Summary
Therapeutic effects of photodynamic therapy (PDT) remain largely limited because of tumor hypoxia. Herein, we report safe and versatile nanocatalysts (NCs) for endogenous oxygen generation and imaging-guided enhanced PDT. The NCs (named as PSP) are prepared by coating Prussian blue (PB) with mesoporous silica to load photosensitizer (zinc phthalocyanine, ZnPc), followed by the modification of polyethylene glycol chains. The inner PB not only acts like a catalase for hydrogen peroxide decomposition but also serves as a photothermal agent to increase the local temperature and then speed up the oxygen supply under near-infrared irradiation. The loaded ZnPc can immediately transform the formed oxygen to generate cytotoxic singlet oxygen upon the same laser irradiation due to the overlapped absorption between PB and ZnPc. Results indicate that the PSP-ZnPc (PSPZP) NCs could realize the photothermally controlled improvement of hypoxic condition in cancer cells and tumor tissues, therefore demonstrating enhanced cancer therapy by the incorporation of PDT and photothermal therapy.
Subject Areas: Drug Delivery System, Chemistry, Inorganic Chemistry, Catalysis, Biological Sciences
Graphical Abstract
Highlights
-
•
All compositions have been approved by the US Food and Drug Administration
-
•
PSP-89Zr serves as a dual-modal PET and PAI imaging agent
-
•
PSP shows catalase-like activity toward H2O2 decomposition under tumor-microenvironment
-
•
Photo-enhanced endogenous O2 generation of PSPZP for augmented photodynamic therapy
Drug Delivery System; Chemistry; Inorganic Chemistry; Catalysis; Biological Sciences
Introduction
As a minimally invasive and toxic strategy, photodynamic therapy (PDT) has been popularly studied in clinical fields for localized and superficial cancer treatment (Hopper, 2000, Lucky et al., 2015, Straten et al., 2017). PDT is easily performed; it utilizes a light source to activate the administered photosensitizing molecules conjugated with molecular oxygen (O2) to generate cytotoxic singlet oxygen (1O2) or other kinds of reactive oxygen species (ROS) to induce cell death (Brown et al., 2004, Dolmans et al., 2003, Huang et al., 2012, Zhang et al., 2008). In addition to directly killing cancer cells, PDT appears to shrink or destroy tumors by damaging blood vessels in the tumor or by activating the immune system to attack cancer cells (Dougherty et al., 1998, Juarranz et al., 2008). The O2-involved therapeutic effect of PDT has remarkably improved the selectivity and side effects of traditional therapeutic agents for cancer therapy (Abbas et al., 2017, Noh et al., 2018, Tian et al., 2011). However, the efficiency of PDT is always limited by the rapid O2 consumption because of the O2-dependent treatment process (Jin et al., 2013, Mitchell et al., 1985, See et al., 1984). More importantly, the rapid proliferation of cancer cells as well as the distorted tumor blood vessels would further lower the O2 supply, leading to hypoxia in the tumor site and hindering the O2-involved cancer therapy (Bristow and Hill, 2008, Vander Heiden et al., 2009, Xu et al., 2016, Yang et al., 2017, Zhang et al., 2015).
Over the past decade, two strategies have been mainly explored for enhancing O2 supply against pre-existing hypoxia in the tumor site. One is to increase the O2 concentration in the tumor site by applying intelligent nanoparticles (NPs) to deliver O2 during blood circulation (Chen et al., 2017, Cheng et al., 2015). The other one is to directly produce O2 in the tumor site by using NPs to catalyze abundant hydrogen peroxide (H2O2, usually ranging from 100 μM to 1 mM) in cancer cells resulting from the excess ROS due to tumor hypoxia (Kuang et al., 2011, Szatrowski and Nathan, 1991). Up to now, all kinds of NP-based nanocatalysts (NCs) have been used for H2O2 decomposition and O2 generation to relieve the tumor hypoxia, such as MnO2, CaO2, carbon nitride (C3N4), carbon dot, and biological catalase (Chen et al., 2015a, Cheng et al., 2016, Fan et al., 2015, Huang et al., 2016, Jia et al., 2018, Zheng et al., 2016). The NCs demonstrate an excellent activity for increasing endogenous O2 supply, and therefore could generate more cytotoxic 1O2 for increase in cancer cell death under near-infrared (NIR) irradiation (Ge et al., 2014). Although NC-based PDT has shown great potential for cancer treatment, the excess use of photosensitizers to ensure decent therapeutic efficacy still causes severe side effects, including strong light sensitivity and multiple pain in patients (Calixto et al., 2016, Triesscheijn et al., 2006). Meanwhile, the unpredictable toxicities and uncontrollable catalytic processes of the reported NCs obviously limits their clinical application (Brohi et al., 2017, Romero-Castillo et al., 2017, Srivastava et al., 2015). Thus there is an urgent demand for the development of biocompatible NCs that exhibit stimuli-responsive O2 supply and simultaneously transform O2 to cytotoxic 1O2 for enhanced PDT efficiency in a synergistic manner.
Here, we report a safe multifunctional platform as NC for photo-controlled O2 generation from endogenous H2O2 decomposition and imaging-guided photo-enhanced cancer therapy. All compositions in the NCs have been approved by the US Food and Drug Administration (FDA). Prussian blue (PB) is selected as the building block due to its imaging function and catalase-like activity for H2O2 decomposition (Cheng et al., 2014, Yang et al., 2012, Yang et al., 2018). It is also expected to control the H2O2 decomposition by local temperature variation of PB irradiated with NIR laser because of the photothermal conversion ability of PB NPs. Mesoporous silica is used to coat PB NPs and provide a space for the loading of targeted molecules. After being modified with polyethylene glycol (PEG) molecules, the resultant PB@SiO2-PEG (named as PSP) NCs will possess good aqueous stability, excellent biocompatibility, and long blood circulation time. Zinc phthalocyanine (ZnPc) is chosen as a photosensitizer model because of its overlapping maximum NIR absorption with PB NPs, which can avoid the time interval between photothermal conversion and PDT of NCs (Lin et al., 2013, Mou et al., 2016). Both in vitro and in vivo results show that the PSP-ZnPc (PSPZP) NCs can enhance photodynamic efficacy for cancer therapy under NIR irradiation since the increase in local temperature of photo-enhanced NCs could improve in situ O2 generation at the tumor site.
