Key Points
Question
Is there evidence for racial/ethnic health inequity with respect to longitudinal patterns of glycemic control among youth with type 1 diabetes?
Findings
In a longitudinal cohort study of 1313 youths (aged <20 years) with type 1 diabetes, patients with black race or Hispanic ethnicity were at higher risk of being in the highest and most rapidly increasing hemoglobin A1c trajectory group over 9 years after diabetes diagnosis when compared with non-Hispanic white patients. These associations persisted only among male patients and those with diagnosis at age 9 years or younger.
Meaning
There is health inequity with regard to glycemic control, particularly among young nonwhite male patients and nonwhite youth diagnosed earlier in life.
This longitudinal cohort study compares trajectories of hemoglobin A1c (HbA1c) increase, considered a measure of glycemic control, among patients younger than 20 years of age with type 1 diabetes according to their self-reported race/ethnicity using data from the SEARCH for Diabetes in Youth study.
Abstract
Importance
Health disparities in the clinical presentation and outcomes among youth with type 1 diabetes exist. Long-term glycemic control patterns in racially/ethnically diverse youth are not well described.
Objectives
To model common trajectories of hemoglobin A1c (HbA1c) among youth with type 1 diabetes and test how trajectory group membership varies by race/ethnicity.
Design, Setting, and Participants
Longitudinal cohort study conducted in 5 US locations. The analysis included data from 1313 youths (aged <20 years) newly diagnosed in 2002 through 2005 with type 1 diabetes in the SEARCH for Diabetes in Youth study (mean [SD] age at diabetes onset, 8.9 [4.2] years) who had 3 or more HbA1c study measures during 6.1 to 13.3 years of follow-up. Data were analyzed in 2017.
Exposures
Self-reported race/ethnicity.
Main Outcomes and Measures
Hemoglobin A1c trajectories identified through group-based trajectory modeling over a mean (SD) of 9.0 (1.4) years of diabetes duration. Multinomial models studied the association of race/ethnicity with HbA1c trajectory group membership, adjusting for demographic characteristics, clinical factors, and socioeconomic position.
Results
The final study sample of 1313 patients was 49.3% female (647 patients) with mean (SD) age 9.7 (4.3) years and mean (SD) disease duration of 9.2 (6.3) months at baseline. The racial/ethnic composition was 77.0% non-Hispanic white (1011 patients), 10.7% Hispanic (140 patients), 9.8% non-Hispanic black (128 patients), and 2.6% other race/ethnicity (34 patients). Three HbA1c trajectories were identified: group 1, low baseline and mild increases (50.7% [666 patients]); group 2, moderate baseline and moderate increases (41.7% [548 patients]); and group 3, moderate baseline and major increases (7.5% [99 patients]). Group 3 was composed of 47.5% nonwhite youths (47 patients). Non-Hispanic black youth had 7.98 higher unadjusted odds (95% CI, 4.42-14.38) than non-Hispanic white youth of being in the highest HbA1c trajectory group relative to the lowest HbA1c trajectory group; the association remained significant after full adjustment (adjusted odds ratio of non-Hispanic black race in group 3 vs group 1, 4.54; 95% CI, 2.08-9.89). Hispanic youth had 3.29 higher unadjusted odds (95% CI, 1.78-6.08) than non-Hispanic white youth of being in the highest HbA1c trajectory group relative to the lowest HbA1c trajectory group; the association remained significant after adjustment (adjusted odds ratio of Hispanic ethnicity in group 3 vs group 1, 2.24; 95% CI, 1.02-4.92). In stratified analyses, the adjusted odds of nonwhite membership in the highest HbA1c trajectory remained significant among male patients and youth diagnosed at age 9 years or younger, but not female patients and youth who were older than 9 years when they were diagnosed (P for interaction = .04 [sex] and .02 [age at diagnosis]).
Conclusions and Relevance
There are racial/ethnic differences in long-term glycemic control among youth with type 1 diabetes, particularly among nonwhite male patients and nonwhite youth diagnosed earlier in life.
