Abstract
Background Warfarin and direct oral anticoagulants (DOACs) are used for the initial treatment and secondary prevention of venous thromboembolism (VTE), and have similar efficacy. Patient concerns and preferences are important considerations when selecting an anticoagulant, yet these are not well studied.
Methods VTE patients ( n = 519) were surveyed from online sources (clotconnect.org, stoptheclot.org and National Blood Clot Alliance Facebook followers [ n = 495]) and a haematology clinic in Vermont ( n = 24).
Results Patients were 83% females and on average (±standard deviation [SD]) 45.7 ± 13.1 years; 65% self-reported warfarin as their initial VTE treatment and 35% a DOAC. Proportions reporting being extremely concerned about the following outcomes were as follows: recurrent VTE 33%, major bleeding 21%, moderate bleeding 16% and all-cause death 29%. When asked about oral anticoagulant characteristics, patients strongly preferred anticoagulants that are reversible (53%), and for which blood drug levels can be monitored (30%). Lower proportions agreed with statements that regular blood testing is inconvenient (18%), that they are comfortable using the newest drug versus an established drug (15%) and that it is difficult to change their diet to accommodate their anticoagulant (17%). In multivariable-adjusted models, patients tended to have had as their initial treatment, and to currently be taking, the oral anticoagulant option they personally preferred.
Discussion Patients held the greatest concern for recurrent VTE and mortality, regardless of which treatment they were prescribed. Potential weaknesses of warfarin (e.g., dietary restrictions, regular monitoring) were generally not considered onerous, while warfarin's advantages (e.g., ability to monitor) were viewed favourably.
Keywords: venous thrombosis, warfarin, direct oral anticoagulants, patient preferences, patient concerns
Introduction
One in 12 U.S. residents will develop venous thromboembolism (VTE) over their lifetimes, 1 of whom 10 to 30% will die within a month of diagnosis. 2 Survivors face a 10% risk of recurrence after 1 year and a 30% risk of recurrence after 5 years. 3 4 5 6 To prevent death and reduce recurrence, patients are treated with an initial 3 to 6 months of anticoagulation (initial treatment), usually with an oral anticoagulant (OAC). Thereafter, anticoagulation is either discontinued or continued long term for secondary prevention, depending on the assessment of an individual's risks for recurrent VTE and bleeding. 7
Traditionally, OAC treatment has been with warfarin, a highly effective anticoagulant with a scalable anticoagulant effect and well-established monitoring and reversal algorithms. 8 However, there are inconveniences associated with using warfarin, in the form of numerous dietary and drug interactions and the need for routine laboratory monitoring of the anticoagulant effect. Since 2010, four new OAC drugs (dabigatran, rivaroxaban, apixaban and edoxaban) have been approved by regulatory agencies for the treatment of VTE in the United States. 9 10 11 12 13 14 They are collectively referred to as direct oral anticoagulants (DOACs). The DOACs have the advantages of fewer drug and food interactions and a reliable anticoagulant effect with fixed dosing, thus not requiring routine laboratory monitoring (and in fact cannot be easily monitored in routine practice settings). Clinical trials of DOACs versus warfarin for the treatment of VTE demonstrate equivalence for the outcomes of recurrent VTE or death, with some demonstrating superiority in terms of less intracranial and major bleeding. 10 11 12 13 14 15 Direct comparisons between warfarin and DOACs for secondary prevention are limited. 16 17
DOACs are now widely used in clinical practice, 18 19 and are included—together with warfarin—as the standard of care for VTE treatment in current guidelines. 7 Given the options of warfarin versus DOACs, when making decisions providers and patients take into consideration factors such as cost, priorities about specific adverse outcomes, convenience and patient preference. 20 Understanding patient concerns and preferences may help inform clinical decisions, assist in provider–patient discussions to individualize anticoagulant treatment decisions and direct avenues of future research.
To understand how individuals with VTE prioritize various outcomes and anticoagulant drug characteristics, we surveyed patients about their attitudes and preferences for VTE outcomes and treatment options.
Materials and Methods
Study Design
VTE patients who participated in this project were recruited in January and February 2016 from three sources: 1 in-person recruitment during clinic visits at the University of Vermont Medical Center's Thrombosis and Hemostasis Program ( www.UVMHealth.org/MedCenterTHP ), 2 online recruitment through the non-profit National Blood Clot Alliance (NBCA) via links posted on their Web site ( www.stoptheclot.org ) and Facebook page, and 3 online recruitment through the non-profit ClotConnect ( www.clotconnect.org ) education Web site. The inclusion criteria for the study were being a VTE patient aged 18 years or older. All data collected were self-reported by the VTE patients.
At the University of Vermont, haematologists and nurse practitioners distributed an envelope containing the survey to VTE patients at the end of their clinic visits. Those patients who chose to participate then mailed the survey in a pre-paid envelope to the University of Minnesota for processing. Returning the survey implied consent; no identifiers were present on the survey, and the clinic was not aware of the identities of the individuals who did or did not return the surveys. The University of Vermont Institutional Review Board approved this protocol.
The online survey was developed using Qualtrics, a secure and Health Insurance Portability and Accountability Act of 1996 (HIPAA) compliant online survey platform. Our goal was to obtain 500 online surveys. Participants provided consent and completed the survey online. The survey was opened on 28 January 2016 and closed on 5 February 2016 after recruitment goals were met. We excluded participants who did not report receiving anticoagulants as part of their initial treatment, yielding 495 usable surveys from online sources. The online component of this project was approved by the University of Minnesota Institutional Review Board.
