Abstract
A series of isoxazole and triazole derivatives, with interesting bioactive scaffolds, were examined for their in vitro antibacterial, antifungal, and antiprotozoal activities. These compounds exhibited antitrypanosomal activity comparable to difluoromethylornithine (DMFO), a drug used in the treatment of human African trypanosomiasis. Isoxazole analogues 1, 3 and 4, and triazole derivatives 16, 17, 28, 37, 40 and 42 showed the highest antitrypanosomal activity with IC50 values of 17.89, 1.82, 10.38, 10.26, 11.77, 9.29, 3.93, 2.11, and 0.93 μM, respectively. Compounds 40 and 42 showed the most potent activity against Leishmania donovani amastigotes with IC50 values of 18.28 and 10.54 μM, respectively. Compound 42 showed the most potent activity against Leishmania donovani macrophage internalized amastigotes with an IC50 value of 8.32 μM. Conjugate triazoles 40–43 displayed potential antimalarial activity against chloroquine-resistant W2 and chloroquine sensitive D6 Plasmodium falciparum strains (IC50 value range from 0.58 to 8.36 μM). Compound 37 showed antibacterial activity against Staphylococcus aureus, MRSA and Mycobacterium intracellulare with IC50 values of 15.53, 14.22 and 47.45 μM, respectively. None of the compounds exhibited antifungal activity.
Keywords: triazole and isoxazole derivatives, antibacterial and antiprotozoal activity
1. Introduction
Over the last decades, progress on the pharmacological activity and synthesis of various substituted triazole/isoxazole derivatives has been added to the scientific literature. Through this research, compounds containing a triazole-ring have showed a broad spectrum of activities, including anti-inflammatory (Sana et al., 2018), analgesic (Montes et al., 2017), antitumor (Kaur et al., 2016), anti-diabetic (Park and Pham 2017), and anticonvulsive (Chelamalla et al., 2017) activity. Additionally, there are several reports that highlight the importance of the triazoles motif against microbes: bacteria, fungi, protozoa, and virus (Ashok et al., 2018; Cao et al., 2017; Keivanloo et al., 2016; Kumar et al., 2013). In fact, the triazole ring is found on several marketed antifungal drugs including, terconazole, fluconazole, posaconazole, voriconazole and itraconazole (Nett et al., 2016). Similarly, the isoxazole motif is found in several clinically approved antibiotics including oxacillin, cloxacillin, dicloxaciillin and flucloxacillin (Sutherland et al., 1970).
Neglected tropical diseases (NTDs) are disabling and potentially life-threatening infections affecting more than a billion people worldwide (Thao et al., 2014). Human African trypanosomiasis (HAT), commonly known as sleeping sickness, is an NTD caused by the Trypanosoma brucei parasite and transmitted to humans through the bite of the tsetse fly (Tiberti et al., 2013). The parasites can travel through the blood-brain barrier and invade the Central Nervous System (CNS), at which time neurological symptoms begin to appear. In the absence of treatment, the disease results in wasting of body tissue, coma, and ultimately death (Gull 2002). The resulting coma is why this infection is called sleeping sickness. Leishmaniasis is a group of tropical diseases caused by a number of species of protozoan parasites belonging to the genus Leishmania. Cutaneous leishmaniasis and visceral leishmaniasis have been reported to be the two major forms of leishmaniasis in humans (Peixoto et al., 2011). These NTDs are clearly related to poverty since this population of patients does not have access to appropriate medication and healthcare. NTDs have a huge impact on the economic growth in developing countries (Kotowski et al., 2015).
While malaria is not cataloged as a NTD, it is considered the leading cause of death and morbidity worldwide among infectious diseases. It is caused by parasites that are transmitted to people through the bites of female mosquitoes infected with a Plasmodium parasite. There are four main species of the malaria parasite which include P. falciparum, P. malariae, P. vivax and P. ovale. According to the World Health Organization (WHO), in 2016 there were approximately 216 million cases of malaria in 91 countries (WHO 2017). The drugs available for the treatment of these endemic diseases are old, expensive, and associated with other problems including safety. The situation is intensified by the quick development of resistance against therapeutic agents, resulting in drug resistant parasites (WHO 2017).
