Abstract
Objective:
To investigate the relationship between aldosterone, use of anti-hypertensive medications, clinical profile and atrial natriuretic peptide (ANP) in hypertensive subjects from the general community.
Patients and Methods:
In a community-based cohort, we analyzed aldosterone plasma levels based on presence (n=477) or absence (n=1073) of hypertension (HTN). In HTN subjects, we evaluated circulating levels of aldosterone according to the number of anti-HTN drugs used, analyzed the associated clinical characteristics and determined the relationship to the counter-regulatory cardiac hormone ANP. Data were collected from August 25 1997 to September 5 2000.
Results:
In the general population, HTN subjects had higher serum aldosterone levels when compared to subjects without HTN (6.4 vs 4.1ng/dL, P < .001). When subjects with HTN were stratified according to the number of anti-HTN medications used, the increase in number of medications (0,1, 2, 3 or more) was associated with higher aldosterone levels (4.8 vs 6.4 vs 7.10 vs 7.9 mg/dL, P= .002), worse metabolic profile and higher prevalence of cardiovascular, renal and metabolic disease. In HTN subjects, ANP plasma levels were inversely related to aldosterone levels when the latest was divided into tertiles.
Conclusions:
In this randomly selected cohort from the general population, aldosterone levels are higher in HTN subjects compared to normotensive subjects. Notably, aldosterone levels increase with the use of anti-HTN medications. Our findings also suggest a relative ANP deficiency with increasing aldosterone levels and use of anti-HTN drugs. These studies have pathophysiological and therapeutic implications for targeting aldosterone in the clinical treatment of HTN.
Keywords: aldosterone, hypertension, anti-hypertensive therapy, atrial natriuretic peptide
INTRODUCTION
Aldosterone is a hormone that plays a fundamental role in intravascular volume and blood pressure (BP) homeostasis. Beyond its physiological role and through activation of the mineralocorticoid receptor (MR), aldosterone may also exert actions leading to organ damage in the heart, kidneys and vasculature.1 Seminal studies by the Calhoun laboratory have importantly advanced aldosterone as a key factor in hypertension (HTN), most importantly in resistant HTN.2,3,4 Indeed, the successful use of the MR antagonist spironolactone in the PATHWAY-2 Trial in RH subjects, led to the conclusion that aldosterone may be the predominant underlying pathophysiological cause of RH through sodium retention.5
We recently reported in a general population study that plasma aldosterone levels, even within normal range, are significantly associated with HTN as well as chronic kidney disease (CKD) and MetS6 and also predicted these diseases in the future.7 The influence of aldosterone in early stage HTN is also supported by Vasan et al. who reported that increased aldosterone levels within the normal range are associated with new onset HTN in non-HTN subjects.8
Most recently, the American College of Cardiology and the American Heart Association (ACC/AHA) released the 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults.9 In part, the new landmark recommendations were a product of the SPRINT Trial.10This seminal study validated the importance of optimizing anti-HTN therapy in order to achieve BP control (systolic BP < 120 mmHg), improve survival and reduce onset of adverse cardiovascular (CV) outcomes in HTN subjects at high CV risk. The relevance of SPRINT to the general US population was recently supported by Bress et al. who reported that a substantial percentage of US adults meets the eligibility criteria for SPRINT supporting its generalizability to the general US population.11 As the 2017 ACC/AHA guidelines propose more aggressive goals for treatment, a high priority is to better characterize the clinical phenotype of adults treated with one or more anti-HTN agents. The in-depth characterization could provide pathophysiologic and therapeutic insights, which may help optimize anti-HTN strategies. Furthermore, with the growing role of aldosterone as a therapeutic target as well as biomarker in HTN, there is also a strong rationale to investigate the relationship between aldosterone levels and anti-HTN therapy in HTN subjects.
Hence, the current study utilized a well-characterized, randomly selected, adult community-based cohort using the Rochester Epidemiology Project in Olmsted County, MN.12 We hypothesized that aldosterone would be increased in subjects with HTN, compared to those without a diagnosis, of HTN. We also tested the hypothesis that aldosterone would be progressively higher with increasing number of anti-HTN drugs used. Lastly, based on previous studies, we hypothesized that in subjects with HTN, plasma aldosterone would be characterized by an inverse relationship with the counter-regulatory hormone atrial natriuretic peptide (ANP).6,13,14,15
PATIENTS and METHODS
Study Population.
The Mayo Clinic Institutional Review Board approved this study and the subjects gave informed consent. Using the resources of the Rochester Epidemiology Project at Mayo Clinic, we analyzed a previously studied random sample of subjects from the general population from Olmsted County, MN.12 Specifically, 4203 eligible residents were eligible and of these 2024 were enrolled. The design, selection criteria and characteristics of this cohort have been previously described.16 A trained nurse abstractors reviewed the medical record for each subject and documented the clinical diagnosis of HTN, myocardial infarction (MI), coronary artery disease and/or diabetes mellitus (DM). Each subject underwent an in-depth physical examination including measurement of BP, height and weight. For the current study, 1550 subjects, who underwent a visit between August 25 1997 and September 5 2000, were analyzed. All subjects had plasma aldosterone and ANP measured and their use and number of drugs or non-use of anti-HTN medications were carefully documented with 1550 of the 2024 having both aldosterone and ANP levels available. Of the 1550 subjects, 1073 subjects were without a diagnosis of HTN (non-HTN subjects) and 477 subjects had a diagnosis of HTN (HTN subjects).