Survey Components
The survey, which is provided as Supplement Material , available online, queried demographics, characteristics of the participants' most recent VTE event and subsequent treatment; level of concern about specific adverse outcomes; and their preferences as related to characteristics of DOACs and warfarin. More specifically, regarding their prior VTE, we asked the year when the event occurred, type of event (pulmonary embolism [PE], deep vein thrombosis [DVT], both or unsure), initial treatment, whether the patient was still on an anticoagulant (and if so, which one) and whether the patient had ever stopped taking an anticoagulant due to bleeding. Using a 5-point Likert scale, we also asked participants to rate their level of concern about specific adverse outcomes (i.e., recurrent VTE, major bleeding, moderate bleeding, death from any cause), with response options ranging from “not at all concerned” to “extremely concerned”. We also assessed patient preferences about characteristics of DOACs and warfarin using a 5-point Likert scale, with response options ranging from “strongly disagree” to “strongly agree”. Though the questions did not specifically state which drug(s) they were referencing, some of the questions were related to disadvantages of warfarin (i.e., regular blood test monitoring, dietary changes), while other questions were regarding present disadvantages of DOACs (i.e., levels cannot be followed, limited present reversibility and less clinical experience with these drugs). At the time of the survey, there were no approved reversal agents for DOACs. Since the survey, idarucizumab has been approved, and other reversal agents are in late-stage clinical trials.
Statistical Analysis
Participant characteristics are provided as overall means ( ± standard deviation [SD]) and proportions, as well as stratified by recruitment method, age categories and VTE initial treatment anticoagulant (i.e., DOAC or warfarin) and current treatment (i.e., DOAC, warfarin or no OAC). We calculated the proportions of (a) participants reporting levels of concern about various potential medical events and (b) perceptions of characteristics of anticoagulants (e.g., perceived difficulty of changing diet to accommodate a medication). Prevalence ratios (PRs; and their 95% confidence intervals [CI]) were estimated using relative risk regression (binomial regression with a log link) 21 to identify participant characteristics associated with a higher (or lower) prevalence of being extremely concerned about specific medical events, and having extreme perceptions (strongly agree/strongly disagree) of anticoagulant characteristics. The interpretation of PRs is similar to the interpretation for odds ratios; however, PRs are preferable in the context of common outcomes, such as in the present analysis. 21 22 Model 1 adjusted for age (continuous), sex and race/ethnicity (white/non-white). Model 2 further adjusted for VTE initial treatment (DOAC or warfarin). Model 3 adjusted for model 1 covariates and current treatment status (DOAC, warfarin, no anticoagulant). Interactions by age, sex, race/ethnicity, VTE initial treatment type (DOAC, warfarin) and VTE subtype (PE [with or without DVT], DVT only) were explored. Results are also reported according to current OAC use status and duration (i.e., not current OAC users, current users <13 months, current users ≥13 months).
Results
Patient Characteristics
A total of 521 patients participated: 495 identified through online sources and 26 through the Vermont clinic (of 49 distributed envelopes). Two people were excluded from the analysis; one who wrote they did not have VTE and one with missing data on key variables. Our analysis is based on the 519 patients with sufficient data available.
The average age ( ± SD) was 45.7 ± 13.1 years (range: 19–85 years), and 82.7% were females ( Table 1 ). There were 90.6% who self-identified as white, 2.9% as black, 2.5% as Hispanic, 0.6% as Asian and 3.5% as other race or not reported. Participants recruited online tended to be younger (45.0 vs. 60.3 years) and were more likely to be females (84.4 vs. 45.8%) than the Vermont clinic patients were.
Table 1. Characteristics of the 519 VTE patients who completed the survey, overall and stratified by recruitment method and age: 2016.
| Overall | Recruitment method | Age category | ||||||
|---|---|---|---|---|---|---|---|---|
| Online | VT clinic | <35 | 35–44 | 45–54 | 55–64 | 65+ | ||
| N | 519 | 495 | 24 | 108 | 144 | 144 | 68 | 55 |
| Age, mean | 45.7 | 45.0 | 60.3 | 28.8 | 39.7 | 48.6 | 59.3 | 70.0 |
| Age category, n (%) | ||||||||
| < 35 | 108 (20.8) | 107 (21.6) | 1 (4.2) | n/a | n/a | n/a | n/a | n/a |
| 35–44 | 144 (27.8) | 143 (28.9) | 1 (4.2) | n/a | n/a | n/a | n/a | n/a |
| 45–54 | 144 (27.8) | 140 (28.3) | 4 (16.7) | n/a | n/a | n/a | n/a | n/a |
| 55–64 | 68 (13.1) | 59 (11.9) | 9 (37.5) | n/a | n/a | n/a | n/a | n/a |
| 65+ | 55 (10.6) | 46 (9.3) | 9 (37.5) | n/a | n/a | n/a | n/a | n/a |
| Female, n (%) | 429 (82.7) | 418 (84.4) | 11 (45.8) | 100 (92.6) | 130 (90.3) | 124 (86.1) | 44 (64.7) | 31 (56.4) |
| Race/ethnicity, n (%) | ||||||||
| White | 470 (90.6) | 450 (90.9) | 20 (83.3) | 99 (91.7) | 132 (91.7) | 124 (86.1) | 63 (92.7) | 52 (94.6) |
| Black or African American | 15 (2.9) | 15 (3.0) | 0 (0.0) | 4 (3.7) | 3 (2.1) | 6 (4.2) | 2 (2.9) | 0 (0.0) |
| Hispanic or Latino | 13 (2.5) | 12 (2.4) | 1 (4.2) | 3 (2.8) | 4 (2.8) | 4 (2.8) | 2 (2.9) | 0 (0.0) |
| Asian American | 3 (0.6) | 2 (0.4) | 1 (4.2) | 0 (0.0) | 1 (0.7) | 2 (1.4) | 0 (0.0) | 0 (0.0) |
| Other/not reported | 18 (3.5) | 16 (3.2) | 2 (8.3) | 2 (1.9) | 4 (2.8) | 8 (5.6) | 1 (1.5) | 3 (5.5) |
| VTE initial treatment | ||||||||
| Warfarin | 338 (65.1) | 319 (64.4) | 19 (79.2) | 57 (52.8) | 99 (68.8) | 88 (61.1) | 50 (73.5) | 44 (80.0) |
| DOAC | 181 (34.9) | 176 (35.6) | 5 (20.8) | 51 (47.2) | 45 (31.3) | 56 (38.9) | 18 (26.5) | 11 (20.0) |
| VTE current treatment | ||||||||
| Warfarin | 218 (42.0) | 206 (41.6) | 12 (50.0) | 30 (27.8) | 64 (44.4) | 65 (45.1) | 31 (45.6) | 28 (50.9) |
| DOAC | 196 (37.8) | 191 (38.6) | 5 (20.8) | 44 (40.7) | 55 (38.2) | 57 (39.6) | 23 (33.8) | 17 (30.9) |
| No anticoagulant | 105 (20.2) | 98 (19.8) | 7 (29.2) | 34 (31.5) | 25 (17.4) | 22 (15.3) | 14 (20.6) | 10 (18.2) |
Abbreviations: DOAC, direct oral anticoagulant; VTE, venous thromboembolism.
