywords: coagulation tests, anticoagulation, trauma management
Background
Anticoagulants used for treatment of arrhythmias and other chronic thrombophilic conditions present a particular challenge to those managing trauma patients at high risk of hemorrhage. The number of patients on anticoagulants presenting to trauma centers is steadily increasing.1–4 When compared with warfarin, novel oral anticoagulants (NOAs) such as dabigatran, rivaroxaban, and apixaban have the benefit of easier dosing,5 6 lower risk of adverse bleeding events,7 8 and clinical management guidelines which favor their utilization.9 10 As such, NOAs are likely to be seen with increasing frequency in trauma patients. However, the lack of readily available and reliable tests to evaluate the presence and level of anticoagulation in patients on NOAs leaves the trauma surgeon with little patient-specific data on which to base management decisions.11–13 Although there have been a number of suggested methods for quantifying the level of anticoagulation associated with NOAs, such as ecarin clotting time (ECT), dilute thrombin time (dTT), and anti-Xa inhibitor monitoring, these tests are not widely available nor used and, at this time, are of limited clinical utility.14 There is some suggestion that NOAs increase activated partial thromboplastin time (aPTT) levels, and that although not quantitative this could be used as a qualitative measure of coagulopathy.15 16 There have also been studies that recommend thromboelastography (TEG) as a reliable method of detecting NOA-associated coagulopathy.17 18 However, most studies have been performed in healthy uninjured volunteers and data that exist on the utility of TEG in trauma patients are limited.11 19–21 This study sought to determine if dabigatran, rivaroxaban, and apixaban were associated with abnormal values on conventional coagulation assays (CCAs) or TEG using a multicenter, prospectively gathered database.
Methods
Data used for this study were collected during a prospective observational study occurring between June 30, 2013 and July 1, 2015 across 16 level 1 trauma centers via the American Association for the Surgery of Trauma-Multi-institutional Trials Committee (AAST-MITC).22 This is a post-hoc analysis of patients admitted on dabigatran, rivaroxaban, or apixaban. Minors (age <18), patients who were pregnant, and prisoners were excluded. Demographics, admission vital signs, mechanism of injury, laboratory values, transfusions, and reversal attempts were collected. Attempts at reversal of coagulopathy included administration of vitamin K, prothrombin complex (PCC), hemodialysis, and transfusion of fresh frozen plasma (FFP), cryoprecipitate or platelets. Laboratory values at admission were compared between medication groups. Repeat laboratory values when present after attempts at reversal were also analyzed. STATA/MP V.14.1 was used to perform the analysis. Continuous variables were reported using mean and SD if normally distributed, and median and IQR if not normally distributed. Univariate analysis used the Pearson’s χ2 for categorical variables. Continuous variables were tested using the non-parametric Kruskal-Wallis test; those that followed a normal distribution were analyzed using one-way analysis of variance (ANOVA). Post-hoc comparisons were performed using the Wilcoxon rank-sum test when the Kruskal-Wallis test was significant, and independent-samples t-test with Bonferroni correction for significant ANOVA results. CCAs (aPTT and prothrombin time (PT)/international normalized ratio (INR)) were compared with TEG results using Spearman’s rank coefficient to determine if any correlation existed. All variables were checked for missing data and any significant values were reported in the Results section.
Results
One hundred and eighty-two patients on NOAs were identified in the database: 50 on dabigatran, 123 on rivaroxaban, and 34 apixaban. Patients were primarily older, non-Hispanic white, and admitted after a fall. Demographics were not significantly different between the medication groups. Very few patients had a documented history of comorbid conditions associated with coagulopathy, such as renal failure (one patient) or cirrhosis (three patients), and their incidence was similar between medication groups (table 1).
Table 1.