Results
Preparation and Characterization of the PSPZP NCs
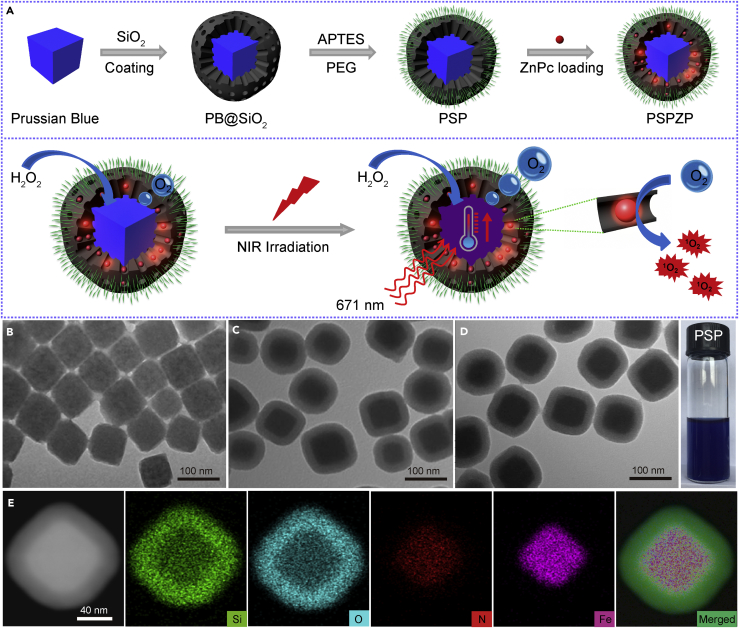
Figure 1A demonstrates the synthetic process of multifunctional PSP NCs with enhanced photo-induced O2 and 1O2 generation. The PB template NPs (see Transparent Methods) possess a uniform size distribution (∼80 nm) and cube-like shape (Figures 1B and S1). After being coated with mesoporous silica through the Stöber method, the as-obtained PB@SiO2 NPs demonstrate a core-shell structure with an average size of 100 nm (Figure 1C). Powder X-ray diffraction pattern and Fourier transform infrared spectroscopy (Figures S2 and S3) confirmed the successful coating of silica on the surface of PB template as evidenced by the existence of asymmetric stretching (1,082 cm−1) and symmetric vibration (797 cm−1) of Si-O-Si and Si-OH (950 cm−1) (Wang et al., 2015). The PB@SiO2 NPs were further functionalized with amino groups (-NH2) with (3-aminopropyl)triethoxysilane, followed by PEGylation with Mal-PEG-SCM (SCM: succinimide; and Mal: maleimide) via amino-SCM click reaction, leading to the formation of biocompatible PSP NCs.
Figure 1.
Synthesis and Characterization of the PSP NCs
(A) Schematic of the synthetic procedure and photo-enhanced therapy of the PSPZP NCs.
(B–D) Transmission electron micrographs of (B) PB NPs, (C) PB@SiO2 NPs, and (D) PSP NCs. The photograph at the right side in (D) shows aqueous PSP dispersion in a vial.
(E) The elemental (Si, O, N, Fe) mappings of PSP NCs.
Transmission electron microscopy (TEM) (Figure 1D) shows that PSP NCs have a good monodispersity with an average size of 120 nm. The elemental mapping data (Figure 1E) of single PSP NCs show that the elements of Fe and N are mainly distributed in the core, whereas Si and O are homogenously distributed in the shell, indicating its core-shell shape with a PB core and SiO2 shell. The hydrodynamic diameter distribution (∼140 nm) of PSP NCs measured by dynamic light scattering is slightly larger than that measured by TEM because of the presence of PEG chains on the surface of NCs (Figure S4). The variation of zeta potential of PB@SiO2 (−32.5 ± 4.3 mV) and PSP NCs (3.2 ± 2.2 mV) reveals that the PEG chains had been successfully immobilized onto the surface of silica (Figure S5). The PEG modification endows excellent stability to the NCs in PBS and saline solutions even after being stored for 20 months (Figure S6).
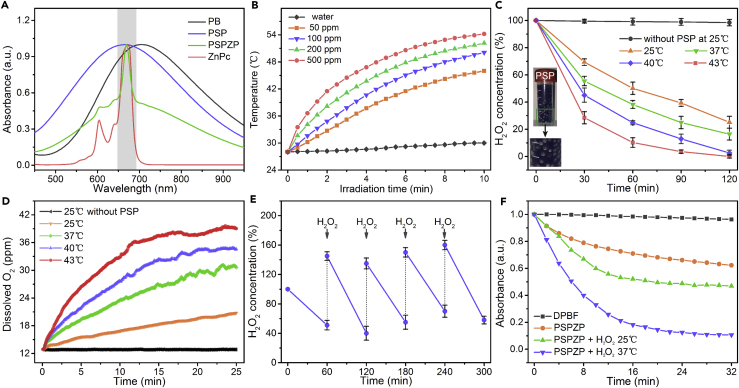
Figure 2A manifests the UV/visible (Vis) absorption spectra of the PB, PSP, ZnPc, and PSPZP NCs. The great overlap between PSP NCs and ZnPc enables PSPZP to function as a combined photothermal/photodynamic agent under the same excitation wavelength using a single NIR laser. Figure 2B shows the photo-induced temperature increase of PSP NCs at different concentrations. Upon 10-min NIR irradiation (671 nm, 0.4 W cm−2), the temperature of the PSP solution (200 ppm) is raised by 25°C, which confirmed the photothermal conversion of PSP NCs (Su et al., 2016, Tian et al., 2017). The UV/Vis absorption spectra of PSP NCs after six heating-cooling cycles have similar intensity, indicating the remarkable stability of NCs under NIR irradiation (Figures S7 and S8). To investigate the ability of PSP NCs for photo-enhanced H2O2 decomposition due to the photothermal conversion of NCs, we carried out time-dependent H2O2 assay under different temperatures. As shown in Figure 2C, more than 50% of H2O2 can be decomposed within 1 hr at room temperature (25°C). The immediate formation of abundant O2 bubbles also reveals the strong catalytic capability of PSP NCs once the NCs were added into H2O2 solution (Figure 2C, inset). With the increase in aqueous temperature (from 25°C to 43°C), the decomposition rate of H2O2 showed an obvious trend of growth. As we know the decomposition reaction of H2O2 is a first-order reaction. The calculated catalytic reaction rate constant under 43°C is 0.0335 min−1, which is 1.93 times than that under 25°C (0.0174 min−1). The generated O2 was measured by an oxygen probe (JPBJ-608 portable Dissolved Oxygen Meters, Shanghai REX Instrument Factory) (Figure 2D). The result showed that the concentration of dissolved O2 at 43°C is 1.95 times that at 25°C. Such improved H2O2 decomposition at higher temperature would make for the increase in PDT efficiency of PSP NCs due to the accelerated O2 supply. Furthermore, the catalytic activity of the PSP NCs has no obvious change after repetitive H2O2 decomposition (Figure 2E), indicating their excellent catalytic stability.
Figure 2.
Characterization of Photothermal and Photodynamic Efficacies
(A) UV/Vis absorption spectra of PB, PSP, ZnPc, and PSPZP (dispersed in DMSO).
(B) Temporal temperature evolutions of PSP solutions at various concentrations under NIR irradiation (671 nm, 0.4 W cm−2).
(C and D) Decomposition (C) and O2 generation (D) of H2O2 treated with PSP NCs or not treated under various temperatures (25°C, 37°C, 40°C, and 43°C).
(E) The catalytic ability of PSP NCs with repetitive addition of H2O2 at room temperature.
(F) Singlet oxygen generation efficiency determined by 1,3-diphenylisobenzonfuran (DPBF) under various conditions.
Error bars were based on the standard error (mean ± SEM, n = 3).