For anti-HTN therapy, we considered the following drugs: beta-blockers (BBs), calcium channel blockers (including dihydropyridines and non-dihydropyridines), vasodilators (including α1-blockers, reserpine and central α2-agonists), angiotensin ll receptor blockers, angiotensin converting enzyme inhibitors and all classes of diuretics such as thiazides, thiazides-like, loop diuretics, potassium-sparing and mineralcorticoid receptor antagonists (MRAs). Lipid-lowering therapy was defined as the use of one or more of the following drugs: statins, fibrates, niacin, ezetimibe and cholestyramine.
To better define associated phenotypes to aldosterone and HTN, body mass index (BMI) was defined with established criteria as previously described.6 Obesity was defined as a BMI ≥ 30 kg/m2. Waist circumference, measured at the top of the umbilicus, was expressed in centimeters (cms) and central obesity was defined as waist circumference >102 cm in men and >88 cm in women. Hypertension was defined according to the use of Joint National Committee VII diagnosis criteria and BP at the visit was measured three times at 5-minute intervals.6 Smoking status was defined as never, prior, or active. Chronic kidney disease was defined as a glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 based on the Modification of Diet in Renal Disease (MDRD) formula. The MetS was defined in accordance with the National Cholesterol Education Program Adult Treatment Panel III, as previously described.6
Plasma Collection.
Blood samples were obtained from subjects in the sitting position and there was no discontinuation of any therapy or change in salt intake prior to the blood collection. Blood was drawn in EDTA tubes and chilled until it was centrifuged at 4°C at 2500 g for 10 minutes. 0.5 mL plasma was aliquoted into polystyrene tubes and stored at − 80°C until assayed.
Aldosterone and Atrial Natriuretic Peptide Assays.
Plasma aldosterone was measured using a competitive radioimmunoassay (RIA) kit (Siemens, Los Angeles, CA) as previously defined.6 Samples were all obtained in the morning around 8.00 am. Plasma ANP was measured as previously described with a RIA using antibody to human ANP (Phoenix Pharmaceutical, Burlingame CA).17
Echocardiography.
Echocardiograms were performed as previously described.16
Statistical analyses.
Characteristics of patients were summarized after separating into various subgroups of interest. These characteristics are presented as number and percent for categorical variables or as mean and standard deviation for continuous variables that were approximately normally distributed. For those continuous variables that were not normally distributed, median and tertiles were used to characterize the distribution. Group differences were tested using logistic regression for categorical variables or linear regression for continuous variables, after normalizing transformation. These models included gender as well as age and BMI as continuous covariates in order to test whether group differences were independent of these factors that were observed to be different between groups. When HTN subjects were stratified into groups based on number of anti-HTN drugs taken, these tests were performed using linear or logistic regression adjusted for age, gender and BMI. Number of HTN drugs was used as an ordinal variable in these analyses, resulting in tests for trend across these groups. In order to investigate the association between aldosterone and ANP while controlling for potential confounders, a generalized linear regression model was used. ANP was log-transformed for this analysis. Age, gender, BMI, MI, heart failure, atrial fibrillation and GFR were used as covariates and adjusted means with corresponding 95% confidence intervals for each tertile of aldosterone, considered as a nominal variable, were presented. In order to take advantage of the ordinal nature of the aldosterone tertiles and create a trend test, the p-value is presented from a model where tertile (1–3) was considered as an ordinal variable. For all analyses, two-sided P-values <.05 were considered to be statistically significant. Analyses were performed using SAS version 9.4 (Cary, NC).
RESULTS
Characteristics of the Study Cohort According to the Diagnosis of Hypertension
As shown in Table 1 compared to non-HTN subjects, HTN subjects were older, had higher ANP levels and higher plasma aldosterone levels. Importantly, when we excluded subjects with aldosterone levels higher the upper normal range of 16.2 ng/dl,6 subjects with hypertension still had significantly higher aldosterone levels than subjects without hypertension [5.65 (1.7, 16.2) ng/dl vs 4.00 (2.5, 16.2) ng/dl, age-sex-BMI-adjusted P<.001]. As expected, the group of HTN subjects presented with higher systolic blood pressure (SBP) and diastolic BP values, lower GFR, higher circulating insulin and glucose levels, more prevalent use of antilipemic treatment, increased BMI, greater prevalence of obesity and abdominal obesity, CKD, MetS, DM, coronary artery disease, MI, HF and cerebrovascular accident. Echocardiography revealed greater prevalence of reduced EF, LVH, cLVH and mild to moderate/severe diastolic dysfunction. In the non-HTN individuals (n=1073), a subgroup of 174 subjects were taking medications classified as anti-HTN in our study.
Table 1.