Demographics, mechanism of injury, admission physiology, and injury severity of patients on novel oral anticoagulants
| Dabigatran | Rivaroxaban | Apixaban | Pvalues | |
| n | 50 | 123 | 34 | |
| Age (SD) | 79 (12) | 74 (16) | 80 (11) | 0.137 |
| Female (%) | 52 | 46 | 56 | 0.493 |
| Renal failure (%) | 0 (0) | 1 (1) | 0 (0) | 0.710 |
| Cirrhosis (%) | 1 (2) | 2 (2) | 0 (0) | 0.729 |
| Race* (%) | 0.777 | |||
| Black | 0 | 1 | 0 | |
| Asian | 2 | 1 | 0 | |
| Hispanic | 8 | 7 | 3 | |
| Non-Hispanic white | 84 | 86 | 97 | |
| Mechanism (%) | 0.843 | |||
| Fall | 70 | 74 | 74 | |
| Motor vehicle accident | 14 | 16 | 18 | |
| Found down | 6 | 2 | 0 | |
| Auto vs. pedestrian | 4 | 2 | 0 | |
| GCS≤8 (%) | 4 | 4 | 3 | 0.954 |
| Shock (SBP<90) (%) | 4 | 7 | 0 | 0.279 |
| ISS median (IQR)† | 9 (4–13) | 5 (4–10) | 6 (4–10) | 0.834 |
| ISS≥10† (%) | 42 | 36 | 41 | 0.737 |
| Reversed (%) | 7 (14) | 31 (25) | 6 (18) | 0.225 |
Missing data <1% unless specifically noted in the table. Data previously presented.22
For Renal Failure and Cirrhosis both the number of patients as well as percent (in parentheses) of the population with the comorbidity are presented.
Values in bold signify number of patients and percent of population in parentheses.
*Race: missing/other: 9 (4%).
†ISS: missing 3 (1.5%).
GCS, Glasgow Coma Scale; ISS, Injury Severity Score; SBP, systolic blood pressure.
Admission INR values were mildly elevated among patients on dabigatran (median 1.3, IQR 1.1–1.4) and rivaroxaban (median 1.3, IQR 1.1–1.6) compared with apixaban (median 1.1, IQR 1.0–1.2). Patients on dabigatran presented with slightly higher than normal aPTT values (median 35, IQR 29.8–46.3), whereas those on rivaroxaban and apixaban did not. Fifty patients had admission TEG results. The median values for R, alpha, and maximum amplitude (MA) were within normal limits and did not differ significantly between groups (table 2). No patients in the study had significant lysis at admission TEG.
Table 2.
Laboratory measures of coagulation among patients on novel oral anticoagulants
| Dabigatran | Rivaroxaban | Apixaban | P values | |
| n | 50 | 123 | 34 | |
| PT value (IQR) | 14.1 (12.1–15.5) | 13.8 (11.8–17.3) | 13.4 (11.3–15) | 0.4406 |
| INR (IQR) | 1.3 (1.1–1.4) | 1.3 (1.1–1.6) | 1.1 (1.0– 1.2) | 0.0113 |
| aPTT (IQR) | 35.0 (29.8– 46.3) | 30.4 (27.0–35.9) | 28.7 (25.7–33.9) | 0.0017 |
| TEG R median (IQR) | 5.3 (3.9–7.5) | 5.6 (4.4–8.0) | 4.4 (4.0–5.0) | 0.2066 |
| TEG alpha median (IQR) | 69.5 (67.6–72.6) | 70.7 (65.7–73.8) | 71.2 (65.6–76.4) | 0.8729 |
| TEG MA median (IQR) | 66.8 (62.9–69.8) | 67.3 (62.2–71.0) | 67.1 (62.4–72.5) | 0.9860 |
Significant values in bold. Normal values: PT=9.7–12.5; aPTT=25–34; R=5–10; alpha=53–72; MA=50–70; lysis=0%–8%.
INR, international normalized ratio; TEG, thromboelastography.
Admission PT and aPTT had a moderate degree of correlation in all medication groups (dabigatran r=0.5281, p=0.0005; rivaroxaban r=0.5365, p<0.0001; apixaban r=0.5634, p<0.0001) (figure 1). PT correlated moderately with the R value on TEG only among patients on rivaroxaban (r=0.5161, p=0.0059) (figure 2). aPTT correlated with the R value on TEG strongly in patients on dabigatran (r=0.6652, p=0.0094) and moderately in patients on rivaroxaban (r=0.5627, p=0.0028) but did not correlate for patients on apixaban (r=0.5000, p=0.2532) (figure 3).
Figure 1.
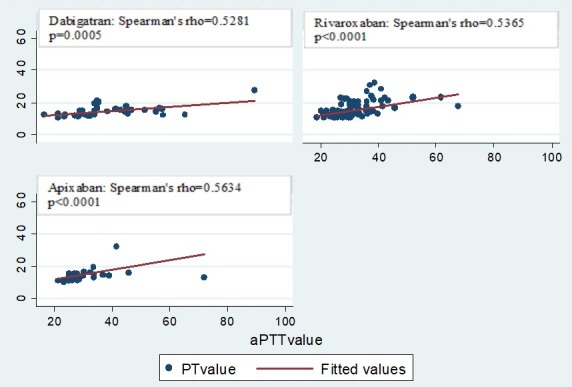
Correlation between PT and aPTT values in patients on novel oral anticoagulants.