Characterization of Photothermal and Photodynamic Efficacy
Full nitrogen sorption isotherms were obtained to measure the porous properties of PSP NCs. As shown in Figure S9, the Brunauer-Emmett-Teller surface area, pore volume, and pore size of PSP NCs are 427.6 m2 g−1, 0.375 cm3 g−1, and 2.82 nm, respectively. The mesoporous shell and large surface area of PSP NCs make them suitable as carriers for photosensitizer loading. The loading capacity was determined by calculating the absorbance change of the characteristic absorption peak of ZnPc (Figure S10). Results showed that each PSP particle could load about 18,800 ZnPc molecules. Given the effective catalytic ability of PSP for O2 generation, PSPZP NCs have great potential as 1O2 supplier for PDT. The produced 1O2 was determined by measuring the bleaching of 1,3-diphenylisobenzonfuran at 421 nm under Ar atmosphere. Notably, in comparison with the absence of H2O2, a significant increase in 1O2 generation by PSPZP was observed in the presence of H2O2 (Figure 2F) at room temperature. Since the inert atmosphere has completely maintained the hypoxic condition in catalytic system, the increased 1O2 generation should come from PSPZP-induced H2O2 decomposition and O2 generation. Importantly, the 1O2 generation can be controlled through the variation of local temperature (from 25°C to 37°C), indicating that the PDT efficiency of PSPZP NCs should be improved by the photo-induced temperature change resulting from the photothermal conversion effect of NCs under NIR irradiation.
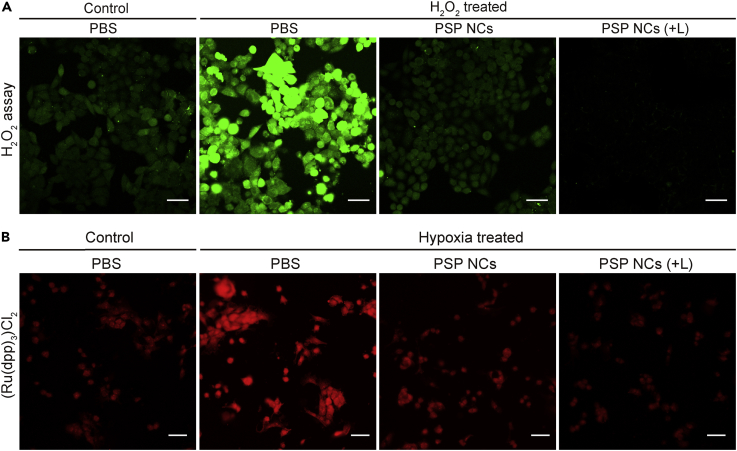
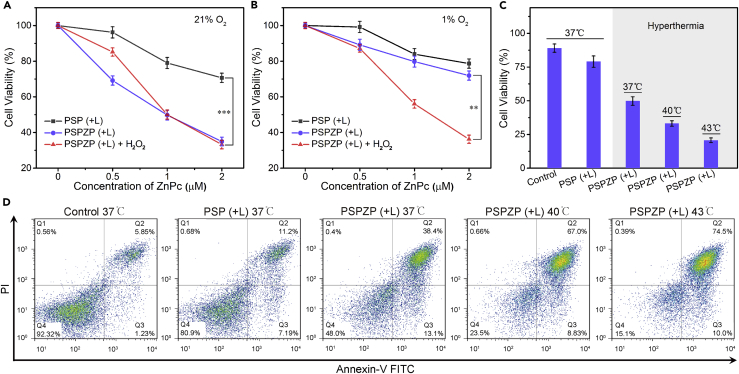
Before conducting the PDT of PSPZP in vitro, the endogenous H2O2 decomposition and O2 generation of PSP NCs was assessed. As shown in Figure 3A, strong green fluorescence shows the high H2O2 level in 4T1 cells in hypoxic condition when treated with 100 μM H2O2. The decrease in fluorescence intensity of the cells treated with PSP NCs indicated that the PSP could efficiently perform endogenous H2O2 decomposition. The disappearance of fluorescence intensity of the cells treated with PSP NCs under NIR irradiation further showed that the external laser could speed up the H2O2 decomposition. In addition, to further evaluate the O2 generation in vitro, an O2 indicator [Ru(dpp)3]Cl2 was applied to monitor the endogenous O2 level (Figure 3B). 4T1 cells incubated under hypoxia condition exhibited the strongest red fluorescence intensity due to the insufficient O2 supply. After being treated with PSP NCs, the reduction in the fluorescence intensity indicates O2 generation due to endogenous H2O2 decomposition, especially with the addition of NIR irradiation. The aforementioned results reveal that PSP NCs can significantly alleviate endogenous hypoxia in vitro via in situ H2O2 decomposition and O2 generation under NIR irradiation. The cytotoxicities of PSP and PSPZP NCs with/without NIR irradiation under hypoxic and normoxic conditions were evaluated. As shown in Figure S11, more than 87% of cells from three cell lines (4T1, HeLa, and A549 cells) survived after 24-hr treatment with PSP at a concentration up to 200 μg mL−1. This indicates that PSP NCs are nontoxic to the tested cells. Meanwhile, 5-min NIR irradiation alone (when the concentration of NCs is 0 μg mL−1) has negligible toxicity in 4T1 cells (Figures 4A and 4B), which shows that the slight decrease in cell viability upon treatment by PSP and PSPZP NCs in hypoxic conditions should come from photothermal therapy (PTT) of NCs under NIR irradiation. The 24-hr treatments with PSPZP NCs in normoxic conditions have more cytotoxicity than treatments in hypoxic conditions. To mimic an H2O2-rich cancer microenvironment, 100 μM H2O2 was added into cell media in hypoxic conditions (Szatrowski and Nathan, 1991). Results show that the introduction of H2O2 could greatly increase the cytotoxicity of PSPZP NCs under NIR irradiation, which should result from the increased 1O2 generation because the photothermal conversion of NCs could speed up the H2O2 decomposition and O2 generation. To further demonstrate the catalytic ability of PSP NCs, mesoporous silica NPs were synthesized through an etching process of PB@SiO2 under alkaline solution (see Transparent Methods). Transmission electron micrographs (Figure S12) show that mesoporous silica without inner PB still maintains its original cubic morphology. Then, ZnPc-loaded mesoporous silica was used to study the O2-dependent PDT ability under hypoxic condition. Results indicated that without inner PB, H2O2 cannot be converted into O2, and thus the killing efficiency will be suppressed (Figure S13). To further confirm the influence of temperature on PDT efficiency, we performed cell viability test of PSPZP NCs under different incubation temperatures. As shown in Figure 4C, with the increase in incubation temperature from 37°C to 43°C, greater cytotoxicities have been observed. Flow cytometry was used to conduct apoptosis analysis of annexin V-fluorescein isothiocyanate and propidium iodide double-stained 4T1 cells treated in different conditions. As shown in Figure 4D, an obvious increased apoptotic rate (Q2 + Q3) is present with the increase of incubation temperature. All in vitro cytotoxic results showed that the local temperature enhanced by the introduction of NIR irradiation plays a key role in increasing the O2 and 1O2 generation, therefore enhancing the PDT efficiency of PSPZP NCs.
Figure 3.
Enhanced H2O2 Decomposition and O2 Generation of PSP NCs in Cells
(A) Confocal laser scanning microscopy (CLSM) images of green fluorescent intracellular H2O2 in 4T1 cells incubated with PBS or PSP NCs treated or not treated by NIR irradiation. Scale bar, 50 μm.