Introduction
Type 1 diabetes (T1D) treatment is centered around the improvement and maintenance of tight glycemic control, as assessed by levels of hemoglobin A1c (HbA1c), to prevent acute and chronic diabetes-related complications.1,2,3 Glycemic control can vary considerably from diabetes onset through adolescence,4,5,6 where fluctuations are known to occur during puberty3,4,7,8,9,10,11,12 and during early adulthood. Poorer glycemic control during early adulthood or from childhood to young adulthood has been attributed to a lack of continuity in diabetes-related clinical care4,11,12 as well as changes in self-care as children and adolescents with T1D grow into adulthood.9,10,13 However, glycemic control in youth and young adults with T1D is critical, as a higher average HbA1c level in this period of development is associated with impaired growth as well as diabetic complications.14,15,16,17
In cross-sectional studies of adolescents and young adults, glycemic control differs by racial and ethnic subgroups.18 African American, American Indian, Hispanic, and Asian or Pacific Islander youth with T1D are more likely to have higher HbA1c levels compared with non-Hispanic white youth.19 In longitudinal studies, nonwhite youth with T1D have increased markers of poor prognosis at diagnosis and 3 years following diagnosis, including higher HbA1c levels, more frequent diabetic ketoacidosis, and severe hypoglycemia.20 A constellation of sociodemographic factors related to race/ethnicity and glycemic control have been proposed, ranging from family dynamics, depressive symptoms, and quality of life13,21,22,23,24,25 to diabetes regimen.26,27,28 The role of socioeconomic position as a mediator of racial/ethnic associations remains controversial.28,29,30,31 Additionally, health care–specific factors such as disparities in health literacy, diabetes-related knowledge, or access to health care are known to contribute to pediatric health disparity but have not been well explored in T1D.32,33
Latent class trajectory modeling has been used to identify subgroups who share a similar trajectory of HbA1c over time.34 Few studies have examined whether racial/ethnic disparities in glycemic control persist over time from childhood into young adulthood among individuals with T1D. Our objective was to first visualize major trajectories of glycemic control from childhood into young adulthood using all data from youth of all racial and ethnic groups and to then characterize specific associations between race/ethnicity and distinct longitudinal patterns of glycemic control. Our hypothesis was that non-Hispanic black and Hispanic youth would be more likely than non-Hispanic white youth to have unfavorable trajectory patterns representing poor glycemic control and that this association may be mediated by clinical factors such as diabetes regimen26,27,28 and by socioeconomic position.29,30,31
Methods
Study Population
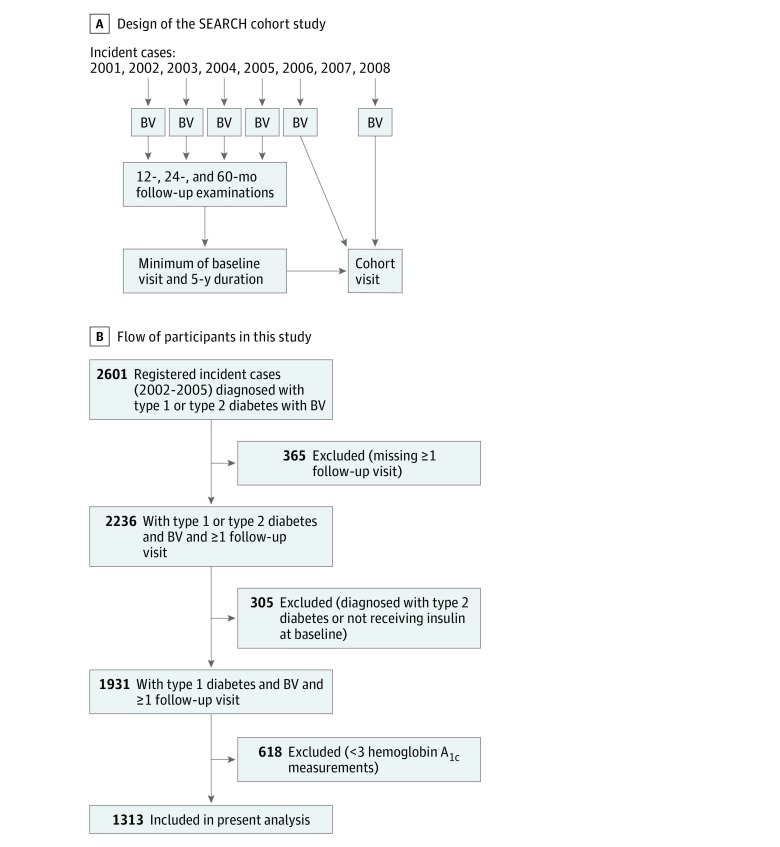