Figure 2.
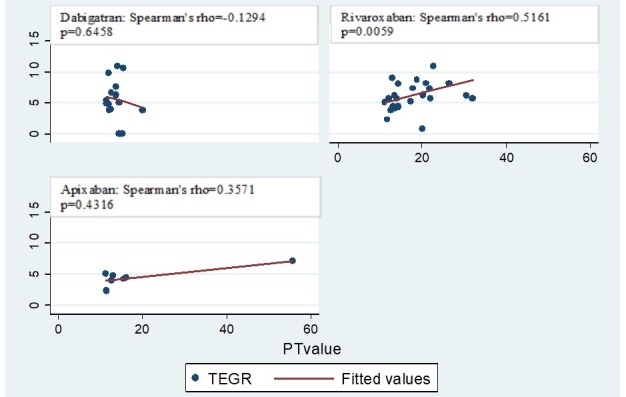
Correlation between PT and R values in patients on novel oral anticoagulants. TEGR, thromboelastogram Reaction (R) time; PT, prothrombin time
Figure 3.
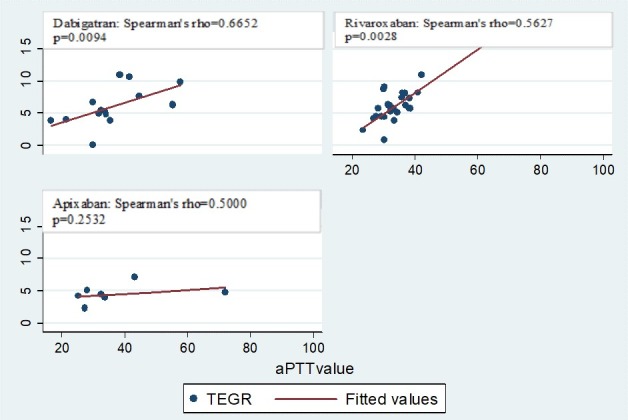
Correlation between aPTT and R values in patients on novel oral anticoagulants. TEGR, thromboelastogram Reaction (R) time; aPTT, activated partial thromboplastin time
Attempts at reversal occurred in 19% of patients overall, 7 dabigatran, 31 rivaroxaban, and 6 apixaban patients. The percentage of patients reversed did not differ significantly between medications groups (table 1). Postreversal CCAs were present in the majority of patients; 6 of 7 dabigatran, 27 of 31 rivaroxaban, and 4 of 6 apixaban (table 3). The mean time from admission to repeat aPTT was 16 (SD ±49) hours and INR 18 (SD ±31) hours. Similar to admission values the postreversal INR of apixaban patients was significantly lower than dabigatran patients (p=0.0096), and the postreversal dabigatran aPTT value remained significantly higher than rivaroxaban and apixaban patients (p=0.0014). However, all median CCA values remained within normal limits in all medication groups (table 3).
Table 3.
Laboratory measures of coagulation after attempted reversal
| Dabigatran | Rivaroxaban | Apixaban | P values | |
| Patients reversed (n) | 7 | 31 | 6 | |
| Best INR (IQR)* | 1.3 (1.2–1.4) | 1.2 (1.1–1.3) | 1.1 (1–1.2) | 0.0096 |
| Best aPTT (IQR)† | 34 (29.8–44.6) | 30.1 (26.9–24.5) | 29.2 (26.1–33.2) | 0.0014 |
| Best TEG R median (IQR)‡ | 5.2 (3.9–6.9) | 5.4 (4.3–7.3) | 4.2 (2.4–5) | 0.2125 |
| Best TEG alpha median (IQR)‡ | 70 (67.8–73.6) | 71.2 (65.6–77.4) | 71.2 (63–77) | 0.8723 |
| Best TEG MA median (IQR)‡ | 66.8 (61.5–70.1) | 67.1 (62.4–72.5) | 65.3 (56.7–69.8) | 0.9364 |
Significant values in bold. Normal values: PT=9.7–12.5; aPTT=25–34; R=5–10; alpha=53–72; MA=50–70; lysis=0%–8%.