Participants self-reported their VTE as PE (22.4%), DVT (28.3%), both PE and DVT (47.2%) or unsure (2.1%). Participants also reported the year when they experienced their most recent VTE: 2016 (2.7%), 2015 (31.2%), 2014 (17.1%), 2011 to 2013 (23.3%), 2006 to 2010 (15.6%), 2001 to 2005 (5.6%) and 2000 or earlier (2.7%). The number of patients who self-reported that their initial treatment was warfarin comprised 65.1%, while 34.9% reported a DOAC ( Table 2 ). Of VTE patients in this study, 12.5% ( n = 65) reported a history of stopping anticoagulant therapy due to bleeding in the past; of these at the time of this survey, 81.5% ( n = 53) were on an OAC.
Table 2. Characteristics of the 519 VTE patients who completed the survey, stratified by initial VTE treatment oral anticoagulant, and current anticoagulant: 2016.
| Initial anticoagulant treatment | Current anticoagulant treatment | ||||
|---|---|---|---|---|---|
| Warfarin | DOAC | Warfarin | DOAC | No OAC | |
| N | 338 | 181 | 218 | 196 | 105 |
| Age, mean | 47.3 | 42.7 | 47.8 | 44.5 | 43.6 |
| Age category, n (%) | |||||
| < 35 | 57 (16.9) | 51 (28.2) | 30 (13.8) | 44 (22.5) | 34 (32.4) |
| 35–44 | 99 (29.3) | 45 (24.9) | 64 (29.4) | 55 (28.1) | 25 (23.8) |
| 45–54 | 88 (26.0) | 56 (30.9) | 65 (29.8) | 57 (29.1) | 22 (21.0) |
| 55–64 | 50 (14.8) | 18 (9.9) | 31 (14.2) | 23 (11.7) | 14 (13.3) |
| 65+ | 44 (13.0) | 11 (6.1) | 28 (12.8) | 17 (8.7) | 10 (9.5) |
| Female, n (%) | 278 (82.3) | 151 (83.4) | 181 (83.0) | 157 (80.1) | 91 (86.7) |
| Race/ethnicity, n (%) | |||||
| White | 306 (90.5) | 164 (90.6) | 195 (89.5) | 178 (90.8) | 97 (92.4) |
| Black or African American | 12 (3.6) | 3 (1.7) | 8 (3.7) | 4 (2.0) | 3 (2.9) |
| Hispanic or Latino | 7 (2.1) | 6 (3.3) | 5 (2.3) | 5 (2.3) | 3 (2.9) |
| Asian American | 2 (0.6) | 1 (0.6) | 1 (0.5) | 2 (1.0) | 0 (0.0) |
| Other/not reported | 11 (3.3) | 7 (3.9) | 9 (4.1) | 7 (3.6) | 2 (1.9) |
| VTE initial treatment | |||||
| Warfarin | n/a | n/a | 205 (94.0) | 67 (34.2) | 66 (62.9) |
| DOAC | n/a | n/a | 13 (6.0) | 129 (65.8) | 39 (37.1) |
Abbreviations: DOAC, direct oral anticoagulant; OAC, oral anticoagulant; VTE, venous thromboembolism.
At the time of the survey, 79.8% were currently taking an OAC. Of these individuals, 52.7% were taking warfarin and 47.3% a DOAC ( Table 2 ). Among the current DOAC users, 65.8% were prescribed a DOAC for their initial treatment, while 34.2% had initially been prescribed warfarin. For current warfarin users, 94.0% reported warfarin as their initial treatment and 6.0% a DOAC.
Concerns about Adverse Events
Of the entire sample, 33.1% reported being extremely concerned about recurrent VTE, 21.4% extremely concerned about major bleeding, 15.6% extremely concerned about moderate bleeding and 28.9% extremely concerned about death from any cause. Level of concern about specific adverse events, stratified by initial anticoagulant therapy, is depicted in Fig. 1 .
Fig. 1.