(B) CLSM images of 4T1 cells stained with O2 indicator ((Ru(dpp)3)Cl2) after different treatments (normoxia with PBS as control, hypoxia with PBS, hypoxia with PSP NCs, and hypoxia with PSP NCs plus NIR irradiation). Scale bar, 50 μm.
Figure 4.
Enhanced Cytotoxicity by PSPZP NCs in Normal and Hypoxic Conditions
(A and B) Viabilities of (A) normoxic and (B) hypoxic 4T1 cells treated with PSP or PSPZP with or without addition of H2O2 under NIR irradiation (671 nm, 0.4 W cm−2, 5 min).
(C) Relative viabilities of 4T1 cells incubated with PSP or PSPZP NCs (PSP concentration in both NCs is 50 μg/mL) in normoxic (37°C) and hypoxic (37°C, 40°C, 43°C) environments under NIR irradiation (671 nm, 0.4 W cm−2, 5 min).
(D) Flow cytometry analysis of 4T1 cells after receiving different treatments.
Statistical analysis was performed using the Student's two-tailed test (∗∗p < 0.01 and ∗∗∗p < 0.001).
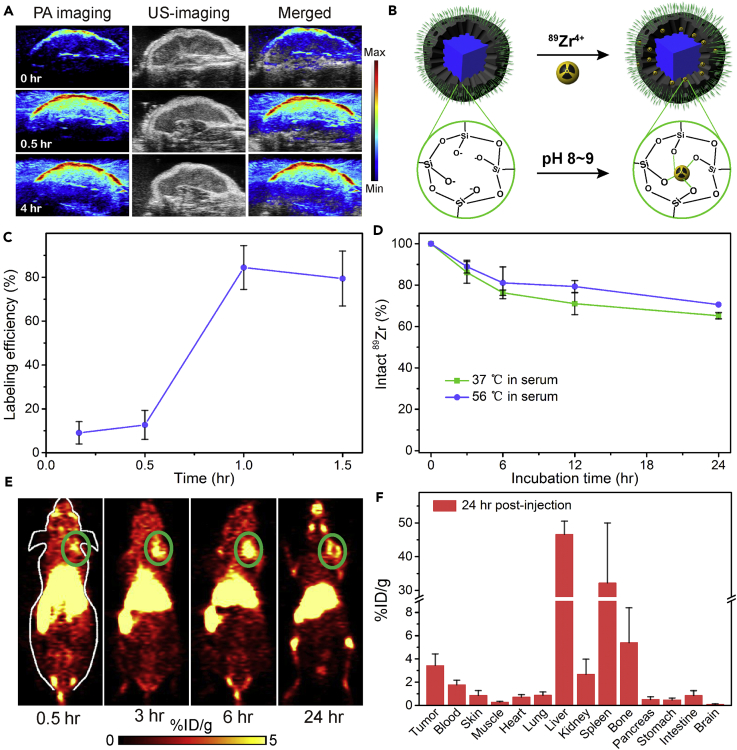
In Vivo Photoacoustic and Positron Emission Tomographic Imaging
As a widely used noninvasive biomedical modality, photoacoustic imaging (PAI) is a newly developed method that employs laser light to acoustically visualize biological tissues (Liba and de La Zerda, 2017). Owing to the strong absorbance in the NIR region, PAI of PSP NCs was assessed in vitro and in vivo. Photoacoustic (PA) signals were first plotted as a function of PSP NCs concentration (Figure S14), which has a linear relationship with a slope of 2.94. 4T1 tumor-bearing mice were selected as model for in vivo imaging. PSP NCs were administered through intravenous injection at a dose of 4 mg kg−1, and the excitation wavelength was fixed at 730 nm. Quantitatively, the average PA signal intensity was increased by 225.8% and 244.7% for tumor at 0.5 and 4 hr post-injection respectively, relative to the signal intensities of the tumor before the injection (Figures 5A and S15).
Figure 5.
In Vivo Photoacoustic and Positron Emission Tomography Imaging of PSP NCs
(A) In vivo 2D ultrasonic and PA imaging of 4T1 tumor in mice injected with PSP NCs acquired at 0 hr and after post-injection (0.5 hr and 4 hr).
(B) Schematic illustration showing the labeling of 89Zr4+ to the deprotonated silanol groups (-Si-O-) from the meso channels.
(C) Quantified labeling yield of 89Zr on PSP NCs at various time points.
(D) Stability test of 89Zr labeling on PSP NCs after incubation in serum at 37°C or 56°C for different periods of time.
(E) PET images of 4T1 tumor-bearing mice taken at various time points (0.5, 3, 6, and 24 hr) after i.v. injection of 89Zr-PSP NCs. The green circles highlight the 4T1 tumor site of mice.
(F) Biodistribution of 89Zr-PSP NCs in different organs at 24 hr after being injected into 4T1 tumor-bearing mice. The unit is the percentage of injected dose per gram of tissue (%ID/g).
Error bars were based on the standard error (mean ± SEM, n = 3).
Positron emission tomography (PET) can offer high sensitivity and accurate quantification when compared with other imaging modalities. It has been widely employed in the field of molecular imaging for disease diagnosis, drug tracking, and treatment monitoring (Thakor and Gambhir, 2013). Radionuclide 89Zr has an optimal half-life (t1/2 = 78.4 hr) and relatively low positron energy (β+avg = 395.5 keV), making it suitable for long-term in vivo tracking of NPs (Yin et al., 2011). For these reasons, 89Zr-based radiopharmaceuticals are now being actively developed for clinical applications, with at least five clinical trials in the United States alone using 89Zr-labeled antibodies (many more in Europe) (Chen et al., 2015b, Goel et al., 2016). PET imaging was further used to evaluate tumor imaging and biodistribution of PSP NCs in vivo. As shown in Figure 5B, 89Zr4+ could be stably loaded into PSP NCs because the deprotonated silanol groups (-Si-O-) in mesoporous silica shell could function as hard Lewis acid sites for stable chelator-free radiolabeling of hard Lewis bases. Herein, results revealed that the labeling yields were 84.5% due to the presence of abundant deprotonated silanol groups inside the mesochannels of mesoporous silica (Figure 5C). To ensure the labeling stability of 89Zr, 89Zr-labeled PSP was mixed with mouse serum and shaken at 37°C and 56°C. By measuring the remaining radioactivities in those samples, we found that 89Zr-PSP was highly stable within 24 hr in serum (Figure 5D). The PSP NCs with stable 89Zr loading were then intravenously injected into mice. As shown in Figure 5E, the obvious tumor contrast was observed 6 hr after injection. These imaging results indicate that the PSP NCs could effectively remain in tumor site due to the enhanced permeability and retention effect of cancerous tumors, and therefore have a great potential for in vivo imaging-guided cancer therapy (Maeda, 2015, Wang et al., 2018). To further study the biodistribution of PSP NCs in vivo, mice treated with 89Zr-PSP NCs were sacrificed 24 hr after injection. The radioactivities in major tissues and organs were measured using a γ-counter (Figure 5F). In addition to the tumors, larger radioactivities were noted in the liver and spleen, which result from the clearance of foreign NPs by macrophage uptake (Liu et al., 2015, Moghimi and Hunter, 2001).