The SEARCH for Diabetes in Youth study began in 2000 with an overarching objective to describe the incidence and prevalence of childhood diabetes among the 5 major racial and ethnic groups in the United States.35 Individuals with diabetes diagnosed before age 20 years were identified from a population-based incidence registry network at 5 US sites (South Carolina; Cincinnati, Ohio, and surrounding counties; Colorado with southwestern Native American sites; Seattle, Washington, and surrounding counties; and Kaiser Permanente, southern California).36 Patients were newly diagnosed with T1D in 2002 through 2005. Patients who could be contacted were asked to complete a short survey and recruited for a baseline visit. If they completed the first visit, they were asked to return for visits at 12, 24, and 60 months to measure risk factors for diabetes complications (Figure 1A). A subset of participants who were aged 10 years and older and had at least 5 years of diabetes duration were recruited for a follow-up cohort visit between 2012 and 2015. The subset of youth who were included in the SEARCH cohort visit were not significantly different from all other youth diagnosed between the years of 2002 and 2008 in terms of average age at diabetes onset, distribution of sex or race and ethnicity, or clinical measures.14
Figure 1. Study Design and Sample Recruitment.
A, Study design of the SEARCH cohort study. B, Flowchart depicting participants in this report, including reasons for exclusion. The final sample included 1313 youths with type 1 diabetes. BV indicates baseline visit.
Inclusion criteria for these analyses consisted of youth diagnosed with T1D between 2002 and 2005. Type 1 diabetes was based on the clinical diagnosis made by a physician or other health care professional at onset and was collected from these health care professionals or abstracted from medical records. Youth with a clinical diagnosis of type 1a, type 1b, or type 1 diabetes were included. Youth who had fewer than 3 measures of HbA1c from research visits during 6.1 to 13.3 years of follow-up were excluded (n = 618). Excluded individuals were not different with regard to HbA1c measures using available data from the study baseline and the cohort visit. The final study sample included 1313 youths with T1D (Figure 1B). The study was approved by institutional review boards with jurisdiction; the parent, the participant, or both provided written consent or assent for all participants (consent of ≥1 parent or legal guardian was required for participants aged <18 years). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Research Visits
Trained personnel administered questionnaires; measured height, weight, and blood pressure; and obtained blood samples. Body mass index was defined as weight (kilograms) divided by height (meters squared) and converted to a z score.37 A blood draw occurred after an 8-hour overnight fast, and medications, including short-acting insulin, were withheld the morning of the visit.
Laboratory Measures
Blood samples were obtained under conditions of metabolic stability, defined as no episodes of diabetic ketoacidosis in the preceding month and the absence of fever and acute infections. They were processed locally and shipped within 24 hours to the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories). Hemoglobin A1c was measured by a dedicated ion exchange high-performance liquid chromatography instrument (TOSOH Bioscience).