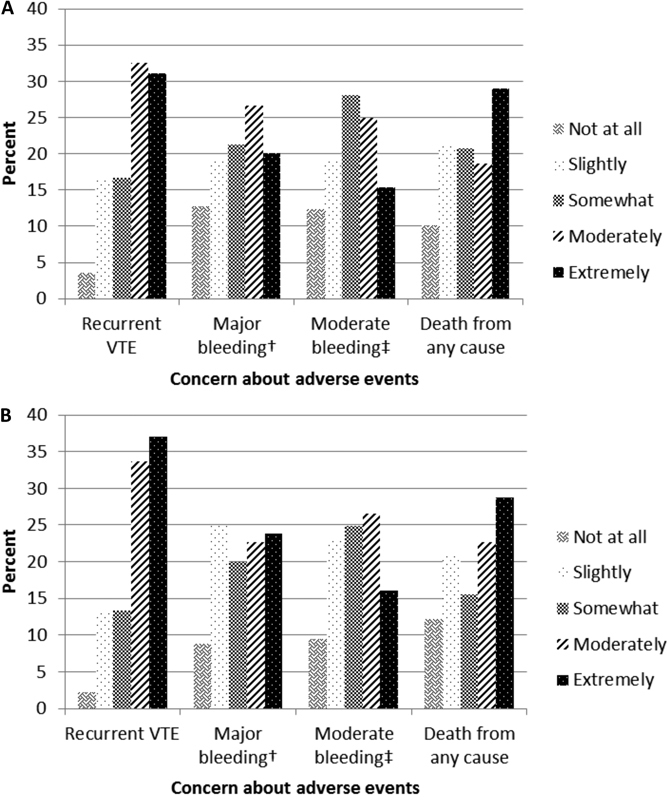
Concern or worry about medical events when taking oral anticoagulants, stratified by whether patients were initially prescribed warfarin or a DOAC: 2016 ( n = 519). ( A ) Initially prescribed warfarin. ( B ) Initially prescribed a DOAC. DOAC, direct oral anticoagulant; VTE, venous thromboembolism. †Major bleeding: for example, a bleed into the head that causes permanent disability. ‡Moderate bleeding: for example, a bleed that requires medical treatment.
Adjusting for race, sex and current treatment status, older patients were less likely to be extremely concerned about recurrent VTE (PR per 10-year increment [95% CI]: model 1 = 0.85 [0.77–0.94]), but were slightly more likely to be extremely concerned about major bleeding (PR per 10-year increment [95% CI]: model 1 = 1.11 [1.01–1.22]), with further adjustment for current treatment status (model 3). Non-white participants tended to be less concerned about moderate bleeding than whites. Patients who were currently on DOACs (vs. warfarin) were less likely to be extremely concerned about major bleeding events (PR [95% CI]: 0.17 [0.10, 0.27]). Those currently taking no anticoagulant (vs. warfarin) were less likely to be extremely concerned about both major bleeding events (0.45 [0.31, 0.65]) and all-cause mortality (0.52 [0.33, 0.82]). There were no other differences in likelihood of being extremely concerned about adverse events according to sex, race or initial VTE treatment ( Table 3 ).
Table 3. Prevalence ratios (95% confidence intervals) for being extremely concerned (vs. having lower levels of concern) about specific adverse outcomes ( n = 519): 2016 .
| Age | Sex | Race | Initial treatment | Current treatment | ||
|---|---|---|---|---|---|---|
| Per 10 y | Male vs. female | Non-white vs. white | DOAC vs. warfarin | DOAC vs. warfarin | No OAC vs. warfarin | |
| Recurrent VTE | ||||||
| Model 1 | 0.85 (0.77, 0.94) | 1.02 (0.71, 1.46) | 1.18 (0.80, 1.73) | |||
| Model 2 | 0.86 (0.77, 0.95) | 1.01 (0.71, 1.45) | 1.16 (0.79, 1.71) | 1.10 (0.86, 1.42) | ||
| Model 3 | 0.85 (0.77, 0.95) | 1.02 (0.71, 1.46) | 1.18 (0.80, 1.73) | 1.01 (0.76, 1.33) | 1.06 (0.77, 1.46) | |
| Major bleeding a | ||||||
| Model 1 | 1.03 (0.91, 1.18) | 0.95 (0.60, 1.50) | 1.49 (0.92, 2.42) | |||
| Model 2 | 1.04 (0.91, 1.19) | 0.95 (0.60, 1.50) | 1.49 (0.92, 2.41) | 1.19 (0.85, 1.68) | ||
| Model 3 | 1.11 (1.01, 1.22) | 0.64 (0.43, 0.95) | 0.92 (0.60, 1.40) | 0.17 (0.10, 0.27) | 0.45 (0.31, 0.65) | |
| Moderate bleeding b | ||||||
| Model 1 | 0.95 (0.81, 1.12) | 0.96 (0.54, 1.69) | 1.93 (1.14, 3.26) | |||
| Model 2 | 0.96 (0.81, 1.13) | 0.96 (0.54, 1.69) | 1.93 (1.14, 3.27) | 1.02 (0.67, 1.54) | ||
| Model 3 | 0.93 (0.79, 1.10) | 0.95 (0.54, 1.68) | 1.88 (1.11, 3.16) | 0.77 (0.50, 1.19) | 0.55 (0.29, 1.02) | |
| Death c | ||||||
| Model 1 | 0.96 (0.86, 1.06) | 1.14 (0.80, 1.63) | 1.24 (0.81, 1.90) | |||
| Model 2 | 0.95 (0.85, 1.06) | 1.14 (0.80, 1.64) | 1.24 (0.81, 1.91) | 0.96 (0.72, 1.28) | ||
| Model 3 | 0.94 (0.84, 1.05) | 1.13 (0.79, 1.62) | 1.25 (0.82, 1.91) | 0.85 (0.64, 1.14) | 0.52 (0.33, 0.82) | |
Abbreviations: DOAC, direct oral anticoagulant; OAC, oral anticoagulant; VTE, venous thromboembolism.