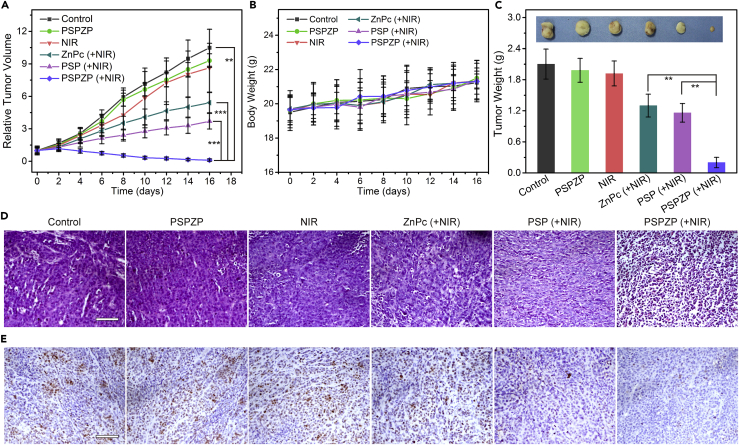
In Vivo PDT and PTT Using Intravenous Injection
To examine the photo-enhanced therapeutic efficacy of PSPZP NCs on treating tumors in vivo, mice bearing 4T1 tumor were administered NCs by intravenous injection, and tumors were then subjected or not subjected to NIR irradiation (671 nm, 0.4 W cm−2) for 5 min at 24 hr post-injection. Tumor-bearing mice were randomly divided into 6 groups with six mice per group. As shown in Figure 6A, mice injected with saline (control) and PSPZP demonstrated the greatest tumor growth. Similarly, NIR irradiation alone has no influence on tumor growth.
Figure 6.
In Vivo PDT and PTT Using Intravenous Injection in a Subcutaneous Tumor Model
(A) Relative tumor volume variation of mice treated under different conditions (n = 6).
(B) Average body weights of mice that received different treatments.
(C) Average weights and typical photographs (inset) of tumors collected from mice at the end of treatments (day 16).
(D) Slices of tumor tissues stained with H&E after different treatments. Scale bar, 50 μm.
(E) Optical microscopic images of tumor sections stained by Ki-67 from different groups. Scale bar, 50 μm.
Statistical analysis was performed using the Student's two-tailed t test (∗∗p < 0.01 and ∗∗∗p < 0.001).
However, tumors in mice treated with ZnPc plus NIR and PSP plus NIR showed obvious reduced growth due to the PDT of ZnPc and PTT of PSP under NIR irradiation, respectively. Notably, a significant tumor inhibition was observed in mice treated with PSPZP plus NIR irradiation. After one round of this treatment, the tumors exhibited no further growth. In addition, mice in all treated groups showed a similar body increase tendency and did not show apparent weight loss (Figure 6B). At day 16, tumors from all groups were harvested and weighed (Figure 6C). The tumor weight in saline-, PSPZP-, or NIR-treated mice is larger than that of mice treated with ZnPc + NIR (PDT) or PSP + NIR (PTT). Importantly, compared with PDT or PTT treatment, the therapeutic efficacy achieved by the combinatory therapy (PSPZP + NIR) appeared to be much higher than the additive efficacy of PDT and PTT used alone based on the tumor growth reduction ratio at day 16, suggesting a synergistic effect of the combinatory therapy. As revealed by micrographs of an H&E-stained tumor slice, prominent cell damage was found in the tumor treated with PSPZP + NIR (Figure 6D). Besides, cancer cells show a higher suppressed proliferation in the PSPZP + NIR group as shown in the Ki-67 staining when compared with other groups (Figure 6E), further indicating the enhanced PDT efficacy. Based on the above results, the PSPZPs NCs demonstrated great potential as an excellent nanoplatform for enhanced PDT due to the photo-enhanced endogenous O2 generation for relieving tumor hypoxia.
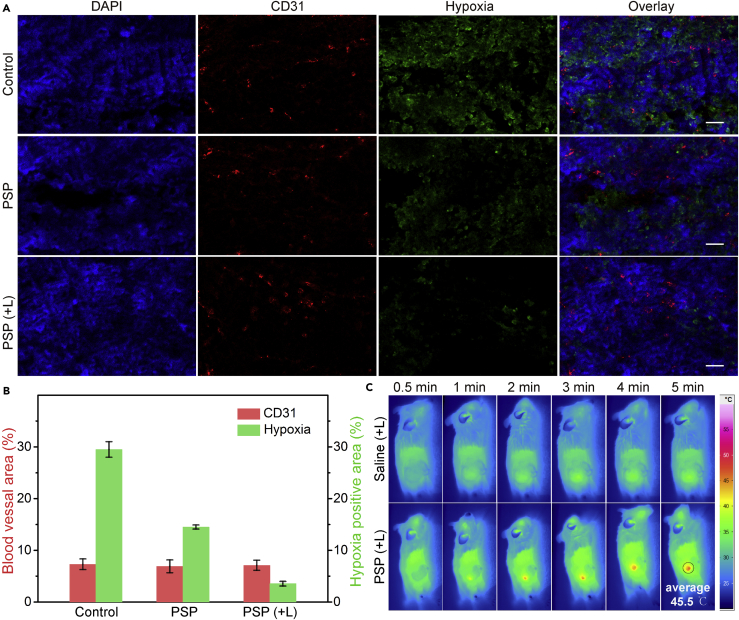
In Vivo Anti-tumor Mechanism and Long-Term Biocompatibility Test
To investigate the ability of NCs to relieve tumor hypoxia in situ for cancer therapy, a hypoxyprobe (pimonidazole) immunofluorescence assay was carried out on tumor slices extracted from mice treated with PSP NCs for 24 hr. Anti-mouse CD31 antibody was used to locate blood vessels inside the tumor. Compared with the control group, the tumor slices from mice treated with PSP NCs showed obviously reduced green fluorescence (pimonidazole-stained hypoxia), indicating that the tumor hypoxia was relieved (Figure 7A). Semi-quantitative statistical analysis of hypoxia-positive areas and blood vessel densities based on nine confocal images further indicated that the PSP NCs, especially with the assistance of NIR irradiation, could greatly reduce tumor hypoxia without affecting blood vessel densities (Figure 7B). Moreover, a significant decrease of green fluorescence was observed in the group treated with PSP NCs plus NIR irradiation. Such photo-enhanced hypoxia improvement should come from the in situ increased endogenous H2O2 decomposition and O2 generation due to the NIR-enhanced local temperature enhancement (Figure 7C). Furthermore, to validate the delivery of PSP NCs to the interior of tumor, in vivo magnetic resonance (MR) imaging was performed as it can provide exquisite anatomical images with high spatial resolution without the restriction of imaging depth (Smith and Gambhir, 2017, Wang et al., 2016). Upon intravenous injection of PSP NCs (20 mg/kg), T1-weighted MR signals gradually lighted up, whereas T2-weighted MR signals decreased in the interior region of tumor sites 24 hr later (Figure S16), indicating that PSP NCs have been delivered to the interior of the tumor, which is considered to be hypoxia (Wilson and Hay, 2011).
Figure 7.
In Vivo Anti-tumor Mechanism of PDT and PTT
(A) Representative immunofluorescence images of tumor slices after hypoxia staining. The hypoxia areas and blood vessels were stained by anti-pimonidazole antibody (green) and anti-CD31 antibody (red), respectively. Scale bar, 50 μm.