Other Measures
Self-reported race and ethnicity were collected based on questions modeled after the 2000 US Census38 and categorized as non-Hispanic white, non-Hispanic black, Hispanic, and other (Asian, Native American, Pacific Islander, other, and unknown). Although the US Census accommodates reporting of multiple races, the SEARCH study did not have sufficient participant numbers to allow evaluation of separate categories of reported multiple-race groups39 and used the National Center for Health Statistics plurality approach, in which data from a study designed to address multiple-race reporting was used to determine which single-race category should be assigned for specific combinations of multiple races reported.38
Insulin regimen was based on mode of insulin delivery, classified as pumps, long-acting with rapid-acting insulin injections with 3 or more injections per day, and any other form of multiple daily injections. Insulin dose was reported as units per kilogram of body weight. Frequency of self-monitoring of blood glucose was self-reported and categorized as less than 1 time per day, 1 to 3 times per day, and 4 or more times per day. Health insurance type was classified as none, private, Medicaid, or other. Parental education was based on the highest educational level attained by either parent and classified as less than high school degree, high school graduate, some college through associate’s degree, and bachelor’s degree or more. Household structure was classified as 2 parent, single parent, or other. Receipt of diabetes care was based on reported number of visits with prespecified diabetes health care professionals, including pediatric endocrinologists, adult diabetologists, and nurse diabetes educators, in the previous 6 months and classified based on the distribution: 0 to 1 visit, 2 to 3 visits, 4 to 5 visits, and 6 or more visits. Receipt of nondiabetes care was based on reported number of visits with prespecified nondiabetes health care professionals (pediatrician, family practice physician, general practice physician, internist, nurse practitioner or physician assistant, traditional healer, dietician, optometrist or ophthalmologist, and psychiatrist, psychologist, or mental health counselor) in the previous 6 months and classified as 0 to 1 visit, 2 to 3 visits, 4 to 6 visits, and 7 or more visits. Satisfaction with diabetes care was based on the response to the question, “How would you rate your diabetes care overall?” (possible responses were excellent, good, fair, poor, and not applicable).
Statistical Analysis
We used group-based trajectory modeling to identify trajectories of HbA1c among youth with T1D using duration of diabetes (months) as the time scale via the PROC TRAJ macro of SAS statistical software version 9.4 (SAS Institute Inc), which fits a semiparametric (discrete mixture) model for longitudinal data using the maximum-likelihood method.40,41,42,43,44 Trajectory analysis uses all available data for a participant and is robust to data that are missing at random. Details about trajectory analysis have been described elsewhere.43,44 The optimal number of groups was determined based on Bayesian information criterion and having at least 5% of the sample in the smallest trajectory group. We named the trajectories based on the baseline HbA1c value (from the initial research visit) and shape of the trajectory over the follow-up visits. We then calculated the posterior predicted probability for each participant of being a member of each trajectory group given his or her observed HbA1c pattern. Participants were assigned to the trajectory group for which they had the greatest posterior probability for group membership. Multinomial regression was used to assess the association of race/ethnicity (non-Hispanic white vs non-Hispanic black vs Hispanic) with HbA1c trajectory group membership. Youths who reported Asian or Pacific Islander, Native American, other, and unknown race/ethnicity (n = 34) were excluded from multinomial modeling. Non-Hispanic white was designated as the referent group.
All covariates were measured at baseline. Model 1 was unadjusted. Model 2 was adjusted for demographic factors (sex, age at diagnosis, and clinic site). Model 3 was additionally adjusted for clinical variables (body mass index z score, insulin regimen, insulin dose, and frequency of self-monitoring of blood glucose). Model 4 was further adjusted for socioeconomic position (highest parental education, household structure, and health insurance type).
Given previous findings of health inequity,45 we tested for sex- and age-related subgroups who may be particularly vulnerable to the effects of heath inequity. Modification of race/ethnicity effects by age and sex was tested by adding an interaction term (race/ethnicity × sex and race/ethnicity × age at diagnosis, respectively) to model 4. The nature of the modification was explored in models stratified by sex and the median age of diagnosis (9 years old). Because of limited sample size, for stratified analyses, race/ethnicity was categorized into non-Hispanic white and other (defined as non-Hispanic black, Hispanic, Asian or Pacific Islander, Native American, other, and unknown).
All analyses were completed in SAS software in 2017. Statistical significance was based on a 2-sided P value of .05. Descriptive analyses used the mean and standard deviation or median and interquartile range (IQR) for nonnormal distributions and for continuous variables and frequencies to describe categorical variables. The means and frequencies of demographic and clinical characteristics were compared using χ2 test for categorical variables and analysis of variance or Kruskal-Wallis test for continuous variables.