*Repeat INR performed after reversal in 6 dabigatran, 27 rivaroxaban, and 4 apixaban patients.
†Repeat aPTT performed after reversal in 6 dabigatran, 24 rivaroxaban, and 3 apixaban patients.
‡Repeat TEG performed after reversal in 3 dabigatran, 11 rivaroxaban, and 2 apixaban patients.
INR, international normalized ratio; MA, maximum amplitude; PT, prothrombin time; TEG, thromboelastography; aPTT, activated partial thromboplastin time.
Very few patients had postreversal TEG values recorded: 3 of 7 dabigatran, 11 of 31 rivaroxaban, and 2 of 6 apixaban patients. The mean time to repeat TEG was 10 (SD ±14) hours. All postreversal TEG measures were within normal limits and were not significantly different between medication groups (table 3). Because of the paucity of patients with postreversal TEG values, no correlation between postreversal TEG and CCA values was performed.
Discussion
NOAs are considered to have a reliable dose–response relationship, and routine testing of serum concentrations is not currently recommended.6 9 23 However, using liquid chromatography-tandem mass spectrometry24 and validated indirect tests such as calibrated anti-Xa assays, multiple studies have reported that NOA levels can vary up to 7 to 20 times between peak and trough measurements depending on the agent.25 26 In the case of dabigatran, further data showed that higher drug concentrations were associated with a higher risk of major bleeding events.27 Additionally, NOA levels vary with age, gender, and creatinine clearance.26 27 It has also been shown that NOAs interact with medications commonly used in the atrial fibrillation/hypercoagulable patient population, including atorvastatin and diltiazem.28 Given this potential variability, it is critically important to be able to detect and quantify the level of NOA-associated coagulopathy to guide clinical decision making after injury and evaluate the efficacy of reversal attempts.
Studies have shown that there are effective tests to measure NOAs, including thrombin time, dTT, and ecarin-based tests (ECT and ecarin chromogenic assay (ECA)), to detect dabigatran levels, and agent-specific anti-Xa activity assays for rivaroxaban and apixaban.12 17 25 Although anti-Xa activity assays are being used more commonly, they are not universally available, particularly with specific agent calibration. These studies often need to be sent out to specialty labs, and cost and turnaround time can reduce their clinical utility greatly. As such, many studies including ours have looked at the feasibility of using more easily and rapidly obtained laboratory measures (CCAs and TEG) to estimate the anticoagulant effects of NOAs.
Multiple studies, primarily in healthy volunteers, have demonstrated modest elevations in aPTT with dabigatran, but the relationship is non-linear at higher concentrations and very modest.12 13 25 PT/INR are also mildly elevated for patients on dabigatran, but these tests are less sensitive than aPTT. In contrast, aPTT is only sometimes elevated in the presence of rivaroxaban, and the relationship is non-linear across all concentrations. Rivaroxaban has also been found to prolong PT, but less so than dabigatran and with a wider range of responses. Neither PT/INR nor aPTT is elevated with apixaban use.12 13 25 29 This is in line with our findings of significantly higher aPTT values among patients taking dabigatran compared with patients on rivaroxaban and apixaban. The median aPTT for dabigatran was also the only value noted in this study to be above laboratory normal values, although this very modest elevation is unlikely to be clinically relevant (table 1). When INR values were compared between the three groups, the median INR level in patients taking apixaban was statistically significantly lower than both dabigatran and rivaroxaban (table 1). However, all median INR values for the three drugs remained within normal limits. In the only other published study addressing NOAs among trauma patients, Ali et al 11 similarly demonstrated increases in aPTT level in patients on dabigatran. The elevations in aPTT were more clinically significant in patients on dabigatran in the Ali et al 11 study, with 100% having levels >36.5 s and a median of 52.7 s, whereas the IQR for patients in the present study on dabigatran was 29.8 to 46.3 s. There are several explanations for the differences between our results. First, the sample size of the published study was severely limited with only 6 dabigatran patients compared with 50 in our study. Additionally, the mean Injury Severity Score (ISS) was higher in the Ali et al 11 study at 18±10 compared with our median ISS of 9 (IQR 4–13) in our dabigatran patients. It is possible that the CCA and TEG results in the Ali et al 11 study were due, in part, to coagulopathy of trauma rather than NOA use.