The control of infectious disease requires continued research on bioactive compounds. In this context, biologically active scaffolds such as heterocyclic compounds possessing triazole and isoxazole derivatives could be used as potential leads. There are few examples of the use of these important heterocyclic motifs against NTDs. Different triazole derivatives showed in vitro and in vivo activity against a variety of strains of T. cruzi and T. brucei brucei. A group of synthesized metal complexes of triazole-pyrimidine derivatives inhibited the in vitro growth of epimastigotes of T. cruzi and procyclic forms of T brucei brucei (Magán et al., 2004). Some isoxazole containing heteroretinoid derivatives showed in vivo antileishmanial activity against Leishmania donovani in hamsters (Suryawanshi et al., 2012). Giannini and Battistuzzi 2015, reported the antiprotozoal activity against T. brucei, T. cruzi and P. falciparum of a series of 3,4,5 isoxazole and 1,4,5 trisubsituted triazole derivatives. These compounds showed nM range activity and excellent binding to the heat shock protein (Hsp90α), confirming the important role that Hsp chaperone proteins play in the live cycle of the parasite. There have been some efforts to conjugate triazole compounds. For example, 3-nitro-1,2,4-triazole with amides and sulfonamides displayed potent T. cruzi activities with IC50 in the nM range (Papadopoulou et al., 2012). Hybrids between 1,2,3-triazole, quinones and sugars showed potential activities against T. cruzi (Jardim et al., 2015).
In continuation of our efforts to find novel active agents against NTDs, a library of triazole and isoxazole compounds were evaluated for their in vitro antitrypanosomal activity, as well as, antileishmanial, antimalarial and antimicrobial properties.
2. Materials and Methods
2.1 Chemistry
The evaluated compounds: twelve isoxazoles 1–12, twenty-four triazoles 13–36 and seven triazole conjugates 37–43 were design and synthesized at the Yarmouk University, Jordan. The general experimental procedure and experimental data for isoxazole and dihydroisoxazole derivatives 1–12 have been reported (Abu-Orabi et al., 1988; Abu-Orabi et al., 1999 and Al-Momani 1995). The synthetic protocols for triazole derivatives 13–36 have been previously described (Abu-Orabi 2002). Compounds 37–39 have been synthesized and their experimental data reported (Tashtuosh et al., 1999). Finally, the synthetic process for compounds 40–43 was previously reported (Abu-Orabi et al., 1988).
2.2 Biological evaluation
2.2.1 In vitro antimicrobial assay
Compounds 1–43 were tested for antimicrobial activity against a panel of microorganisms obtained from the American Type Culture Collection (Manassas, VA, USA) and included the fungi: Candida albicans ATCC 90028, Candida glabrata ATCC 90030, Candida krusei ATCC 6258, Cryptococcus neoformans ATCC 90113, Aspergillus fumigatus ATCC 204305, the bacteria Staphylococcus aureus ATCC 29213, methicillin-resistant S. aureus ATCC 33591 (MRSA), Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, and Mycobacterium intracellulare ATCC 23068. The bioassays were performed as previously described (Ma et al., 2004 and Bharate et al., 2007). Ciprofloxacin and amphotericin B were used as positive drug controls.
2.2.2 In vitro antitrypanosomal assays
Blood stage forms of Trypanosoma brucei brucei was grown in IMDM medium supplemented with 10% fetal bovine serum. The culture was maintained at 37 °C in 5% CO2 incubator. Two day old culture of T. brucei was diluted to 5,000 parasites/ml. Diluted T. brucei parasite culture was dispenses in clear flat bottom culture well plates and treated with test compounds. The antitrypanosomal screening assay was based on Alamar blue based fluorometric growth analysis at concentration range of 10–0.4 μg/ml. Active compounds were further screened at concentration range 10–0.0032 μg/ml. Pentamidine and difluoromethylornithine were used as positive drug controls. IC50 and IC90 values were computed from dose response growth inhibition curve by XLfit version 5.2.2 (Jain et al., 2016).