(B) The relative hypoxia-positive areas and blood vessel densities as recorded from nine images using the ImageJ software.
(C) Full-body thermographic images of 4T1-tumor-bearing mice treated with saline and PSP NCs within 5-min NIR irradiation.
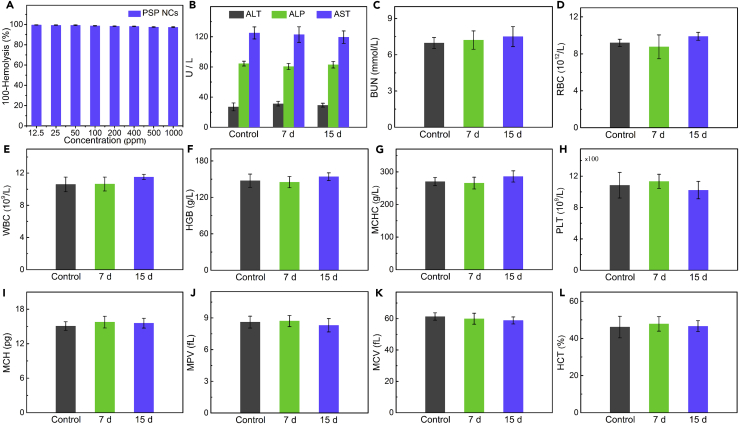
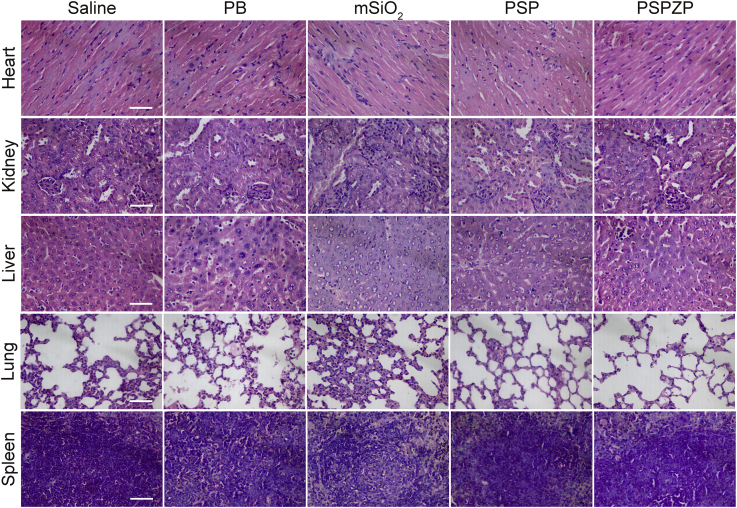
The hemolysis of PSPZP NCs was studied to test their safety in vivo. As shown in Figure 8A, no visible hemolytic effects (<3%) were observed even at a PSP concentration up to 1,000 μg mL−1. The long-term toxicity of PSPZP NCs on healthy tissues was also investigated via in vivo blood biochemistry test, blood routine analysis, and H&E staining. The blood of healthy BALB/c mice was drawn from the tail artery at different time points (7 and 15 days) after intravenous injection of PSPZP NCs, whereas an untreated group was used as the control. For blood biochemistry test, alanine transaminase, alkaline phosphatase, aspartate aminotransferase, and blood urea nitrogen served as hepatic and renal function markers. As shown in Figures 8B and 8C, no significant changes were observed between control, 7 days, and 15 days, indicating the good hepatic and renal safety property of PSPZP NCs. For blood routine analysis, parameters such as red blood cells, white blood cell, hemoglobin, mean corpuscular hemoglobin concentration, platelets, mean corpuscular hemoglobin, mean platelet volume, mean corpuscular volume, and hematocrit were measured (Figures 8D–8L). All the nine markers are well within the normal ranges, suggesting the negligible in vivo toxicity of PSPZP at the treatment dose within 15 days (Liu et al., 2016). The potential toxicity of nanoscale therapeutic agent has been a major safety concern for clinical applications. To test the biocompatibility of the NCs in vivo, we performed histological analysis on various tissues (heart, kidney, liver, lung, and spleen) from mice injected with PBS, PB NPs, mSiO2, PSP NCs, and PSPZP NCs to identify signs of acute toxicity. Tissues were harvested from mice 120 hr after NC injection, fixed in 10% formalin, embedded in paraffin, sectioned, and stained with H&E. As shown in Figure 9, no obvious tissue damage or inflammatory lesion in these main organs was observed, which indicates that all samples have no obvious damage in normal tissues. Thanks to the FDA-approved compositions in the nanostructure, the reported NCs herein are nontoxic and safe and suitable for biomedical applications.
Figure 8.
Long-Term Biocompatibility Test
(A) Hemolysis of PSPZP NCs at various concentrations.
(B) The levels of alanine transaminase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST) of untreated mice (control) and PSPZP NC-injected mice at 7 and 15 days.
(C) The level of blood urea nitrogen (BUN) of untreated mice (control) and PSPZP NC-injected mice at 7 and 15 days.
(D–L) Hematology data of red blood cell (RBC) (D), white blood cell (WBC) (E), hemoglobin (HGB) (F), mean corpuscular hemoglobin concentration (MCHC) (G), platelet (PLT) (H), mean corpuscular hemoglobin (MCH) (I), mean platelet volume (MPV) (J), mean corpuscular volume (MCV) (K), and hematocrit (HCT) (L) in the untreated mice (control) and in mice treated with the PSPZP NCs. Error bars were based on the standard error (mean ± SEM, n = 3).
Figure 9.
H&E Staining
H&E-stained tissue sections of mouse heart, kidney, liver, lung, and spleen obtained from saline-injected animals, and those injected with PB NPs, mSiO2, PSP NCs, and PSPZP NCs. Scale bar, 50 μm.
Discussion
In summary, multifunctional and safe PSP NCs have been successfully synthesized by the surface coating of biocompatible PB NPs with mesoporous silica shell modified with PEG chains. Combining each function of building blocks, the as-synthesized PSP NCs not only maintain the properties of the individual constituents but also display cooperative properties for photo-enhanced H2O2 decomposition and O2 generation. In vitro and in vivo results show that the single laser-induced 1O2 generation could in situ relieve cancer hypoxia for enhanced cancer therapy by combined photothermal therapy/PDT. The NIR absorption and the 89Zr loading of PSP endow NCs the dual-modal PET and PAI imaging function to identify the tumor location and monitor the treatment progress. Such designed NCs demonstrate great promise toward advanced nanoplatforms for simultaneous imaging diagnostics and high-efficacy therapy.
Limitations of Study
This work proposes a new possibility to construct multifunctional system through photo-induced local temperature enhancement for modulating catalytic reaction toward tumor-overexpressed hydrogen peroxide for augmented PDT. Although demonstrated experimentally, the low tumor accumulation and lack of targeting agent limit the further optimization of treatment efficiency for such a multifunctional system.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the MOST Grant 2016YFA0101202; The National Natural Science Foundation of China, 21571168, 31471268, and U1232211; National Key R&D Program of China 2016YFA0401801; Innovative Program of Development Foundation of Hefei Center for Physical Science and Technology (2016FXCX005); CAS/SAFEA International Partnership Program for Creative Research Teams and CAS Hefei Science Center (2016HSC-IU011); the NIH (P30CA014520); and the American Cancer Society (125246-RSG-13-099-01-CCE). The authors are also thankful for the support of the Core Facility Center for Life Sciences of University of Science and Technology of China for imaging support.