Note: Model 1: age (continuous per 10-year increments); sex (referent = female); race (referent = Caucasian).
Model 2: model 1 + initial treatment (referent = warfarin).
Model 3: model 1 + current treatment (referent = warfarin).
Described as bleeding into the head that causes permanent disability.
Described as bleeding that requires medical treatment.
Death from any cause.
Level of concern about specific adverse events, further stratified by whether the participants were currently taking OACs, and among users' duration (<13 and ≥13 months) is presented in Supplementaryl Table 1 , available online only. Overall, current OAC users who had been using OACs for less than 13 months expressed the highest levels of concern. Among these individuals, 46.2% were extremely concerned about recurrent VTE, 41.4% about death from any cause, 28.3% about major bleeding and 20.7% about moderate bleeding.
Anticoagulant Characteristic Preferences
Descriptive information about anticoagulant preferences is provided in Fig. 2 , stratified by whether the patient was initially prescribed warfarin ( Fig. 2A ) or a DOAC ( Fig. 2B ). Multi-variable results are presented in Table 4 . Participants were asked about their degree of agreement or disagreement with the following statement: “I am comfortable using a blood thinner where the levels cannot be followed”; 29.9% strongly disagreed with this assertion. These individuals tended to be older (PR per 10-year increment: model 1 = 1.19 [1.08, 1.31]), and were less likely to have been prescribed a DOAC in their initial treatment phase (PR DOAC versus warfarin : 0.35 [0.23, 0.53]), or to currently be on a DOAC (0.17 [0.10, 0.27; Table 4 ).
Fig. 2.
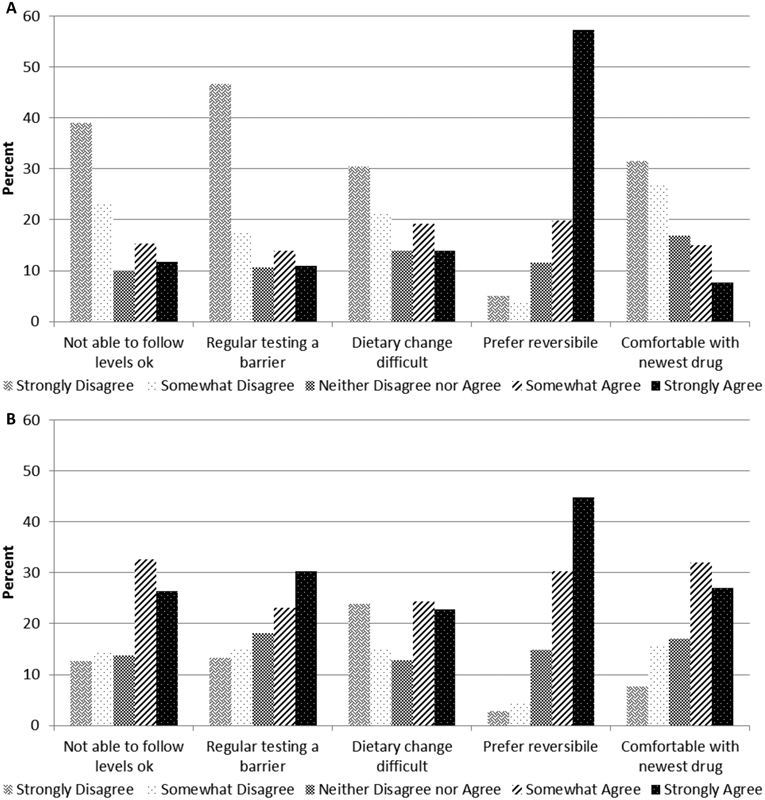
Preferences about characteristics of oral anticoagulants, a stratified by whether patients were initially prescribed warfarin or a direct oral anticoagulant (DOAC): 2016 ( n = 519). ( A ) Initially prescribed warfarin. (B) Initially prescribed a DOAC. a Full questions from survey: I am comfortable using a blood thinner where the levels cannot be followed. • Regular blood tests to monitor a blood thinner's level would make me less likely to use that blood thinner. • It is difficult for me to change my diet, so I can take a particular medication. • I prefer a blood thinner that is reversible. • I am comfortable using the newest drug versus an older but more established drug.
Table 4. Prevalence ratios (95% confidence intervals) for preferences of anticoagulants ( n = 519): 2016 .