Clinical Characteristics of Subjects with and without Diagnosis of Hypertension
| Non-HTN Subjects | HTN Subjects | ||
|---|---|---|---|
| Characteristics | (N=1073) | (N=477) | P Valuea |
| Age, y | 62 ± 10 | 67 ± 10 | <.001 |
| Female Gender, n (%) | 615 (57%) | 270 (57%) | .92 |
| Aldosterone, ng/dL, median (min, max) | 4.10 (2.5, 51.1) | 6.40 (1.7, 91.0) | <.001 |
| Aldo in tertiles, n (%) | <.001 | ||
| 1 | 400 (37%) | 114 (24%) | |
| 2 | 393 (37%) | 128 (27%) | |
| 3 | 280 (26%) | 235 (49%) | |
| ANP, pg/ml | 11.10 (7.40, 15.70) | 13.40 (8.60, 19.40) | .01 |
| BMI, kg/m2 | 28 ± 5 | 30 ± 6 | <.001 |
| Obesity, n (%) | 287 (27%) | 213 (45%) | <.001 |
| Waist circumference > 102 cm in males, > 88 cm in females, n (%) | 303 (28%) | 213 (45%) | <.001 |
| Systolic Blood Pressure, mmHg | 129 ± 20 | 143 ± 23 | <.001 |
| Diastolic Blood Pressure, mmHg | 72 ± 10 | 75 ± 11 | <.001 |
| Total Cholesterol, mg/dl | 205 ± 37 | 200 ± 35 | .03 |
| HDL Cholesterol, mg/dl | 47 ± 15 | 45 ± 15 | .40 |
| LDL Cholesterol, mg/dl | 129 ± 33 | 123 ± 31 | .007 |
| Triglycerides, mg/dl | 143 ± 83 | 156 ± 93 | .10 |
| Calculated GFR, mL/min, (MDRD formula) | 82.32 ± 16.95 | 74.62 ± 20.29 | <.001 |
| CKD, n (%) | 117 (11%) | 119 (25%) | <.001 |
| Creatinine, mg/dL | 0.80 (0.70, 0.90) | 0.90 (0.70, 1.00) | <.001 |
| Insulin, μU/mL | 4.70 (3.40, 7.30) | 6.70 (4.40, 10.00) | <.001 |
| Serum Glucose, mg/dL | 92 (87, 99) | 97 (90, 105) | .001 |
| Diabetes, n (%) | 58 (5%) | 69 (14%) | <.001 |
| Metabolic Syndrome, n (%) | 179 (17%) | 154 (32%) | .001 |
| Current/Former Smoker, n (%) | 538 (50%) | 213 (45%) | .20 |
| Atrial Fibrillation/Flutter, n (%) | 47 (4%) | 37 (8%) | .40 |
| Coronary Artery Disease, n (%) | 107 (10%) | 98 (21%) | .002 |
| Heart Failure, n (%) | 13 (1%) | 28 (6%) | <.001 |
| Myocardial Infarction, n (%) | 34 (3%) | 43 (9%) | .003 |
| CVA, n (%) | 13 (1%) | 18 (4%) | .01 |
| Antilipemic Therapy, n (%) | 153 (14%) | 128 (27%) | <.001 |
| Anti-HTN Therapy, n (%) | 174 (16%) | 418 (88%) | <.001 |
| EF< 40%, n (%) | 8 (1%) | 16 (3%) | .01 |
| LVH, n(%) | 242 (28%) | 177 (49%) | <.001 |
| cLVH, n(%) | 118 (14%) | 120 (34%) | <.001 |
| Diastolic Dysfunction, n (%) | <.001 | ||
| No | 747 (77%) | 207 (50%) | |
| Mild | 167 (17%) | 148 (35%) | |
| Mod/Severe | 56 (6%) | 62 (15%) |
Age-sex-body mass index adjusted P value. Continuous variables are expressed as mean ± standard deviation or median (first quartile − third quartile). BMI= body mass index, ANP= atrial natriuretic peptide, HDL= high density lipoprotein, LDL= low density lipoprotein, GFR= glomerular filtration rate, MDRD= Modification of Diet in Renal Disease (formula), CKD= chronic kidney disease, CVA = cerebrovascular accident, Anti-HTN= anti-hypertensive, EF= ejection fraction, LVH= left ventricular hypertrophy, cLVH= concentric LVH
Characteristics of HTN Subjects Stratified by Number of Anti-Hypertensive Medications Taken
As reported in Table 2 A, B, C within the group of HTN subjects (n=477), 228 subjects were treated with 1 anti-HTN drug, 145 subjects were taking 2 drugs, 45 subjects were taking 3 or more anti-HTN drugs and 59 subjects were taking no drug. More specifically, within HTN subjects treated with 1 drug, the most common prescribed anti-HTN treatment was a diuretic. When HTN subjects were taking 2 anti-HTN medications, the most common combination was a diuretic with a BB. Within the group taking 3 or more drugs, the most common combination consisted of diuretic (non-MRA), BB and calcium channel blocker. Overall, 50% of the HTN subjects were taking a diuretic while 35% were taking a BB.
Table 2A.
Anti-HTN Drugs Prescribed in Subjects with Diagnosis of HTN Taking One Drug (n=228)
Table 2B.
Anti-HTN Drugs Prescribed in Subjects with Diagnosis of HTN Taking Two Drugs (n=145)
| Drug Combination | Frequencya | Percentb |
|---|---|---|
| Beta Blocker + Diuretic | 51 | 10.7 |
| Ace Inhibitor + Diuretic | 32 | 6.7 |
| Calcium Channel Blocker + Diuretic | 16 | 3.4 |
| Beta Blocker + Ace Inhibitor | 13 | 2.7 |
| Angiotensin Receptor Blocker + Diuretic | 8 | 1.7 |
| Beta Blocker + Calcium Channel Blocker | 8 | 1.7 |
| Calcium Channel Blocker + Ace Inhibitor | 7 | 1.5 |
| Calcium Channel Blocker + Vasodilator | 4 | 0.8 |
| Diuretic + Vasodilator | 2 | 0.4 |
| Calcium Channel Blocker + Angiotensin Receptor Blocker | 2 | 0.4 |
| Ace Inhibitor + Vasodilator | 1 | 0.2 |
| Beta Blocker + Vasodilator | 1 | 0.2 |
Table 2C.