2.2.3 In vitro antileishmanial assays
Promastigote culture of Leishmanial donovani was grown in RPMI medium with 10% FBS with pH 7.4 at 26 °C. Axenic amastigote culture of Leishmanial donovani was grown in RPMI medium with 10% FBS with pH 5.5 at 37 °C in 5% CO2 incubator. The antileishmanial activity of the compounds was tested in vitro against promastigotes, axenic amastigotes and macrophage internalized amastigote form of Leishmania donovani parasite. Promastigotes and axenic amastigotes assays were based on alamar blue fluorometric growth analysis (Manda et al., 2014). Differentiated THP1 cells were been used in macrophage internalized-amastigote assay. Macrophage internalized amastigote method was based on parasite rescued and transformation assay described earlier, pentamidine and amphotericin B were used as positive standards (Jain et al., 2012).
2.2.4 In vitro antimalarial assays
Antimalarial activity was determined in vitro against chloroquine sensitive (D6, Sierra Leone) and resistant (W2, Indo China) strains of Plasmodium falciparum by a colorimetric assay that determines the parasitic lactate dehydrogenase (pLDH) activity, as described earlier (Hamid et al., 2004).
2.2.5 In vivo antimalarial assays
Plasmodium berghei (NK-65) parasite was obtained from MR4 (Malaria Research Reference Reagents Resource). Male Swiss albino mice weighing ~20 gram were procured from ENVIGO, USA. Animal studies were done under protocol (Protocol approval number 15-007) approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Mississippi, USA. Blood with high parasitemia was collected by cardiac puncture in Acid-Citrate-Dextrose (ACD) solution from these infected mice washed and diluted with sterile saline to adjust the infected RBC’s concentration to 8×107 RBCs/ml. The animals were randomly divided in two different groups with 5 animals in each group and all the mice were inoculated i.p. with 0.5 ml of infected blood for producing the infection. The mice were treated with the test compound oral once daily dose of 11.1 mg/kg from day 0 to 2 post infection. Dose was selected based on maximum tolerated dose of compound 43 in mice.
2.2.6 Cytotoxicity assays
The cultures of Human Acute Monocytic Leukemia Cell Line (THP1 cells) and vero cells were maintained in RPMI medium with 10% FBS in 37 °C 5% CO2 incubator. For the THP1 cytotoxicity assay, four days’ old culture of THP1 cells was diluted with RPMI medium treated with phorbol 12-myristate 13-acetate at 2.5 ng/ml concentration for transformation of the cells to adherent macrophages (Jain et al., 2016). The cytotoxicity assay was done on these differentiated THP1 cells, alamarBlue® cell viability reagent was used to detect fluorometric changes at concentration range of 10–0.4 μg/ml (Jain et al., 2012). Cytotoxicity assay for vero cells was based on the neutral red assay, which was described earlier (Yang et al., 2006).
3. Results and Discussion
A series of twelve isoxazoles 1–12 (Figure 1), twenty-four triazoles 13–36 (Figure 2) and seven triazole conjugates 37–43 (Figure 3) were evaluated for their antibacterial, antifungal, antimalarial antitrypanosomal, antileishmanial and cytotoxicity activities. Out of forty-three tested compounds, twenty-two showed promising antitrypanosomal activity. Four triazole conjugated derivatives (40–43) showed in vitro antimalarial activity. Most of the antitrypanosomal compounds showed comparable activity to the positive control drug difluoromethylornithine (DFMO).
Figure 1.
Isoxazole and dihydroisoxazole derivatives (1–12)
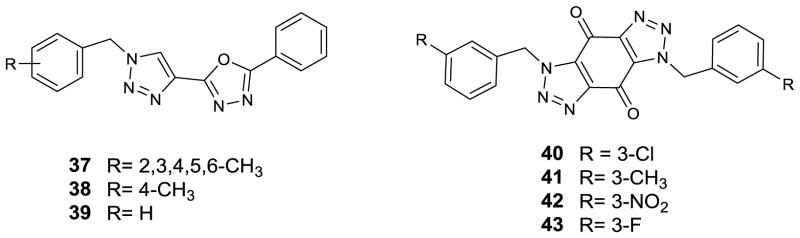
Figure 2.