| Age | Sex | Race | Initial treatment | Current treatment | ||
|---|---|---|---|---|---|---|
| Per 10 y | Male vs. female | Non-white vs. white | DOAC vs. warfarin | DOAC vs. warfarin | No OAC vs. warfarin | |
| I am comfortable using a blood thinner where the levels cannot be followed (strongly disagreed vs. all other options) | ||||||
| Model 1 | 1.19 (1.08, 1.31) | 0.55 (0.35, 0.85) | 1.06 (0.67, 1.70) | |||
| Model 2 | 1.13 (1.02, 1.24) | 0.57 (0.37, 0.88) | 1.02 (0.65, 1.60) | 0.35 (0.23, 0.53) | ||
| Model 3 | 1.11 (1.01, 1.22) | 0.64 (0.43, 0.95) | 0.92 (0.60, 1.40) | 0.17 (0.10, 0.27) | 0.45 (0.31, 0.65) | |
| Regular blood tests make less likely to use that blood thinner (strongly agreed vs. all other options) | ||||||
| Model 1 | 0.97 (0.84, 1.13) | 1.28 (0.80, 2.06) | 1.08 (0.56, 2.07) | |||
| Model 2 | 1.04 (0.90, 1.19) | 1.19 (0.76, 1.85) | 1.11 (0.59, 2.06) | 2.86 (1.96, 4.19) | ||
| Model 3 | 1.05 (0.91, 1.21) | 1.04 (0.67, 1.62) | 1.10 (0.60, 2.01) | 6.59 (3.58, 12.16) | 3.06 (1.47, 6.36) | |
| It is difficult for me to change my diet so I can take a particular medication (strongly agreed vs. all other options) | ||||||
| Model 1 | 0.89 (0.75, 1.04) | 0.78 (0.42, 1.43) | 1.26 (0.68, 2.32) | |||
| Model 2 | 0.91 (0.78, 1.07) | 0.76 (0.41, 1.38) | 1.27 (0.70, 2.33) | 1.60 (1.09, 2.35) | ||
| Model 3 | 0.93 (0.80, 1.09) | 0.74 (0.40, 1.35) | 1.28 (0.71, 2.31) | 2.88 (1.70, 4.87) | 3.07 (1.74, 5.42) | |
| I prefer a blood thinner that is reversible (strongly agreed vs. all other options) | ||||||
| Model 1 | 1.03 (0.96, 1.09) | 1.01 (0.81, 1.25) | 0.85 (0.61, 1.20) | |||
| Model 2 | 1.01 (0.94, 1.08) | 1.00 (0.80, 1.25) | 0.85 (0.60, 1.18) | 0.78 (0.65, 0.95) | ||
| Model 3 | 1.01 (0.94, 1.08) | 1.01 (0.82, 1.25) | 0.84 (0.61, 1.17) | 0.66 (0.54, 0.80) | 0.79 (0.63, 0.98) | |
| I am comfortable using the newest drug vs. an older but more established drug (strongly agreed vs. all other options) | ||||||
| Model 1 | 1.01 (0.86, 1.19) | 1.31 (0.76, 2.24) | 1.03 (0.47, 2.22) | |||
| Model 2 | 1.11 (0.95, 1.30) | 1.24 (0.76, 2.04) | 1.04 (0.49, 2.17) | 3.80 (2.43, 5.96) | ||
| Model 3 | 1.11 (0.95, 1.31) | 1.05 (0.63, 1.74) | 1.17 (0.57, 2.41) | 6.94 (3.51, 13.70) | 2.38 (1.00, 5.68) | |
Abbreviations: DOAC, direct oral anticoagulant; OAC, oral anticoagulant.
Note: Model 1: age (continuous per 10-year increments); sex (referent = female); race (referent = Caucasian).
Model 2: model 1 + initial treatment (referent = warfarin).
Model 3: model 1 + current treatment (referent = warfarin).
Participants were also asked about their degree of agreement or disagreement with the following statement: “Regular blood tests to monitor a blood thinner's level would make me less likely to use that blood thinner.” Only 17.7% of participants strongly agreed. Accordingly, these individuals were more likely prescribed a DOAC versus warfarin for their initial treatment (PR: 2.86 [1.96, 4.19] and to currently be on a DOAC [6.59 (3.58, 12.16)] or no anticoagulant [3.06 (1.47, 6.36)], as opposed to warfarin). Conversely, approximately 35% strongly disagreed with the statement.
Similarly, 17.0% strongly agreed with the assertion: “It is difficult for me to change my diet so I can take a particular medication.” These individuals were also more likely to be prescribed a DOAC versus warfarin for initial treatment (1.60 [1.09, 2.35]), and to currently be on a DOAC (2.88 [1.70, 4.87]) or no anticoagulant (3.07 [1.74, 5.42]) versus warfarin. Of the full sample, 28.2% strongly disagreed with the statement.
In terms of reversibility, 52.9% strongly agreed with the statement: “I prefer a blood thinner that is reversible.” These individuals were less likely to be prescribed a DOAC versus warfarin for their initial treatment (0.78 [0.65, 0.85]), or to be currently on a DOAC (0.66 [0.54, 0.80]) or no anticoagulant (0.79 [0.63, 0.98]).
Finally, participants were asked about their degree of agreement or disagreement with the following statement: “I am comfortable using the newest drug versus an older but more established drug.” Only 14.5% strongly agreed with the statement, while 23.2% strongly disagreed. Participants who strongly agreed with this statement were 3.80 (2.43, 5.96) times more likely to have been prescribed a DOAC than warfarin for their initial treatment, and to presently be on a DOAC (6.94 [3.51, 13.70]), as compared with warfarin.
Results were similar in sensitivity analyses where we stratified by data source (online vs. Vermont clinic) and VTE subtype (PE with or without DVT vs. DVT only), and there was no evidence of interaction by age ( p interaction > 0.2).
Supplementaryl Table 2 (available online only) presents results stratified by whether the participants were currently taking OACs, and among users' duration (<13 and ≥13 months). Overall, results of these subgroups were similar to results for the full analysis.
Discussion
The opinions of patients with VTE regarding their preferences for treatment choices and degree of concern about specific outcomes are poorly understood. The results of this study, which included VTE patients that were predominantly females and younger than typical VTE patient populations, provide information about factors that may influence patient choice of treatments. We found that, overall, patients with VTE were more concerned about recurrent VTE than bleeding, and that reversibility of an anticoagulant and the ability to monitor anticoagulant levels were important to them. Further, most did not consider regular monitoring and dietary restrictions onerous. These findings bring into question whether what are perceived as advantages of DOACs by the medical community are really also viewed by patients as advantages.