Anti-HTN Drugs Prescribed in Subjects with Diagnosis of HTN Taking Three or More Drugs (n=45)
| Drug Combination | Frequencya | Percentb |
|---|---|---|
| Beta Blocker + CCB + Diuretic | 8 | 1.7 |
| Beta Blocker + Ace Inhibitor + Diuretic | 6 | 1.3 |
| Diuretic + Vasodilator + ARB | 4 | 0.8 |
| Ace Inhibitor + Diuretic + Vasodilator | 4 | 0.8 |
| CCB + Diuretic + ARB | 4 | 0.8 |
| CCB + Ace Inhibitor + Diuretic | 4 | 0.8 |
| Beta Blocker + Diuretic + ARB | 4 | 0.8 |
| CCB + Diuretic + Vasodilator | 3 | 0.6 |
| CCB + Ace Inhibitor + Diuretic + Vasodilator | 2 | 0.4 |
| Beta Blocker + CCB + Ace Inhibitor | 2 | 0.4 |
| Beta Blocker + CCB + Ace Inhibitor + Diuretic | 2 | 0.4 |
| Beta Blocker + Ace Inhibitor + Diuretic + Vasodilator | 1 | 0.2 |
| Beta Blocker + CCB + Vasodilator | 1 | 0.2 |
Frequency refers to number of subjects.
Percent is calculated on the total subjects with a diagnosis of hypertension. BB= beta blockers, CCB= calcium channel blockers, ACEI= angiotensin converting enzyme inhibitors, ARB= angiotensin II receptor blockers.
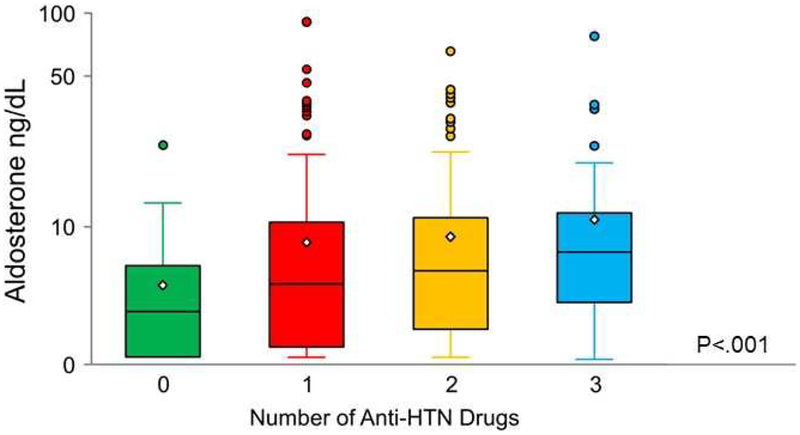
Aldosterone progressively increased according to the number of anti-HTN medications (Figure 1). Table 3 reports the cohort of HTN subjects stratified by number of anti-HTN medications. In all HTN subjects, mean SBP was above the SPRINT goal of 120 mmHg. Importantly, increasing use of anti-HTN medications was associated with a modest increase in ANP, higher BMI, lower HDL cholesterol, higher triglycerides, lower GFR, higher insulin and glucose levels. Further, it was also associated with increased prevalence of clinical comorbidities such as obesity, CKD, DM, CV diseases and use of lipid lowering agents. In contrast to these co-morbidities, increasing use of anti-HTN medications did not correlate with increasing cardiac structure and functional abnormalities that included EF, LVH, cLVH and diastolic dysfunction.
Figure 1:
Plasma aldosterone levels in subjects with hypertension stratified by number of anti-hypertensive drugs taken. Median (line in box), Q1 (lower box), Q3 (upper box) and mean (diamond). The whiskers extend to 1.5×IQR above and below the box (or the max or min values if there are no points outside of 1.5×IQR from the box). The circles are subjects that are above 1.5× IQR. Unadjusted P< .001. Anti-HTN= anti-hypertensive.
Table 3.
Subjects with Hypertension Stratified by Number of Anti-Hypertensive Drugs
| 0 | 1 | 2 | 3 or > | ||
|---|---|---|---|---|---|
| Variable | (N=59) | (N=228) | (N=145) | (N=45) | P Valuea |
| Age, y | 66 ± 11 | 65 ± 9 | 70 ± 10 | 69 ± 11 | <.001 |
| Female Gender, n (%) | 31 (53%) | 144 (63%) | 78 (54%) | 17 (38%) | .02 |
| Aldosterone, ng/dL, median (min, max) | 4.80 (2.5, 21.1) | 6.40 (1.7, 91.0) | 7.10 (2.5, 67.5) | 7.90 (2.5, 79.2) | .002 |
| Aldo in tertiles, n (%) | .004 | ||||
| 1 | 20 (34%) | 59 (26%) | 28 (19%) | 7 (16%) | |
| 2 | 19 (32%) | 59 (26%) | 39 (27%) | 11 (24%) | |
| 3 | 20 (34%) | 110 (48%) | 78 (54%) | 27 (60%) | |
| ANP, pg/ml | 11.80 (7.30, 15.60) | 12.75 (8.05, 18.50) | 14.80 (10.60, 21.10) | 15.60 (9.10, 25.90) | .005 |
| BMI, kg/m2 | 29 ± 6 | 29 ± 6 | 30 ± 6 | 32 ± 6 | <.001 |