Triazole derivatives (13–36)
Figure 3.
Triazole conjugated derivatives (37–43)
Antimicrobial activity
The antifungal and antibacterial activities were evaluated against a panel of pathogenic fungi (Candida albicans, C. glabrata, C. krusei, Cryptococcus neoformans, and Aspergillus fumigatus) and a panel of pathogenic bacteria (Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Escherichia coli, Pseudomonas aeruginosa and Mycobacterium intracellulare) associated with opportunistic infections. Only compound 37 possessed antibacterial activity against S. aureus, and MRSA with IC50 values of 15.53 and 14.22 μM, respectively. Compounds 37, 40, 42 and 43 showed significant activity against M. intracellulare with IC50 values of 47.45, 44.83, 43.16 and 7.60 μM, respectively. None of the compounds possessed antifungal activity.
Antitrypanosomal activity
Tables 1–3 show the screening results for antitrypanosomal activity of all compounds. Isoxazole 3 showed the most potent activity against T. brucei with an IC50 value of 1.82 μM (Table 1). Replacing the tertiary butyl ester on the R1 with hydrazinocarbonyl 4 led to reduced activity with a value of 10.38 μM. Changing the methyl group on the benzene ring to methoxy 1, while both R1 and R2 were kept as in 3, did not significantly improve the potency. Modification of the isoxazole in 4 to a 4,5-dihydroisoxazole like in compound 8 lead to lose the biological activity against T. brucei. However, modification replacing the hydrazinocarbonyl R1 with a substituted benzene ring and hydrazinocarbonyl R2 with a benzyl ester in the dihydroisoxazole core yielded compound 10, which showed an IC50 value of 11.51 μM against T. brucei. Replacing the isoxazole moiety of compound 4 with the triazole moiety and keeping the hydrazinocarbonyl in R1 and R2 showed no significant change in the bioactivity against T. brucei. In this series 13–24 halogenated monosubstitution in the aromatic ring showed the highest IC50 values for compounds 15 to 18 (Table 2). Further modification with a trimethyl substitution 22 led to an IC50 value of 22.28 μM. Inserting a bulky group at position R1 and R2 of the triazole ring, such as the pivaloyloxy in compound 34 led the highest potency for this series, with an IC50 value of 7.44 μM. In general, compounds (13–36) containing electronegative groups, such as, halogens showed antitrypanocidal activity. No clear patterns were found to explain the biological effects of the substituents on the benzyl ring.
Table 1.
In vitro antiprotozoal activity of isoxazole (1–7) and dihydroisoxazole (8–12) derivatives
| Compound | Leishmania donovani macrophage internalized amastigote | Trypanosoma brucei | ||
|---|---|---|---|---|
| IC50, μM | IC90, μM | IC50, μM | IC90, μM | |
| 1 | NA | NA | 17.895 ± 1.079 | 23.640 ± 0.843 |
| 2 | NA | NA | NA | NA |
| 3 | 28.836 ± 0.087 | >28.952 | 1.824 ± 0.086 | 4.488 ± 0.819 |
| 4 | NA | NA | 10.385 ± 3.308 | 24.792 ± 5.784 |
| 5 | NA | NA | NA | NA |
| 6 | NA | NA | NA | NA |
| 7 | NA | NA | NA | NA |
| 8 | NA | NA | NA | NA |
| 9 | NA | NA | NA | NA |
| 10 | NA | NA | 11.509 ± 2.265 | >22.347 |
| 11 | NA | NA | NA | NA |
| 12 | NA | NA | NA | NA |
| Amp B | 0.272 ± 0.100 | 0.441 ± 0.019 | NT | NT |
| DFMO | NT | NT | 21.299 ± 2.013 | 59.175 ±5.748 |
NA: not active; NT: not tested; Amp B = Amphotericin B; DFMO = difluoromethylornithine
Table 3.