TEG is increasingly used in trauma settings, and recent literature has demonstrated an improved ability of TEG to predict transfusion and bleeding in severely injured trauma patients.30 There have also been studies that suggest that TEG can reliably detect NOAs, primarily as a prolongation of R time when compared with healthy controls not exposed to drug.17 18 21 The study by Ali et al 11 did not show significant differences in TEG-identified coagulopathy in patients with preinjury pharmacologic anticoagulation compared with those without the exposure, but their study was limited by a small sample size. In our larger study, we were also not able to detect any significant coagulopathy among NOA patients at admission TEG (table 2). There are several explanations for the inability of TEG to detect coagulopathy in NOA patients in our study. First, there is a tendency toward hypercoagulability in women, patients with atrial fibrillation, and the elderly, characteristics which were all common in our study group (female 52%, arrhythmia 57%, mean age 77±14).27 31 32 It is possible that the trend toward hypercoagulability prevented the detection of the anticoagulant effects of the NOAs in our study population. Second, although the R values in the previously referenced studies were elevated compared with controls, the averages were not consistently outside of normal range.18 It is possible that the presenting R time in our patients is elevated over their native R time but not enough to be outside of normal range, as was seen in the healthy volunteers. However, a small portion of the study population had repeat TEG values during their hospitalization, and although the small sample size precluded a statistically significant comparison the gross values did not appear to be different from admission values despite withholding the NOA. Lastly, trauma itself can result in an early hypercoagulable state; therefore, it is possible that the admission TEG values demonstrate this effect, with the hypercoagulable state after injury potentially masking or attenuating NOA-induced coagulopathy.32
Based on prior studies demonstrating strong correlation between CCAs and TEG30 among injured patients with traumatic coagulopathy, we sought to determine if there was any correlation between PT or aPTT and R value on TEG as this was the most commonly elevated TEG value in NOA studies.17 18 21 We found a consistent degree of correlation between the two CCAs (PT and aPTT) through all medication groups (figure 1). However there was no consistent correlation between CCAs and R time on TEG (figures 2 and 3). The strongest correlation was found between aPTT and R time in patients on dabigatran (figure 3), which is expected based on prior studies suggesting that elevations in aPTT and prolongation or R time are most pronounced and reliable among patients on dabigatran.12 13 17 18 21 25 It is possible that we were unable to detect a strong consistent correlation due to the overall small sample size, particularly for apixaban. It is also possible that the lack of clinically significant coagulopathy in any group precluded the identification of meaningful correlations.
There is some controversy on how to reverse patients on NOAs, and there is varying evidence for factor replacement via PCC, FFP, or specific recombinant factors as confirmation for their clinical utility is inconsistent and sparse.14 20 In this study, 19% of patients underwent an attempt at reversal, the majority had repeat CCAs, and a minority had repeat TEG values recorded (table 3). Similar to admission results, the postreversal measures of coagulation remained within normal limits. Due to the very small sample size and heterogeneity in reporting postreversal lab values between medication groups, we did not perform a statistical comparison between prereversal and postreversal lab values. However, there did not appear to be any clinically significant difference between the median values for INR, aPTT, R, alpha, or MA at admission compared with those after reversal. This is in contrast to a study in healthy volunteers which demonstrated an elevation in PT with administration of rivaroxaban and reversal of the elevation with administration of PCC.20 It is possible that attempts at reversal are achieving reductions in measures of both CCA and TEG values, but due to the very low sample size in this study and the heterogeneity of reversal strategies between centers we were unable to detect it.
This study has several limitations. First, and most importantly, we are limited by the relatively low sample size and are underpowered to detect very small elevations in measures of coagulation. However, it is unlikely that such subtle increases in coagulation parameters would be of great clinical significance. Second, it is a post-hoc analysis, and so any conclusions must be drawn cautiously. This data set also lacks controls of similar elderly patients not on anticoagulants and does not have baseline values of coagulation among the study population when off NOAs. Lastly, due to the infrequency of reversal and heterogeneity of reversal strategies in this study, it is not possible to determine if the current studies (CCA and TEG) could be used to gauge the impact of reversal in patients on NOAs.
In conclusion, among this group of trauma patients on NOAs seen at 16 level 1 trauma centers, neither CCAs nor TEG was able to detect clinically significant coagulopathy at admission, nor were they able to detect a significant impact of reversal attempts. There was also no reliable correlation between CCA and TEG values. With the lack of reliability of the more readily available coagulation assays, current decisions on reversing trauma patients on NOAs must be made based solely on the history of drug administration. Better qualitative and quantitative tests of coagulation such as ECA, ECT, and calibrated drug-specific anti-Xa levels should be strongly considered in the workup of trauma patients on NOAs and used to guide reversal attempts. Future studies should focus on correlating ECA/ECT and calibrated anti-Xa levels values with clinical outcomes and efficacy of reversal agents and protocols.