Given the novelty of DOACs, very little research has evaluated VTE patient concerns and preferences about characteristics of DOACs compared with warfarin. In the present study, the outcomes of greatest concern were recurrent VTE, major bleeding events and all-cause mortality. Level of concern was similar regardless of whether the patient had been initially prescribed a DOAC or warfarin. In the Netherlands, a study of 135 VTE patients using warfarin was conducted, which presented patients with four different advantages of DOACs and, using “trade-off technique” methodology, asked whether they would switch from warfarin given each individual advantage. 23 Of these patients, 65% said they would switch to a DOAC if it resulted in fewer food and drug interactions, 57% if there was decreased bleeding risk and 36% if there was no need for laboratory control. Greater efficacy was not significantly associated with patients saying they would switch from warfarin to a DOAC. Across scenarios, neither gender nor treatment duration was associated with self-reported likelihood of switching. 23 These results are similar to another study conducted in the Netherlands, in the context of AF patients. 24 These analyses of hypothetical questions compliment the present study, which looked at characteristics and preferences of individuals actively taking either DOACs or warfarin. One notable difference between the findings is that patients in the Netherlands viewed food and drug interactions as very important, whereas in the present study only 17% agreed with the statement: “It is difficult for me to change my diet so I can take a particular medication.”
There is a general assumption that the conveniences of DOACs (i.e., fewer dietary and drug interactions, no drug-level monitoring) result in preferred use by patients compared with warfarin, despite their disadvantages (i.e., less real-world safety and efficacy data, lack of ability to determine if drug levels are in the therapeutic range and less established protocols for anticoagulant reversal). Our data reveal otherwise; patients generally did not view the inconveniences of warfarin overly burdensome, but were wary about the aforementioned uncertainties related to DOACs. These results did not vary by current anticoagulant status or duration taking anticoagulants. According to current guidelines, 7 the decision as to which anticoagulant is chosen for initial and long-term VTE treatment is “expected to be sensitive to patient preferences”. Our results need to be interpreted in the light of the fact that at the time of the survey there were no approved reversal agents for DOACs. Since the survey, idarucizumab has been approved, and other reversal agents are in late-stage clinical trials. However, clinical experience with DOAC antidotes is limited at present.
From a scientific standpoint, the most effective anticoagulant is one that treats VTE and prevents recurrence, with little or no excessive bleeding and no off-target effects. From a more patient-centric approach, patient values and concerns must be taken into consideration, as the most effective anticoagulant is ineffective if patients do not take the medication. Despite perceived limitations, warfarin is a highly effective anticoagulant and, when properly managed in the appropriately educated patient, is fairly safe. 8 Presently, the DOACs have met clinical equivalence standards in the treatment of VTE. 9 10 11 12 13 14 Additional data are needed to document the safety and effectiveness of DOACs versus warfarin for the treatment of VTE in real-world settings in VTE patient subgroups that were not adequately represented in the randomized controlled trials (e.g., those with cancer). 15 It is possible that, as additional information accrues, either DOACs or warfarin will emerge as superior for select patient subgroups. As we await definitive answers, research such as that presented herein can enhance understanding of considerations that influence patients' decision making and can help inform conversations between providers and their VTE patients.
This study has noteworthy strengths and limitations. A key strength is the recruitment of participants from both VTE patient organizations and a thrombosis and haemostasis clinic. Participants from the online sources allowed for a snapshot of anticoagulant preferences and concerns across a wide geographic range. However, our results from participants recruited through the VTE patient organizations may not be fully generalizable, since to be included in the study the patients had to have access to the internet, and elect to visit a VTE patient Web site or follow the NBCA on Facebook. While VTE can affect individuals of all sexes and ages, it is predominantly a disease of ageing with a relatively equal distribution by sex. 25 Our sample had a higher percentage of women (82%) and was younger (average age of 46 years) than seen in the typical distribution of VTE patients. Both www.stoptheclot.org and www.clotconect.org are very popular, with approximately 1 million and 120,000 page views each month, respectively. Further, NBCA has more than 12,000 Facebook followers. In sensitivity analyses, results were similar when data were stratified by data source (Web site vs. clinic), providing support for the generalizability of our results. Another limitation of this study is that all information was self-reported; however, overall participant responses were internally consistent. Additionally, the data are cross-sectional, and it is possible patients reported preferences in accord with the drug they were currently prescribed, rather than their preferences at the time of initial prescription. Lastly, this study did not evaluate anticoagulant cost, or adherence and persistence. Additional research is needed to evaluate whether cost or any of the patient preferences evaluated herein are associated prospectively with adherence and persistence. Such investigations may inform interventions to improve anticoagulant usage among VTE patients, and in doing so improve patient outcomes.
Conclusions
In summary, to optimize outcomes for persons affected by VTE, identifying a treatment that is safe, effective and will be adhered to is paramount. Individual-level patient characteristics and preferences are important when selecting the appropriate anticoagulation therapy for a particular patient. In the present study of 519 VTE patients, who were predominantly female and had an average age of 46 years, we observed that patients held the greatest concern for recurrent VTE, major bleeding events and all-cause mortality. Concern for specific adverse events was similar across patients, regardless of whether they were initially prescribed a DOAC or warfarin. The advantages of the DOACs (i.e., less major bleeding, minimal food interactions and no need for monitoring levels) were less appreciated by patients than some of the disadvantages (e.g., inability to monitor levels). Reversibility was also an important consideration for patients. These findings provide insight into how VTE patients weigh various factors when selecting, together with their clinician, an anticoagulant for VTE initial treatment and secondary prevention. Further, these data highlight the need for development of monitoring parameters for all anticoagulants, and for the establishment reversal strategies.