In vitro antiprotozoal activity of triazole conjugates (37–43)
| Compound | L. donovani promastigotes | L. donovani Axenic amastigotes | L. donovani macrophage internalized amastigote | T. brucei | P. falciparum | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50, μM | IC90, μM | IC50, μM | IC90, μM | IC50, μM | IC90, μM | IC50, μM | IC9, μM | IC50 (D6), μM | IC50 (W2), μM | |
| 37 | NA | NA | 21.796 ± 0.001 | >26.777 | 23.537 ±2.286 | >26.777 | 3.935 ± 0.079 | 4.940 ± 0.092 | NA | NA |
| 38 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 39 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 40 | 14.625 ± 5.757 | >22.766 | 18.281 ± 0.592 | 22.447 ± 0.016 | NA | NA | 2.117 ± 0.147 | 6.766 ± 1.918 | 6.322 ± 0.363 | 7.611 ± 0.838 |
| 41 | NA | NA | NA | NA | NA | NA | 4.741 ± 1.735 | 13.514 ±0.988 | 8.367 ± 3.041 | 6.606 ± 0.732 |
| 42 | 16.445 ± 3.868 | >21.722 | 10.536 ± 0.824 | 18.149 ± 1.651 | 8.321 ± 0.327 | 12.879 ±5.811 | 0.926 ± 0.034 | 1.200 ± 0.026 | 1.909 ± 0.403 | 2.886 ± 0.701 |
| 43 | NA | NA | NA | NA | NA | NA | 7.595 ± 2.119 | 10.468 ± 3.234 | 0.697 ± 0.187 | 0.583 ± 0.140 |
| Amp B | 0.192 | 0.312 | 1.729 | 2.004 | 0.272 ± 0.100 | 0.441 ±0.019 | NT | NT | NT | NT |
| Pent | 2.036 | 3.668 | 15.345 | 16.872 | 6.531 | >16.872 | NT | NT | NT | NT |
| DFMO | NT | NT | NT | NT | NT | NT | 21.299 ±2.013 | 59.175 ±5.748 | NT | NT |
| CQ | NT | NT | NT | NT | NT | NT | NT | NT | 0.052 | 0.46 |
NA: not active; NT: not tested; Amp B = Amphotericin B; Pent = pentamidine; DFMO = difluoromethylornithine; CQ = chloroquine
Table 2.
In vitro antiprotozoal activity of triazole derivatives (13–36)
| Compound | T. brucei | |
|---|---|---|
| IC50, μM | IC90, μM | |
| 13 | 18.277 ± 4.703 | 27.023 ± 0.523 |
| 14 | 22.279 ± 5.081 | 32.159 ± 0.653 |
| 15 | 18.340 ± 6.856 | 28.058 ± 1.866 |
| 16 | 10.268 ± 0.396 | 21.665 ± 1.312 |
| 17 | 11.774 ± 1.997 | 24.452 ± 0.095 |
| 18 | 14.174 ± 9.934 | >28.235 |
| 19 | 25.813 ± 5.853 | >34.099 |
| 20 | NA | NA |
| 21 | NA | NA |
| 22 | 22.278 ± 6.227 | >31.511 |
| 23 | NA | NA |
| 24 | NA | NA |
| 25 | NA | NA |
| 26 | NA | NA |
| 27 | NA | NA |
| 28 | 9.293 ± 3.071 | 17.689 ± 7.967 |
| 29 | NA | NA |
| 30 | NA | NA |
| 31 | NA | NA |
| 32 | 41.026 ± 1.059 | >42.514 |
| 33 | 32.838 ± 0.892 | >34.100 |
| 34 | 7.445 ± 1.209 | 9.486 ± 1.436 |
| 35 | 40.218 ± 1.615 | >42.514 |
| 36 | NA | NA |
| DFMO | 21.299 ± 2.013 | 59.175 ±5.748 |
NA: not active; NT: not tested; DFMO = difluoromethylornithine
In the series of conjugate triazole-oxadiazole compounds (37–39), compound 37 showed the highest activity with an IC50 value of 3.93 μM. The quinone-triazole conjugate compounds (40–43) showed in overall the highest bioactive potency for all the series against T. brucei with IC50 values of 2.12, 4.74, 0.93, and 7.60 μM, respectively (Table 3). Compound 42 has a strong deactivating substituent and was the most potent against T. brucei. Cytotoxicity screening was done for all tested compounds. None of the compounds showed more than 50 % inhibition on differentiated THP1 cells with the exception of compound 42 with an IC50 value of 10.10 μM.