Footnotes
Contributors: LMK, AB and RC conceived of the study, created the study design, performed the analysis and interpretation, authored and revised the article. PB, CVB, MB, MMC, RDC, JH-N, KI, SK, ALK, TK, EJL, EMM, FOM, JM, RN, DP, JQ, OR, and MS all contributed to data acquisition.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: University of California San Diego IRB committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional data from this study population are available to the participating centers in this multi-institutional trial.
References
- 1. Dossett LA, Riesel JN, Griffin MR, Cotton BA. Prevalence and implications of preinjury warfarin use: an analysis of the National Trauma Databank. Arch Surg 2011;146:565–70. 10.1001/archsurg.2010.313 [DOI] [PubMed] [Google Scholar]
- 2. Maxwell CA, Miller RS, Dietrich MS, Mion LC, Minnick A. The aging of America: a comprehensive look at over 25,000 geriatric trauma admissions to United States hospitals. Am Surg 2015;81:630–6. [DOI] [PubMed] [Google Scholar]
- 3. Siracuse JJ, Robich MP, Gautam S, Kasper EM, Moorman DW, Hauser CJ. Antiplatelet agents, warfarin, and epidemic intracranial hemorrhage. Surgery 2010;148:724–30. 10.1016/j.surg.2010.07.014 [DOI] [PubMed] [Google Scholar]
- 4. Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes 2012;5:615–21. 10.1161/CIRCOUTCOMES.112.967299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baglin T. Clinical use of new oral anticoagulant drugs: dabigatran and rivaroxaban. Br J Haematol 2013;163:160–7. 10.1111/bjh.12502 [DOI] [PubMed] [Google Scholar]
- 6. Dentali F, Riva N, Crowther M, Turpie AG, Lip GY, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation 2012;126:2381–91. 10.1161/CIRCULATIONAHA.112.115410 [DOI] [PubMed] [Google Scholar]
- 7. Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 2012;110:453–60. 10.1016/j.amjcard.2012.03.049 [DOI] [PubMed] [Google Scholar]
- 8. Ogbonna KC, Jeffery SM. Risk versus benefit of non-vitamin K dependent anticoagulants compared to warfarin for the management of atrial fibrillation in the elderly. Drugs Aging 2013;30:513–25. 10.1007/s40266-013-0075-y [DOI] [PubMed] [Google Scholar]
- 9. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et al. . Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 10. Macle L, Cairns J, Leblanc K, Tsang T, Skanes A, Cox JL, Healey JS, Bell A, Pilote L, Andrade JG, et al. . 2016 Focused update of the canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol 2016;32:1170–85. 10.1016/j.cjca.2016.07.591 [DOI] [PubMed] [Google Scholar]
- 11. Ali JT, Daley MJ, Vadiei N, Enright Z, Nguyen J, Ali S, Aydelotte JD, Teixeira PG, Coopwood TB, Brown CV, et al. . Thromboelastogram does not detect pre-injury anticoagulation in acute trauma patients. Am J Emerg Med 2017;35:632–6. 10.1016/j.ajem.2016.12.061 [DOI] [PubMed] [Google Scholar]
- 12. Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest 2017;151:127–38. 10.1016/j.chest.2016.08.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 2014;64:1128–39. 10.1016/j.jacc.2014.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gehrie E, Tormey C. Novel oral anticoagulants: efficacy, laboratory measurement, and approaches to emergent reversal. Arch Pathol Lab Med 2015;139:687–92. 10.5858/arpa.2013-0677-RS [DOI] [PubMed] [Google Scholar]
- 15. Scridon A, Şerban RC. Laboratory monitoring: a turning point in the use of new oral anticoagulants. Ther Drug Monit 2016;38:12–21. 10.1097/FTD.0000000000000247 [DOI] [PubMed] [Google Scholar]
- 16. Liew A, Eikelboom JW, O'Donnell M, Hart RG. Assessment of anticoagulation intensity and management of bleeding with old and new oral anticoagulants. Can J Cardiol 2013;29(7 Suppl):S34–S44. 10.1016/j.cjca.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 17. Bliden KP, Chaudhary R, Mohammed N, Muresan AA, Lopez-Espina CG, Cohen E, Raviv G, Doubleday M, Zaman F, Mathew B, et al. . Determination of non-Vitamin K oral anticoagulant (NOAC) effects using a new-generation thrombelastography TEG 6s system. J Thromb Thrombolysis 2017;43:437–45. 10.1007/s11239-017-1477-1 [DOI] [PubMed] [Google Scholar]