| Obesity, n (%) | 20 (34%) | 101 (44%) | 65 (45%) | 27 (60%) | .002 |
| Waist circumference > 102 cm in males, > 88 cm in females, n (%) | 21 (36%) | 101 (44%) | 67 (46%) | 24 (53%) | .02 |
| Systolic Blood Pressure, mmHg | 152 ± 22 | 141 ± 22 | 139 ± 22 | 152 ± 22 | .05 |
| Diastolic Blood Pressure, mmHg | 81 ± 9 | 75 ± 11 | 73 ± 11 | 77 ± 11 | <.001 |
| Total Cholesterol, mg/dL | 213 ± 34 | 202 ± 35 | 191 ± 33 | 197 ± 41 | .02 |
| HDL Cholesterol, mg/dL | 49 ± 19 | 46 ± 13 | 42 ± 15 | 42 ± 16 | .05 |
| LDL Cholesterol, mg/dL | 136 ± 32 | 125 ± 31 | 116 ± 28 | 118 ± 29 | <.001 |
| Triglycerides, mg/dL | 137 ± 64 | 149 ± 79 | 166 ± 80 | 184 ±185 | .004 |
| Calculated GFR, ml/min, (MDRD formula) | 78.74 ± 19.79 | 77.28 ± 18.05 | 70.76 ± 23.23 | 68.18 ± 18.40 | .005 |
| CKD, n (%) | 11 (19%) | 37 (16%) | 51 (35%) | 20 (44%) | .001 |
| Creatinine, mg/dL | 0.80 (0.70, 1.00) | 0.80 (0.70, 1.00) | 0.90 (0.80, 1.20) | 1.00 (0.80, 1.30) | .02 |
| Insulin, μU/mL | 5.60 (4.20, 8.90) | 6.25 (4.00, 10.00) | 7.20 (4.90, 10.30) | 7.90 (5.50, 12.00) | .06 |
| Serum Glucose, mg/dL | 95 (88, 101) | 95 (89, 104) | 101 (93, 110) | 102 (94, 119) | .005 |
| Diabetes, n (%) | 5 (8%) | 22 (10%) | 29 (20%) | 13 (29%) | .002 |
| Metabolic Syndrome, n (%) | 14 (24%) | 64 (28%) | 61 (42%) | 15 (33%) | .26 |
| Current/Former Smoker, n (%) | 27 (48%) | 92 (40%) | 69 (48%) | 25 (56%) | .22 |
| Atrial Fibrillation/Flutter, n (%) | 3 (5%) | 7 (3%) | 16 (11%) | 11 (24%) | .002 |
| Coronary Artery Disease, n (%) | 7 (12%) | 32 (14%) | 39 (27%) | 20 (44%) | <.001 |
| Heart Failure, n (%) | 0 (0%) | 5 (2%) | 13 (9%) | 10 (22%) | <.001 |
| Myocardial Infarction, n (%) | 2 (3%) | 17 (7%) | 17 (12%) | 7 (16%) | .13 |
| Stroke, n (%) | 1 (2%) | 4 (2%) | 10 (7%) | 3 (7%) | .06 |
| Antilipemic Therapy, n (%) | 11 (19%) | 59 (26%) | 39 (27%) | 19 (42%) | 0.05 |
| Echocardiographic Parameters | |||||
| Ejection Fraction <40%, n (%) | 1 (2%) | 5 (2%) | 6 (4%) | 4 (9%) | .14 |
| LVH, n(%) | 23 (51%) | 78 (44%) | 53 (50%) | 23 (79%) | .59 |
| cLVH, n (%) | 19 (42%) | 49 (28%) | 35 (33%) | 17 (59%) | .92 |
| Diastolic Dysfunction, n (%) | .60 | ||||
| No | 21 (41%) | 118 (56%) | 53 (44%) | 15 (42%) | |
| Mild | 22 (43%) | 73 (35%) | 40 (33%) | 13 (36%) | |
| Mod/Severe | 8 (16%) | 19 (9%) | 27 (23%) | 8 (22%) |
Age-sex-body mass index adjusted P value. Continuous variables are expressed as mean ± standard deviation or median (first quartile − third quartile). BMI= body mass index, ANP= atrial natriuretic peptide, HDL= high density lipoprotein, LDL= low density lipoprotein, GFR= glomerular filtration rate, MDRD= Modification of Diet in Renal Disease (formula), CKD= chronic kidney disease, LVH= left ventricular hypertrophy, cLVH= concentric LVH.
In consideration of the effect of diuretic therapy on intravascular volume and, consequently, the renin-angiotensin-aldosterone system, we compared aldosterone levels in hypertensive subjects on diuretics (n= 234) versus hypertensive subjects on medications other than diuretics (n= 184). The median (Q1, Q3) aldosterone levels were, 9.3 (8.5–10.2) vs 5.0 (4.5–5.6) ng/dL respectively, age-sex-BMI adjusted P<.001.