Results
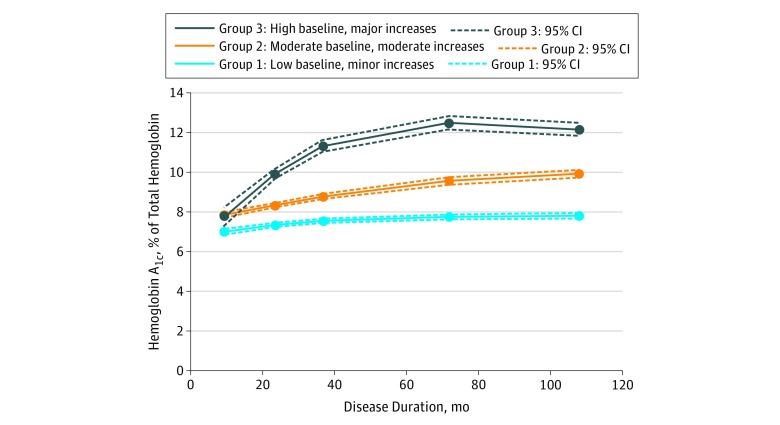
The sample of 1313 youths with T1D was 49.3% female (647 patients); 77.0% were non-Hispanic white (1011 patients); 10.7%, Hispanic (140 patients); 9.8%, non-Hispanic black (128 patients); and 2.6%, other race/ethnicity (34 patients) (Table 1). At the baseline visit, the mean (SD) age was 9.7 (4.3) years and the mean (SD) diabetes duration was 9.2 (6.3) months. Group-based trajectory modeling identified 3 distinct HbA1c trajectories over a mean (SD) follow-up of 108 (16) months (9.0 [1.4] years) of diabetes duration: group 1, low baseline and mild increases (50.7% [666 patients]); group 2, moderate baseline and moderate increases (41.7% [548 patients]); and group 3, moderate baseline and major increases (7.5% [99 patients]) (Figure 2).
Table 1. Baseline Characteristics of 1313 Participants With Type 1 Diabetes by Hemoglobin A1c Trajectory Group.
| Characteristic | No. (%) | P Valuea | |||
|---|---|---|---|---|---|
| Total Participants (N = 1313) | Group 1: Low Baseline and Mild Increases (n = 666) | Group 2: Moderate Baseline and Moderate Increases (n = 548) | Group 3: Moderate Baseline and Major Increases (n = 99) | ||
| Age at diagnosis, mean (SD), y | 8.9 (4.2) | 8.8 (4.5) | 8.5 (3.9) | 11.3 (3.5) | <.001 |
| Age at baseline, mean (SD), y | 9.7 (4.3) | 9.6 (4.5) | 9.3 (3.9) | 12.2 (3.5) | <.001 |
| Diabetes duration, mean (SD), mo | 9.2 (6.3) | 9.0 (6.4) | 9.3 (6.1) | 10.4 (6.4) | .13 |
| Female | 647 (49.3) | 316 (47.5) | 280 (51.1) | 51 (51.5) | .40 |
| Nonwhite race/ethnicityb | 302 (23.0) | 102 (15.3) | 153 (27.9) | 47 (47.5) | <.001 |
| Race/ethnicityb | |||||
| Non-Hispanic white | 1011 (77.0) | 564 (84.7) | 395 (72.1) | 52 (52.5) | <.001 |
| Non-Hispanic black | 128 (9.8) | 34 (5.1) | 69 (12.6) | 25 (25.3) | |
| Hispanic | 140 (10.7) | 56 (8.4) | 67 (12.2) | 17 (17.2) | |
| Other | 34 (2.6) | 12 (1.8) | 17 (3.1) | 5 (5.1) | |
| Parental education | |||||
| Less than high school | 48 (3.7) | 20 (3.0) | 20 (3.7) | 8 (8.1) | <.001 |
| High school graduate | 180 (13.8) | 61 (9.2) | 93 (17.1) | 26 (26.3) | |
| Some college (through associate’s degree) | 441 (33.8) | 184 (27.8) | 219 (40.3) | 38 (38.4) | |
| Bachelor’s degree or more | 636 (48.7) | 397 (60.0) | 212 (39.0) | 27 (27.3) | |
| Insurance | |||||