Antileishmania activity
Antileishmania activity data is shown for active compounds in Tables 1 and 3. For isoxazoles derivatives and triazoles conjugates, analogues 37, 40, and 42 exhibited the highest inhibition values for L. donovani axenic amastigote with IC50 value of 21.80, 18.28 and 10.54 μM, respectively. Analogues 37 and 42 have high activity on the macrophage internalized amastigote form of Leishmania donovani parasite with IC50 values of 23.54 and 8.32 μM, respectively.
Antimalarial activity
All compounds 1–43 were tested against P. falciparum D6 and W2 strains. Activity was found only for compounds 40–43. These compounds showed promising action against both chloroquine-resistant (P. falciparum W2) and chloroquine-sensitive strains of malaria (P. falciparum D6) (Table 3). Compound 43 showed the most potent activity with IC50 values of 0.70 and 0.59 μM, for D6 and W2, respectively. Replacing the 3-fluoro substitution with chloro 40, methyl 41, or nitro 42 groups reduced the activity of this series. All the compounds were evaluated for cytotoxicity against mammalian kidney cell line (Vero) and selectivity index was calculated. Selectivity index values less than 1.2 indicated that antimalarial activity was not due to cytotoxicity. Antimalarial activity of compound 43, the most potent of all the series was tested in vivo in P. berghei mouse malaria model for survival (Figure 4). Chloroquine served as the positive control. Compound 43 increased the survival time to 16.2 days when compared to the vehicle control group of mice at a dose of 11.1 mg/kg per day for 3 days.
Figure 4.
Survival chart for mice in antimalarial study (43)
In conclusion, we report the antimalarial, antileishmanial, antibacterial activities of 43 isoxazole and triazole derivatives. Triazole compounds 13–36 were active against T. brucei, while compound 37, having triazole and oxadiazole rings, showed promising activities against all pathogens. Compounds 40–43 produced the most potent antileishmanial and antimalarial activities. Further explorations of these triazole derivatives can provide lead compounds for antimicrobial agents.
Supplementary Material
Acknowledgments
The authors thank Dr. Shabana Khan for performing the antimalarial assays. The work was supported by grant number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or the NIH. Furthermore, this investigation was conducted in a facility constructed with support from research facilities improvement program C06RR14503 from the NIH National Center for Research Resources (NCRR).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Abu-Orabi ST. 1, 3-Dipolar cycloaddition reactions of substituted benzyl azides with acetylenic compounds. Molecules. 2002;7:302–314. [Google Scholar]
- Abu-Orabi ST, Al-Hamdany R, Al-Momany LA, Ta’an EA. Reactions of isoxazoline and isoxazole derivatives with hydrazine hydrate. Asian J Chem. 1999;11:1276. [Google Scholar]
- Abu-Orabi ST, Jibril I, Obiedat R, Hatamleh L. Cycloaddition reactions of 2, 4, 6-trimethoxybenzonitrile oxide with disubstituted acetylenes. J Chem Eng Data. 1988;33:540–541. [Google Scholar]
- Abu-Orabi ST, Saleh M, Al-Momani L, Jibril I, Yousef Y. Synthesis of mono-and bis-triazoles via 1, 3-dipolar cycloaddition reactions of azide derivatives with naphtho- and benzoquinone. Jordan J Chem. 2006;1:109–120. [Google Scholar]
- Al-Momani LA. Master Dissertation. Yarmouk University; 1995. Synthesis, study and reactions of isoxazole and isoxazoline compounds via 1,3-dipolar cycloadditions of aromatic nitrile oxides. [Google Scholar]