Relationship between Aldosterone and ANP plasma levels
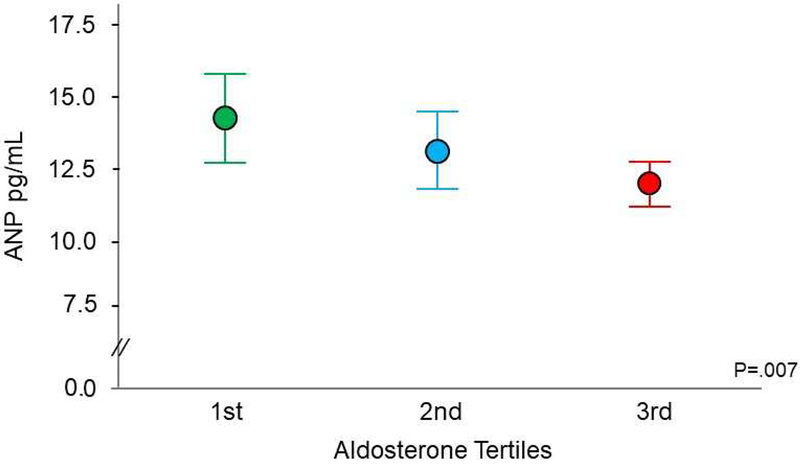
Given the counter-regulatory roles played by aldosterone and ANP, we sought to determine the relationship between these two hormones in the context of HTN. Aldosterone level was divided into tertiles: the 1st tertile ranged from 2.5 to 3.1 ng/dL, the 2nd tertile ranged from 3.2 to 6.5 ng/dL and the 3rd tertile ranged from 6.6 to 79.2 ng/dL whereas ANP was analyzed as a continuous variable. As illustrated in Figure 2, within all hypertensive subjects, ANP decreased as aldosterone increased and we found a strong inverse relationship between aldosterone and ANP that remained significant after adjustment for age, sex, BMI, MI, heart failure, atrial fibrillation and GFR, all of which may affect ANP circulating levels (P=.007). In consideration of the potential confounding effect of diuretics on the production of aldosterone and ANP, we also analyzed the relationship between these two hormones in hypertensive subjects who were not on diuretics and the inverse relationship remained similar (adjusted P=.04).
Figure 2:
Inverse relationship between plasma aldosterone and atrial natriuretic peptide in hypertensive subjects. Aldosterone analyzed by tertiles and atrial natriuretic peptide as continuous variable (mean and 95% CI). Age-gender-BMI-MI-heart failure-atrial fibrillation-GFR adjusted P = .007. ANP= atrial natriuretic peptide
DISCUSSION
The current study is the first to analyze circulating aldosterone and its relationship with HTN, the use of anti-HTN medications and ANP in the general population. From this randomly selected population, we report that 31% of the general community had an established diagnosis of HTN and that plasma aldosterone was higher in these subjects compared to subjects without a diagnosis of HTN. Importantly, in the post SPRINT Trial era of intensifying HTN treatment to achieve optimal BP control and improve outcomes we found that aldosterone levels were progressively higher with the increasing number of anti-HTN medications. Moreover, subjects with HTN on diuretics had significantly higher aldosterone levels compared to those on other anti-hypertensive medications. This study also reports an inverse relationship between ANP and aldosterone levels. Indeed, in subjects on anti-hypertensive medications, modest increases in plasma ANP corresponds to higher increase in plasma aldosterone, underscoring an imbalance between these two counter-regulatory BP-regulating hormones.
Aldosterone, through the well-characterized MR present on the renal tubular epithelium, is a hormone that induces reabsorption of sodium thus preserving intravascular volume and BP at times of sodium deficit. In modern society with the presence of high sodium diets and increasing longevity, a pathophysiological role for aldosterone has emerged. Indeed, inappropriately increased levels of aldosterone even within the normal range are associated with HTN, renal and metabolic disease and also predicts future onset of these disease entities within the general population.6,7 In the current investigation utilizing a randomly selected and well-characterized general population cohort of adults, we observed that higher plasma aldosterone levels were present in HTN subjects and this increase in aldosterone remained significant even after adjusting for age, gender and BMI.
The SPRINT Trial represents a seminal large-scale clinical trial that defines the impact of intensive BP control to achieve SBP levels below 120 mmHg in over 9000 HTN subjects.10 The major finding was that intensive therapy to achieve BP goals improved survival and reduced adverse CV outcomes. One observation in the SPRINT Trial was that an average of 2.5 medications was required to achieve the BP goal of < 120 mmHg. We believe that the potential of more intensive use of anti-HTN medications to reduce BP in HTN markedly increases the priority of understanding unwanted consequences and/or associations of the use of such medications such as neurohumoral responses to therapy.
Here, we analyzed aldosterone levels in HTN subjects on no, 1, 2 or 3 or more anti-HTN medications. A highly significant association between aldosterone and the number of anti-HTN medications was observed, even after adjusting for age, gender and BMI. In the HTN subjects from this cohort, the most commonly used anti-HTN agents were diuretics. The most common combination was diuretics and BB. Only 6 subjects were on MRAs. Subjects on diuretics had higher aldosterone levels when compared to subjects on medications other than diuretics. Importantly, with the increasing number of anti-HTN agents we observed an increase in aldosterone levels and a higher prevalence of CV, renal and metabolic diseases. These findings were prominent in those HTN subjects taking 3 or more anti-HTN medications. An important conclusion from our study is that aldosterone excess is clearly a feature of treated HTN which progressively increases with the use of more anti-HTN drugs. What remains to be investigated is whether the progressive increase in aldosterone levels is secondary to the use of BP lowering medication and/or reflects hormonal activation secondary to the severity of HTN and/or dysmetabolic state. In addition, further studies are necessary to evaluate the potential activation of various neurohumoral pathways associated with different anti-hypertensive therapy.