Author Contributions
Conceptualization, Q.C., W.C. and Z.G.; Methodology, D.W., R.S., H.Wang and G.X.; Investigation, D.W., R.S. and P.X.; Experiments, D.W., R.S., J.Z., S.S. and H.Wu; Writing – Original Draft, D.W. and R.S.; Writing – Review & Editing, D.W. and H.Wang; Funding Acquisition, Q.C., Z.G., and W.C.; Resources, Q.C., W.C., Z.G. and T.E.B.; Supervision, Q.C., Z.G., and W.C.; D.W., R.S. and J.Z. conducted the project and contributed equally. Dedicated to University of Science and Technology of China (USTC) on the occasion of its 60th anniversary.
Declaration of Interests
The authors declare no competing interest.
Published: November 30, 2018
Footnotes
Supplemental Information includes Transparent Methods and 16 figures and can be found with this article online at https://doi.org/10.1016/j.isci.2018.10.005.
Contributor Information
Hui Wang, Email: hw39@hmfl.ac.cn.
Weibo Cai, Email: wcai@uwhealth.org.
Zhen Guo, Email: zhenguo@ustc.edu.cn.
Qianwang Chen, Email: cqw@ustc.edu.cn.
Supplemental Information
References
- Abbas M., Zou Q., Li S., Yan X. Self-assembled peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Adv. Mater. 2017;29:1605021. doi: 10.1002/adma.201605021. [DOI] [PubMed] [Google Scholar]
- Bristow R.G., Hill R.P. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer. 2008;8:180. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- Brohi R.D., Wang L., Talpur H.S., Wu D., Khan F.A., Bhattarai D., Rehman Z.U., Farmanullah F., Huo L.J. Toxicity of nanoparticles on the reproductive system in animal models: a review. Front. Pharmacol. 2017;8:00606. doi: 10.3389/fphar.2017.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.B., Brown E.A., Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- Calixto G., Bernegossi J., Freitas L., Fontana C., Chorilli M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: a review. Molecules. 2016;21:342. doi: 10.3390/molecules21030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Tian J., He W., Guo Z. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 2015;137:1539–1547. doi: 10.1021/ja511420n. [DOI] [PubMed] [Google Scholar]
- Chen F., Goel S., Valdovinos H.F., Luo H., Hernandez R., Barnhart T.E., Cai W. In vivo integrity and biological fate of chelator-free zirconium-89-labeled mesoporous silica nanoparticles. ACS Nano. 2015;9:7950–7959. doi: 10.1021/acsnano.5b00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Luo H., Liu Y., Zhang W., Li H., Luo T., Zhang K., Zhao Y., Liu J. Oxygen-self-produced nanoplatform for relieving hypoxia and breaking resistance to sonodynamic treatment of pancreatic cancer. ACS Nano. 2017;11:12849–12862. doi: 10.1021/acsnano.7b08225. [DOI] [PubMed] [Google Scholar]
- Cheng L., Gong H., Zhu W., Liu J., Wang X., Liu G., Liu Z. PEGylated Prussian blue nanocubes as a theranostic agent for simultaneous cancer imaging and photothermal therapy. Biomaterials. 2014;35:9844–9852. doi: 10.1016/j.biomaterials.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Cheng H., Jiang C., Qiu X., Wang K., Huan W., Yuan A., Wu J., Hu Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015;6:8785. doi: 10.1038/ncomms9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Zhu J.Y., Li S.Y., Zeng J.Y., Lei Q., Chen K.W., Zhang C., Zhang X.Z. An O2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy. Adv. Funct. Mater. 2016;26:7847–7860. [Google Scholar]
- Dolmans D.E., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Dougherty T.J., Gomer C.J., Henderson B.W., Jori G., Kessel D., Korbelik M., Moan J., Peng Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Bu W., Shen B., He Q., Cui Z., Liu Y., Zheng X., Zhao K., Shi J. Intelligent MnO2 nanosheets anchored with upconversion nanoprobes for concurrent pH-/H2O2-responsive UCL imaging and oxygen-elevated synergetic therapy. Adv. Mater. 2015;27:4155–4161. doi: 10.1002/adma.201405141. [DOI] [PubMed] [Google Scholar]
- Ge J., Lan M., Zhou B., Liu W., Guo L., Wang H., Jia Q., Niu G., Huang X., Zhou H. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014;5:4596. doi: 10.1038/ncomms5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S., Chen F., Luan S., Valdovinos H.F., Shi S., Graves S.A., Ai F., Barnhart T.E., Theuer C.P., Cai W. Engineering intrinsically zirconium-89 radiolabeled self-destructing mesoporous silica nanostructures for in vivo biodistribution and tumor targeting studies. Adv. Sci. 2016;3:1600122. doi: 10.1002/advs.201600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000;1:212–219. doi: 10.1016/s1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Huang P., Lin J., Wang X., Wang Z., Zhang C., He M., Wang K., Chen F., Li Z., Shen G. Light-triggered theranostics based on photosensitizer-conjugated carbon dots for simultaneous enhanced-fluorescence imaging and photodynamic therapy. Adv. Mater. 2012;24:5104–5110. doi: 10.1002/adma.201200650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.C., Chia W.T., Chung M.F., Lin K.J., Hsiao C.W., Jin C., Lim W.H., Chen C.C., Sung H.W. An implantable depot that can generate oxygen in situ for overcoming hypoxia-induced resistance to anticancer drugs in chemotherapy. J. Am. Chem. Soc. 2016;138:5222–5225. doi: 10.1021/jacs.6b01784. [DOI] [PubMed] [Google Scholar]
- Jia Q., Ge J., Liu W., Zheng X., Chen S., Wen Y., Zhang H., Wang P. A Magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv. Mater. 2018;30:6069. doi: 10.1002/adma.201706090. [DOI] [PubMed] [Google Scholar]
- Jin C.S., Lovell J.F., Chen J., Zheng G. Ablation of hypoxic tumors with dose-equivalent photothermal, but not photodynamic, therapy using a nanostructured porphyrin assembly. ACS Nano. 2013;7:2541–2550. doi: 10.1021/nn3058642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarranz Á., Jaén P., Sanz-Rodríguez F., Cuevas J., González S. Photodynamic therapy of cancer. basic principles and applications. Clin. Transl. Oncol. 2008;10:148–154. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- Kuang Y., Balakrishnan K., Gandhi V., Peng X. Hydrogen peroxide inducible DNA cross-linking agents: targeted anticancer prodrugs. J. Am. Chem. Soc. 2011;133:19278–19281. doi: 10.1021/ja2073824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liba O., de La Zerda A. Photoacoustic tomography: breathtaking whole-body imaging. Nat. Biomed. Eng. 2017;1:0075. [Google Scholar]