Acknowledgements
We are grateful to the survey respondents, the NCBA, Clot Connect, and the University of Vermont Thrombosis and Hemostasis Program (especially Karen Libby RN and Emily Parenteau APRN) who volunteered their time to facilitate this project.
Funding Statement
Funding/Support This work was supported by non-sponsored McKnight Land-Grant Professorship funds to Dr. Lutsey and R01 HL131579.
Footnotes
Conflicts of Interest Dr. Stephan Moll has consulted for Janssen (manufacturers Xarelto/rivaroxaban), Boehringer-Ingelheim (Pradaxa/dabigatran etexilate and Praxbind/idarucizumab) and Portola (betrixaban and andexanet). The commercial entities had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. No other authors have any conflicts of interest to disclose.
What is known about this topic?
Both warfarin and direct oral anticoagulants (DOACs) are now widely used to treat venous thromboembolism (VTE).
It is unclear how VTE patients perceive oral anticoagulant treatment options.
What does this paper add?
A total of 519 VTE patients, who were mostly females and were younger than typical VTE patients, were surveyed about their perceptions and concerns related to oral anticoagulants.
VTE patients were most concerned about recurrent VTE and all-cause mortality.
Patients viewed as important reversibility of an anticoagulant and the ability to monitor anticoagulant levels, while most did not consider regular monitoring and dietary restrictions onerous.
These findings bring into question whether what are perceived as advantages of DOACs by the medical community are also really viewed by patients as advantages.
Supplementary Material
References
- 1.Bell E J, Lutsey P L, Basu S et al. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med. 2016;129(03):3.39E21–3.39E28. doi: 10.1016/j.amjmed.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White R H. The epidemiology of venous thromboembolism. Circulation. 2003;107(23) 01:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 3.Lutsey P L, Virnig B A, Durham S B et al. Correlates and consequences of venous thromboembolism: the Iowa Women's Health Study. Am J Public Health. 2010;100(08):1506–1513. doi: 10.2105/AJPH.2008.157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prandoni P, Lensing A W, Cogo A et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(01):1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Heit J A, Mohr D N, Silverstein M D, Petterson T M, O'Fallon W M, Melton L J., III Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160(06):761–768. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]
- 6.Prandoni P, Noventa F, Ghirarduzzi A et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92(02):199–205. doi: 10.3324/haematol.10516. [DOI] [PubMed] [Google Scholar]
- 7.Kearon C, Akl E A, Ornelas J et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(02):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Middeldorp S, Prins M H, Hutten B A. Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism. Cochrane Database Syst Rev. 2014;8(08):CD001367. doi: 10.1002/14651858.CD001367.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison L, McGinnis J, Crowther M, Ginsberg J, Hirsh J. Assessment of outpatient treatment of deep-vein thrombosis with low-molecular-weight heparin. Arch Intern Med. 1998;158(18):2001–2003. doi: 10.1001/archinte.158.18.2001. [DOI] [PubMed] [Google Scholar]
- 10.Schulman S, Kearon C, Kakkar A K et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 11.Bauersachs R, Berkowitz S D, Brenner B et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 12.Büller H R, Décousus H, Grosso M A et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 13.Agnelli G, Buller H R, Cohen A et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(09):799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 14.Schulman S, Kakkar A K, Goldhaber S Z et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(07):764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 15.van der Hulle T, Kooiman J, den Exter P L, Dekkers O M, Klok F A, Huisman M V. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(03):320–328. doi: 10.1111/jth.12485. [DOI] [PubMed] [Google Scholar]
- 16.Schulman S, Kearon C, Kakkar A K et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(08):709–718. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 17.Raskob G, Ageno W, Cohen A T et al. Extended duration of anticoagulation with edoxaban in patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol. 2016;3(05):e228–e236. doi: 10.1016/S2352-3026(16)00023-5. [DOI] [PubMed] [Google Scholar]
- 18.Schulman S, Singer D, Ageno W, Casella I B, Desch M, Goldhaber S Z. NOACs for treatment of venous thromboembolism in clinical practice. Thromb Haemost. 2017;117(07):1317–1325. doi: 10.1160/TH17-01-0065. [DOI] [PubMed] [Google Scholar]
- 19.Larsen T B, Skjøth F, Kjældgaard J N, Lip G YH, Nielsen P B, Søgaard M. Effectiveness and safety of rivaroxaban and warfarin in patients with unprovoked venous thromboembolism: a propensity-matched nationwide cohort study. Lancet Haematol. 2017;4(05):e237–e244. doi: 10.1016/S2352-3026(17)30054-6. [DOI] [PubMed] [Google Scholar]
- 20.Janzic A, Kos M. Cost effectiveness of novel oral anticoagulants for stroke prevention in atrial fibrillation depending on the quality of warfarin anticoagulation control. Pharmacoeconomics. 2015;33(04):395–408. doi: 10.1007/s40273-014-0246-7. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(03):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 22.Rothman K J, Greenland S, Lash T L. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. Modern Epidemiology. 3rd ed. [Google Scholar]
- 23.Brekelmans M P, Kappelhof M, Nieuwkerk P T, Nierman M, Buller H R, Coppens M. Preference for direct oral anticoagulants in patients treated with vitamin K antagonists for venous thromboembolism. Neth J Med. 2017;75(02):50–55. [PubMed] [Google Scholar]
- 24.Boom M S, Berghuis E M, Nieuwkerk P T, Pinedo S, Büller H R. When do patients prefer a direct oral anticoagulant over a vitamin K antagonist? Neth J Med. 2015;73(08):368–372. [PubMed] [Google Scholar]
- 25.Heit J A, Spencer F A, White R H. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41(01):3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