- Ashok D, Gundu S, Aamate VK, Devulappaly MG, Bathini R, Manga V. Dimers of coumarin-1,2,3-triazole hybrids bearing alkyl spacer: Design, microwave-assisted synthesis, molecular docking and evaluation as antimycobacterial and antimicrobial agents. J Mol Struct. 2018;1157:312–321. [Google Scholar]
- Bharate SB, Khan SI, Yunus NA, Chauthe SK, Jacob MR, Tekwani BL, Khan IA, Singh IP. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg Med Chem. 2007;15:87–96. doi: 10.1016/j.bmc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Cao X, Wang W, Wang S, Bao L. Asymmetric synthesis of nnovel triazole derivatives and their in vitro antiviral activity and mechanism of action. Eur J Med Chem. 2017;139:718–725. doi: 10.1016/j.ejmech.2017.08.057. [DOI] [PubMed] [Google Scholar]
- Chelamalla R, Makula A, Manda S. Design, synthesis and in silico studies of new 5-substituted-2-(2-(5-aryl-1H-1,2,4-triazole-3-ylthio)acetyl) hydrazine carbothioamide/carboxamides for anticonvulsant activity. Lett Drug Des Discovery. 2017;14:1155–1163. [Google Scholar]
- Giannini G, Battistuzzi G. Exploring in vitro and in vivo Hsp90 inhibitors activity against human protozoan parasites. Bioorg Med Chem Lett. 2015;25:462–465. doi: 10.1016/j.bmcl.2014.12.048. [DOI] [PubMed] [Google Scholar]
- Gull K. The Cell Biology of Parasitism in Trypanosoma brucei: Insights and Drug Targets from Genomic Approaches? Curr Pharm Des. 2002;8:241–256. doi: 10.2174/1381612023396212. [DOI] [PubMed] [Google Scholar]
- Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol In Vitro. 2004;18:703–710. doi: 10.1016/j.tiv.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Jain SK, Jacob MR, Walker LA, Tekwani BL. Screening North American plant extracts in vitro against Trypanosoma brucei for discovery of new antitrypanosomal drug leads. BMC Complementary Altern Med. 2016;16:131. doi: 10.1186/s12906-016-1122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Sahu R, Walker LA, Tekwani BL. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J Visualized Exp. 2012;70:e4054. doi: 10.3791/4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardim GAM, Reis WJ, Ribeiro MF, Ottoni FM, Alves RJ, Silva TL, Goulart MOF, Braga AL, Menna-Barreto RFS, Salomão K, de Castro SL, da Silva EN., Junior On the investigation of hybrid quinones: synthesis, electrochemical studies and evaluation of trypanocidal activity. RSC Adv. 2015;5:78047–78060. [Google Scholar]
- Kaur R, Dwivedi AR, Kumar B, Kumar V. Recent developments on 1,2,4-triazole nucleus in anticancer compounds: a review. Anti-Cancer Agents Med Chem. 2016;16:465–489. doi: 10.2174/1871520615666150819121106. [DOI] [PubMed] [Google Scholar]
- Keivanloo A, Bakherad M, Abbasi F, Besharati-Sedani T, Amin AH. Efficient synthesis of novel 1,2,3-triazole-linked quinoxaline scaffold via copper-catalyzed click reactions. RSC Adv. 2016;6:105433–105441. [Google Scholar]
- Kotowski N, Jardim R, Dávila AMR. Improved orthologous databases to ease protozoan targets inference. Parasit Vectors. 2015;8:494. doi: 10.1186/s13071-015-1090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Kavitha HP. Synthesis and biological applications of triazole derivatives. Mini-Rev Org Chem. 2013;10:40–65. [Google Scholar]
- Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z, Pasco DS, Walker LA, Khan IA. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob Agents Chemother. 2004;48:4450–4452. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magán R, Marín C, Rosales MJ, Barrera MA, Salas JM, Sánchez-Moreno M. Activities of Pt (II) and Ru (III) triazole-pyrimidine complexes against Trypanosoma cruzi and T. brucei brucei. Pharmacology. 2004;70:83–90. doi: 10.1159/000074672. [DOI] [PubMed] [Google Scholar]