- Lin J., Wang S.J., Huang P., Wang Z., Chen S.H., Niu G., Li W.W., He J., Cui D.X., Lu G.M. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano. 2013;7:5320–5329. doi: 10.1021/nn4011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Shi S., Liang C., Shen S., Cheng L., Wang C., Song X., Goel S., Barnhart T.E., Cai W., Liu Z. Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy. ACS Nano. 2015;9:950–960. doi: 10.1021/nn506757x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yang Y., Zhu W., Yi X., Dong Z., Xu X., Chen M., Yang K., Lu G., Jiang L., Liu Z. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials. 2016;97:1–9. doi: 10.1016/j.biomaterials.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Lucky S.S., Soo K.C., Zhang Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Mitchell J.B., McPherson S., DeGraff W., Gamson J., Zabell A., Russo A. Oxygen dependence of hematoporphyrin derivative-induced photoinactivation of Chinese hamster cells. Cancer Res. 1985;45:2008–2011. [PubMed] [Google Scholar]
- Moghimi S.M., Hunter C. Capture of stealth nanoparticles by the body's defences. Crit. Rev. Ther. Drug Carrier Syst. 2001;18:527–550. [PubMed] [Google Scholar]
- Mou J., Lin T., Huang F., Chen H., Shi J. Black titania-based theranostic nanoplatform for single NIR laser induced dual-modal imaging-guided PTT/PDT. Biomaterials. 2016;84:13–24. doi: 10.1016/j.biomaterials.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Noh I., Lee D., Kim H., Jeong C.U., Lee Y., Ahn J.O., Hyun H., Park J.H., Kim Y.C. Enhanced photodynamic cancer treatment by mitochondria-targeting and brominated near-infrared fluorophores. Adv. Sci. 2018;5:1700481. doi: 10.1002/advs.201700481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Castillo L., Posadas I., Ceña V. Exploring the in vivo toxicity of nanoparticles. Can. J. Chem. 2017;95:917–926. [Google Scholar]
- See K.L., Forbes I.J., Betts W.H. Oxygen dependency of photocytotoxicity with haematoporphyrin derivative. Photochem. Photobiol. 1984;39:631–634. doi: 10.1111/j.1751-1097.1984.tb03902.x. [DOI] [PubMed] [Google Scholar]
- Smith B.R., Gambhir S.S. Nanomaterials for in vivo imaging. Chem. Rev. 2017;117:901–986. doi: 10.1021/acs.chemrev.6b00073. [DOI] [PubMed] [Google Scholar]
- Srivastava V., Gusain D., Sharma Y.C. Critical review on the toxicity of some widely used engineered nanoparticles. Ind. Eng. Chem. Res. 2015;54:6209–6233. [Google Scholar]
- Straten V.D., Mashayekhi V., Bruijn H., Oliveira S., Robinson D. Oncologic photodynamic therapy: basic principles, current clinical status and future directions. Cancers (Basel) 2017;9:19. doi: 10.3390/cancers9020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Teng Z., Yao H., Wang S., Tian Y., Zhang Y., Liu W., Tian W., Zheng L., Lu N. A multifunctional PB@mSiO2-PEG/DOX nanoplatform for combined photothermal-chemotherapy of tumor. ACS Appl. Mater. Interfaces. 2016;8:17038–17046. doi: 10.1021/acsami.6b01147. [DOI] [PubMed] [Google Scholar]
- Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- Thakor A.S., Gambhir S.S. Nanooncology: the future of cancer diagnosis and therapy. CA Cancer J. Clin. 2013;63:395–418. doi: 10.3322/caac.21199. [DOI] [PubMed] [Google Scholar]
- Tian B., Wang C., Zhang S., Feng L., Liu Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano. 2011;5:7000–7009. doi: 10.1021/nn201560b. [DOI] [PubMed] [Google Scholar]
- Tian W., Su Y., Tian Y., Wang S., Su X., Liu Y., Zhang Y., Tang Y., Ni Q., Liu W. Periodic mesoporous organosilica coated prussian blue for MR/PA dual-modal imaging-guided photothermal-chemotherapy of triple negative breast cancer. Adv. Sci. 2017;4:1600356. doi: 10.1002/advs.201600356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triesscheijn M., Baas P., Schellens J., Stewart F.A. Photodynamic therapy in oncology. Oncologist. 2006;11:1034–1044. doi: 10.1634/theoncologist.11-9-1034. [DOI] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Guo Z., Zhou J., Chen J., Zhao G., Chen R., He M., Liu Z., Wang H., Chen Q. Novel Mn3[Co(CN)6]2@SiO2@Ag Core-shell nanocube: enhanced two-photon fluorescence and magnetic resonance dual-modal imaging-guided photothermal and chemo-therapy. Small. 2015;11:5956–5967. doi: 10.1002/smll.201502102. [DOI] [PubMed] [Google Scholar]
- Wang D., Zhou J., Chen R., Shi R., Zhao G., Xia G., Li R., Liu Z., Tian J., Wang H. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials. 2016;100:27–40. doi: 10.1016/j.biomaterials.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Wang D., Wu H., Zhou J., Xu P., Wang C., Shi R., Wang H., Wang H., Guo Z., Chen Q. In situ one-pot synthesis of MOF-polydopamine hybrid nanogels with enhanced photothermal effect for targeted cancer therapy. Adv. Sci. 2018;5:1800287. doi: 10.1002/advs.201800287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W.R., Hay M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Xu R., Wang Y., Duan X., Lu K., Micheroni D., Hu A., Lin W. Nanoscale metal-organic frameworks for ratiometric oxygen sensing in live cells. J. Am. Chem. Soc. 2016;138:2158–2161. doi: 10.1021/jacs.5b13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Hu S., Zhang Y., Cai X., Huang Y., Wang F., Wen S., Teng G., Gu N. A hydrogen peroxide-responsive O(2) nanogenerator for ultrasound and magnetic-resonance dual modality imaging. Adv. Mater. 2012;24:5205–5211. doi: 10.1002/adma.201202367. [DOI] [PubMed] [Google Scholar]
- Yang G., Xu L., Chao Y., Xu J., Sun X., Wu Y., Peng R., Liu Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017;8:902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.L., Tian W., Wang Q., Zhao Y., Zhang Y.L., Tian Y., Tang Y.X., Wang S.J., Liu Y., Ni Q.Q. Oxygen-evolving mesoporous organosilica coated prussian blue nanoplatform for highly efficient photodynamic therapy of tumors. Adv. Sci. (Weinh) 2018;5:1700847. doi: 10.1002/advs.201700847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z., Hao H., Cai W. PET tracers based on zirconium-89. Curr. Radiopharm. 2011;4:131–139. doi: 10.2174/1874471011104020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Murakami T., Ajima K., Tsuchida K., Sandanayaka A.S., Ito O., Iijima S., Yudasaka M. Fabrication of ZnPc/protein nanohorns for double photodynamic and hyperthermic cancer phototherapy. Proc. Natl. Acad. Sci. U S A. 2008;105:14773–14778. doi: 10.1073/pnas.0801349105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhao K., Bu W., Ni D., Liu Y., Feng J., Shi J. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. 2015;54:1770–1774. doi: 10.1002/anie.201408472. [DOI] [PubMed] [Google Scholar]
- Zheng D.W., Li B., Li C.X., Fan J.X., Lei Q., Li C., Xu Z., Zhang X.Z. Carbon-dot-decorated carbon nitride nanoparticles for enhanced photodynamic therapy against hypoxic tumor via water splitting. ACS Nano. 2016;10:8715–8722. doi: 10.1021/acsnano.6b04156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.