- 18. Dias JD, Norem K, Doorneweerd DD, Thurer RL, Popovsky MA, Omert LA. Use of thromboelastography (teg) for detection of new oral anticoagulants. Arch Pathol Lab Med 2015;139:665–73. 10.5858/arpa.2014-0170-OA [DOI] [PubMed] [Google Scholar]
- 19. Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, Zhelev Z, Hyde C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev 2015;2:Cd010438 10.1002/14651858.CD010438.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9. 10.1161/CIRCULATIONAHA.111.029017 [DOI] [PubMed] [Google Scholar]
- 21. Solbeck S, Ostrowski SR, Stensballe J, Johansson PI. Thrombelastography detects dabigatran at therapeutic concentrations in vitro to the same extent as gold-standard tests. Int J Cardiol 2016;208:14–18. 10.1016/j.ijcard.2016.01.148 [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi L, Barmparas G, Bosarge P, Brown CV, Bukur M, Carrick MM, Catalano RD, Holly-Nicolas J, Inaba K, Kaminski S, et al. . Novel oral anticoagulants and trauma: The results of a prospective American Association for the Surgery of Trauma Multi-Institutional Trial. J Trauma Acute Care Surg 2017;82:827–35. 10.1097/TA.0000000000001414 [DOI] [PubMed] [Google Scholar]
- 23. Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring Oral Direct Inhibitors (ODIs) of thrombin and factor Xa: A recommendation from the subcommittee on control of anticoagulation of the scientific and standardisation committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2013:756–60. 10.1111/jth.12149 [DOI] [PubMed] [Google Scholar]
- 24. Ebner M, Peter A, Spencer C, Härtig F, Birschmann I, Kuhn J, Wolf M, Winter N, Russo F, Zuern CS, et al. . Point-of-care testing of coagulation in patients treated with non-vitamin k antagonist oral anticoagulants. Stroke 2015;46:2741–7. 10.1161/STROKEAHA.115.010148 [DOI] [PubMed] [Google Scholar]
- 25. Samuelson BT, Cuker A. Measurement and reversal of the direct oral anticoagulants. Blood Rev 2017;31:77–84. 10.1016/j.blre.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Testa S, Legnani C, Tripodi A, Paoletti O, Pengo V, Abbate R, Bassi L, Carraro P, Cini M, Paniccia R, et al. . Poor comparability of coagulation screening test with specific measurement in patients receiving direct oral anticoagulants: results from a multicenter/multiplatform study. J Thromb Haemost 2016;14:2194–201. 10.1111/jth.13486 [DOI] [PubMed] [Google Scholar]
- 27. Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, Ezekowitz MD, Nehmiz G, Wang S, Wallentin L, et al. . The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 2014;63:321–8. 10.1016/j.jacc.2013.07.104 [DOI] [PubMed] [Google Scholar]
- 28. Stangier J, Rathgen K, Stähle H, Reseski K, Körnicke T, Roth W. Coadministration of dabigatran etexilate and atorvastatin: assessment of potential impact on pharmacokinetics and pharmacodynamics. Am J Cardiovasc Drugs 2009;9:59–68. [DOI] [PubMed] [Google Scholar]
- 29. Douxfils J, Tamigniau A, Chatelain B, Goffinet C, Dogné JM, Mullier F. Measurement of non-VKA oral anticoagulants versus classic ones: the appropriate use of hemostasis assays. Thromb J 2014;12:24 10.1186/1477-9560-12-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, et al. . Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg 2012;256:476–86. 10.1097/SLA.0b013e3182658180 [DOI] [PubMed] [Google Scholar]
- 31. Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, Huang H, Pinto R, Speers V, Reis M, et al. . Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res 2009;42:1210–7. 10.1590/S0100-879X2009001200015 [DOI] [PubMed] [Google Scholar]
- 32. Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullins RJ. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma 2005;58:475–81. discussion 80-1 10.1097/01.TA.0000153938.77777.26 [DOI] [PubMed] [Google Scholar]