- Manda S, Khan SI, Jain SK, Mohammed S, Tekwani BL, Khan IA, Vishwakarma RA, Bharate SB. Synthesis, antileishmanial and antitrypanosomal activities of N-substituted tetrahydro-β-carbolines. Bioorg Med Chem Lett. 2014;24:3247–3250. doi: 10.1016/j.bmcl.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Montes GC, Monteiro-da Silva BN, Rezende B, Sudo RT, Ferreira VF, de Carvalho-da Silva F, Pinto AdC, Vasconcellos-da Silva B, Zapata-Sudo G. The hypnotic, anxiolytic, and antinociceptive profile of a novel μ-opioid agonist. Molecules. 2017;22:800. doi: 10.3390/molecules22050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Andes DR. Antifungal Agents: spectrum of activity, pharmacology, and clinical indications. Infect Dis Clin North Am. 2016;30:51–83. doi: 10.1016/j.idc.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Papadopoulou MV, Bloomer WD, Rosenzweig HS, Chatelain E, Kaiser M, Wilkinson SR, McKenzie C, Ioset JR. Novel 3-nitro-1H-1,2,4-triazole-based amides and sulfonamides as potential antitrypanosomal agents. J Med Chem. 2012;55:5554–5565. doi: 10.1021/jm300508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HI, Pham NTA. 1,2,3-triazole derivatives as antidiabetic agent, and methods for the preparation thereof. 2017045941 A20170428 Korean Patent. 2017
- Peixoto JA, Andrade e Silva ML, Crotti AEM, Sola Veneziani RC, Gimenez VMM, Januario AH, Groppo M, Magalhães LG, Dos Santos FF, Albuquerque S, da Silva Filho AA, Cunha WR. Antileishmanial activity of the hydroalcoholic extract of Miconia langsdorffii, isolated compounds, and semi-synthetic derivatives. Molecules. 2011;16:1825–1833. doi: 10.3390/molecules16021825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi SN, Tiwari A, Chandra N, Gupta S. Chemotherapy of leishmaniasis. Part XI: Synthesis and bioevaluation of novel isoxazole containing heteroretinoid and its amide derivatives. Bioorg Med Chem Lett. 2012;22:6559–6562. doi: 10.1016/j.bmcl.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Sutherland R, Croydon EAP, Rolinson GN. Flucloxacillin, a new isoxazolyl penicillin, compared with oxacillin, cloxacillin, and dicloxacillin. Br Med J. 1970;4:455–460. doi: 10.1136/bmj.4.5733.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq S, Alam O, Amir M. Synthesis, anti-inflammatory, p38α MAP kinase inhibitory activities and molecular docking studies of quinoxaline derivatives containing triazole moiety. Bioorg Chem. 2018;76:343–358. doi: 10.1016/j.bioorg.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Tashtuosh H, Abu-Orabi S, Ta’an E, Al-Talab M. Synthesis and Spectroscopic Properties of 2-[1-benzyl-1,2,3,-triazolo-4]-5-aryl-1,3,4-oxadiazoles. Asian J Chem. 1999;11:441. [Google Scholar]
- Thao NP, No JH, Luyen BTT, Yang G, Byun SY, Goo J, Kim KT, Cuong NX, Nam NH, Minh CV, Schmidt TJ, Kang JS, Kim YH. Secondary metabolites from Vietnamese marine invertebrates with activity against Trypanosoma brucei and T. cruzi. Molecules. 2014;19:7869–7880. doi: 10.3390/molecules19067869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiberti N, Matovu E, Hainard A, Enyaru JC, Lejon V, Robin X, Turck N, Ngoyi DM, Krishna S, Bisser S, Courtioux B, Buscher P, Kristensson K, Ndung’u JM, Sanchez JC. New biomarkers for stage determination in Trypanosoma brucei rhodesiense sleeping sickness patients. Clin Transl Med. 2013;2:1. doi: 10.1186/2001-1326-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Malaria WHO Fact Sheet No. 94. WHO; Geneva, Switzerland: 2017. [Accessed November 2017]. http://www.who.int/mediacentre/factsheets/fs094/en/ [Google Scholar]
- Yang CR, Zhang Y, Jacob MR, Khan SI, Zhang YJ, Li XC. Antifungal activity of C-27 steroidal saponins. Antimicrob Agents Chemother. 2006;50:1710–1714. doi: 10.1128/AAC.50.5.1710-1714.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.