Aldosterone and ANP play a counter-regulatory role in body fluid and BP homeostasis with aldosterone being sodium retaining and BP increasing, while ANP is natriuretic, BP lowering, suppresses aldosterone production and may antagonize the MR.18 Indeed, the carriers of the ANP genetic variant rs5068, which is associated with higher circulating levels of ANP, have lower BP values and risk of HTN.19,20 We previously reported an inverse relationship between aldosterone and ANP in a general population cohort that included a mix of HTN and normotensive adults.6 In the current study, subjects with hypertension have higher aldosterone and ANP levels when compared to subjects without hypertension. While this analysis allows us to compare the clinical characteristics of the two groups, it does not provide information regarding the relationship between the two hormones. In hypertensive subjects, the magnitude of aldosterone increase is greater than the modest increase in ANP resulting in a reduction on the ANP/aldosterone ratio. Importantly, here, we demonstrated for the first time thatin HTN subjects, an elevation in aldosterone levels analyzed according to tertiles correspond to adecrease in ANP levels such that an inverse relationship between two hormones was found. Our findings are supported by studies that reported a relative NP deficiency in subjects with HTN and metabolic disease.13,14 It would be tempting to speculate that a reduction in ANP production may be secondary to lower intravascular volume due to diuretic use. Notably, when we analyzed hypertensive subjects who were not on diuretics, the inverse relationship between aldosterone and ANP was confirmed. Further, more advanced stages of HTN especially in obesity associated HTN, are characterized by intravascular volume expansion despite the use of diuretics suggesting that these medications might not contribute to lower levels of ANP in HTN.21 In addition, type II DM, which is commonly found in association with HTN, is characterized by elevated insulin levels that may up-regulate the NP clearance receptor (NPR-C) thus diminishing circulating ANP levels.22 Obesity represents another metabolic disease often associated with HTN and it is also characterized by lower levels of natriuretic peptides.14 Importantly, aldosterone production is complexly regulated by several factors (renin, serum potassium levels, adrenocorticotropic hormone). The inverse relationship between aldosterone and ANP observed in our analysis along with the relative ANP deficiency reported in previous studies might be one of the numerous elements playing a role in aldosterone regulation. Future in vitro and in vivo studies are needed to investigate more in-depth the interaction between these two hormones.
The current study has several strengths. First, our cohort consisted of a large number (n=1550) of randomly selected adult subjects in the general population and not volunteers. Secondly, our subjects were well characterized with plasma aldosterone and ANP measurements, extensive phenotyping and echocardiography. Third, our cohort consisted of 45 years old and older subjects, constituting a sample of individuals at high-risk for developing CV, renal and metabolic disease. Moreover, this cohort is similar to subjects in the SPRINT Trial and is of high relevance to the 2017 ACC/AHA BP Guideline that focus on 10-year CV risk, which is largely driven by age.9,23
This study also has limitations. In our study, plasma renin activity, potassium and adrenocorticotropic hormone plasma levels were not available and subjects were not assessed for primary and secondary aldosteronism. In order to exclude those subjects with a potential diagnosis of primary or secondary aldosteronism we performed further analyses in subjects with aldosterone levels below the upper normal range 16.2 ng/dl.6 Importantly, in this sub-group, subjects with hypertension had aldosterone levels significantly higher than subjects without hypertension. The previous study by Buglioni et al, confirm our findings as it showed a positive association between aldosterone levels and hypertension in a similar cohort excluding subjects with aldosterone levels > 16.2 ng/dl.6 Future studies in well characterized cohorts of hypertensive subjects are warranted to further investigate the complex relationship between aldosterone, anti-hypertensive drugs and ANP. As the Olmsted County MN population is mostly Caucasian, we cannot extend our conclusions to African American or other ethnic populations. Further investigations in African Americans would be of relevance as recent studies have established a relative NP deficiency in this ethnic group and studies have also reported excessive aldosteronism in African Americans that may increase the risk for metabolic disease as well as HTN.24,25,26,27 Additionally, we do not know the sodium intake of each subject. However, recent studies have reported that the average dietary sodium intake in Minnesota is unchanged over the past two decades and exceeds the recommended upper limit of 2300 mg/day.28 Finally, our study was cross-sectional and analyzed the relationship between aldosterone, hypertension, anti-hypertensive medications and ANP. Further mechanistic studies are warranted to investigate the pathophysiological process underlying the associations observed.
Conclusions
In spite of remarkable achievements in HTN research, Mensah and colleagues at the National Heart Lung and Blood Institute recently emphasized that “hypertension remains the leading cause of global death and disability from heart disease and stroke and a major contributor to all-cause mortality worldwide.”29 The growing health burden of HTN reinforces the need to understand mechanisms, diagnostic and therapeutic opportunities for prevention and treatment of HTN. From this perspective, our findings have important pathophysiologic and clinical implications in human HTN. We report the elevation of circulating aldosterone levels in HTN subjects randomly selected from the general population, and importantly, this elevation is progressively higher with the number of anti-HTN medications taken. The prevalence of CV, renal and metabolic disease was the highest among the hypertensive subjects with the highest levels of aldosterone. Moreover, in our cohort of HTN subjects, greater aldosterone levels were associated with lower circulating ANP. Additional studies are warranted to validate the importance of measuring both plasma aldosterone and ANP in the setting of treated HTN. Further investigations are also warranted to define whether antagonizing aldosterone30 and/or compensating for the relative ANP deficit31,32 may be effective strategies for the prevention and treatment of HTN.
Acknowledgments
Source of Funding: This study was supported by grants from the National Institutes of Health: PO1 HL76611, RO1 AG034676,RO1 HL55502, RO1 HL136340 and Scientist Development Grant from the American Heart Association 16SDG29930003.
ABBREVIATIONS
- ANP
atrial natriuretic peptide
- BB
beta-blocker
- BMI
body mass index
- BP
blood pressure
- CKD
chronic kidney disease
- CV
cardiovascular
- DM
diabetes mellitus
- GFR
glomerular filtration rate
- HTN
hypertension
- MetS
metabolic syndrome
- MI
myocardial infarction
- MR
mineralocorticoid receptor
- MRA
mineralocorticoid receptor antagonist
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
REFERENCES
- 1.Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992. December;120(6):893–901. [PubMed] [Google Scholar]
- 2.Calhoun DA. Aldosterone and cardiovascular disease: smoke and fire. Circulation. 2006. December 12;114(24):2572–2574. [DOI] [PubMed] [Google Scholar]
- 3.Dudenbostel T, Ghazi L, Liu M, et al. Body mass index predicts 24-hour urinary aldosterone levels in patients with resistant hypertension. Hypertension. 2016. October;68(4):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaddam K, Corros C, Pimenta E, et al. Rapid reversal of left ventricular hypertrophy and intracardiac volume overload in patients with resistant hypertension and hyperaldosteronism: a prospective clinical study. Hypertension. 2010. May;55(5):1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomized, double-blind, crossover trial. Lancet. 2015. November 21;386(10008):2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buglioni A, Cannone V, Cataliotti A, et al. Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension. 2015. January;65(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buglioni A, Cannone V, Sangaralingham SJ, et al. Aldosterone predicts cardiovascular, renal, and metabolic disease in the general community: A 4-year follow-up. J Am Heart Assoc. 2015. December 23;4(12):e00250514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. New Engl J Med. 2004. July 1;351(1):33–41. [DOI] [PubMed] [Google Scholar]
- 9.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017. November 7. [DOI] [PubMed] [Google Scholar]
- 10.Wright JT Jr., Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bress AP, Tanner RM, Hess R, et al. Generalizability of SPRINT results to the US adult population. J Am Coll Card. 2015;67:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012. December;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macheret F, Heublein D, Costello-Boerrigter LC, et al. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J Am Coll Card. 2012. October 16;60(16):1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004. February 10; 109(5):584–600. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj NS, Gutiérrez OM, Arora G, et al. Racial Differences in Plasma Levels of N-Terminal Pro-B-Type Natriuretic Peptide and Outcomes: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA Cardiol. 2018. January 1;3(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redfield MM, Jacobsen SJ, Burnett JC Jr., et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003. January 8;289(2):194–202. [DOI] [PubMed] [Google Scholar]
- 17.Burnett JC Jr., Kao PC, Hu DC, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science.1986. March 7;231(4742):1145–1147. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa H, Oberwinkler H, Nikolaev VO, et al. Atrial natriuretic peptide locally counteracts the deleterious effects of cardiomyocyte mineralocorticoid receptor activation. Circ HF. 2014. September;7(5):814–821. [DOI] [PubMed] [Google Scholar]
- 19.Cannone V, Boerrigter G, Cataliotti A, et al. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol. 2011. August 2;58(6):629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannone V, Cefalu’ AB, Noto D, et al. The atrial natriuretic peptide genetic variant rs5068 is associated with a favorable cardiometabolic phenotype in a Mediterranean population. Diabetes Care. 2013. September;36(9):2850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall JE, do Carmo JM, da Silva AA, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015. March; 13:116(6):991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordicchia M, Ceresiani M, Pavani M, et al. Insulin/glucose induces natriuretic peptide clearance receptor in human adipocytes: a metabolic link with the cardiac natriuretic pathway. Am J Physiol Regul Integr Comp Physiol. 2016. July 1;311(1):R104–114. [DOI] [PubMed] [Google Scholar]
- 23.Greenland P, Peterson E. The New 2017 ACC/AHA Guidelines “Up the Pressure” on Diagnosis and Treatment of Hypertension. JAMA. 2017. December 5;318(21):2083–2084. [DOI] [PubMed] [Google Scholar]
- 24.Gupta DK, de Lemos JA, Ayers CR, et al. Racial differences in natriuretic eptide levels: The Dallas Heart Study. JACC Heart Fail. 2015. July; 3(7):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musani SK, Vasan RS, Bidulescu A, et al. Aldosterone, c-reactive protein, and plasma b-type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: findings from the Jackson Heart Study. Diabetes Care. 2013. October;36(10):3084–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TJ. Natriuretic Peptide Deficiency-When There Is Too Little of a Good Thing. JAMA Cardiol. 2018. January 1;3(1):7–9 [DOI] [PubMed] [Google Scholar]
- 27.Gupta DK, Daniels LB, Cheng S, et al. Differences in Natriuretic Peptide Levels by Race/Ethnicity (From the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2017. September 15;120(6):1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer KA, Harnack LJ, Luepker RV, et al. Twenty-two-year population trends in sodium and potassium consumption: the Minnesota Heart Survey. J Am Heart Assoc. 2013. October 2;2(5):e000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mensah GA, Galis ZS, Fine LJ, et al. Building on a legacy of hypertension research. Charting our future together. Hypertension. 2017. January;69(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flatt DM, Brown MC, Mizeracki AM, et al. Mineralocorticoid receptor antagonists in the management of heart failure and resistant hypertension. JAMA Cardiol. 2016. August 1;1(5):607–612. [DOI] [PubMed] [Google Scholar]
- 31.Ruilope LM, Dukat A, Bohm M, et al. Blood-pressure reduction with LCZ696, a novel-acting inhibitor of the angiotensin II receptor and neprilysin: a randomized, double-blind, placebo-controlled, active comparator study. Lancet. 2010;10:375(9722):1255–1266. [DOI] [PubMed] [Google Scholar]
- 32.Meems LMG, Burnett JC Jr. Innovative Therapeutics: Designer Natriuretic Peptides. JACC Basic Transl Sci. 2016. December;1(7):557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]