| None | 19 (1.5) | 8 (1.2) | 8 (1.5) | 3 (3.0) | <.001 |
| Private | 1052 (80.7) | 586 (88.4) | 402 (74.3) | 64 (64.7) | |
| Medicaid | 211 (16.2) | 61 (9.2) | 119 (22.0) | 31 (31.3) | |
| Other | 21 (1.6) | 8 (1.2) | 12 (2.2) | 1 (1.1) | |
| Family structure | |||||
| Two-parent household | 961 (73.6) | 543 (81.9) | 366 (67.4) | 52 (52.5) | <.001 |
| Single-parent household | 311 (23.8) | 109 (16.4) | 161 (29.7) | 41 (41.4) | |
| Other structure | 33 (2.53) | 11 (1.7) | 16 (3.0) | 6 (6.1) | |
| Insulin regimen | |||||
| Pump | 106 (8.15) | 67 (10.1) | 36 (6.6) | 3 (3.1) | .01 |
| Long with short or rapid insulin, ≥3 times/d | 418 (32.1) | 225 (33.9) | 164 (30.3) | 29 (29.6) | |
| Long with other combinationc | 779 (59.8) | 371 (56.0) | 342 (63.1) | 66 (67.4) | |
| Insulin dose, mean (SD), units/kg | 0.63 (0.42) | 0.59 (0.46) | 0.66 (0.38) | 0.73 (0.38) | .001 |
| Blood glucose monitoring, times/d | |||||
| <1 | 10 (0.8) | 14 (2.1) | 11 (2.0) | 4 (4.0) | <.001 |
| 1-3 | 148 (11.5) | 64 (9.6) | 58 (10.6) | 26 (26.5) | |
| ≥4 | 1134 (88.8) | 588 (88.3) | 478 (87.4) | 68 (70.4) | |
| Body mass index z score, mean (SD) | 0.58 (0.97) | 0.40 (0.92) | 0.66 (1.00) | 0.68 (1.10) | .02 |
| Diabetes care visits in past 6 mo, No.d | |||||
| Mean (SD)d | 3.9 (2.9) | 3.9 (2.9) | 4.1 (3.0) | 3.6 (2.6) | .31 |
| 0-1 | 173 (13.2) | 95 (14.3) | 65 (11.9) | 13 (13.1) | .12 |
| 2-3 | 479 (36.5) | 228 (34.2) | 211 (38.5) | 40 (40.4) | |
| 4-5 | 383 (29.2) | 211 (31.7) | 142 (25.9) | 30 (30.30) | |
| ≥6 | 278 (21.2) | 132 (19.8) | 130 (23.7) | 16 (16.2) | |
| Other care visits in past 6 mo, No.d | |||||
| Mean (SD)d | 5.0 (4.1) | 4.8 (3.9) | 5.1 (4.3) | 5.0 (4.5) | .44 |
| 0-1 | 175 (13.3) | 83 (12.5) | 73 (13.3) | 19 (19.2) | .23 |
| 2-3 | 384 (29.3) | 207 (31.1) | 153 (27.9) | 24 (24.2) | |
| 4-6 | 424 (33.1) | 225 (33.8) | 182 (33.2) | 27 (27.3) | |
| ≥7 | 320 (24.4) | 151 (22.7 | 140 (25.6) | 29 (29.3) | |
| Satisfaction with diabetes caree | |||||
| Excellent | 938 (72.4) | 505 (77.2) | 382 (70.6) | 51 (54.8) | <.001 |
| Good | 288 (22.4) | 127 (19.4) | 133 (24.6) | 28 (30.1) | |
| Fair | 49 (3.8) | 16 (2.5) | 21 (3.9) | 12 (12.9) | |
| Poor | 5 (0.4) | 1 (0.2) | 3 (0.6) | 1 (1.1) | |
P values based on use of χ2 test and analysis of variance or Kruskal-Wallis test, as appropriate based on model assumptions.
Self-reported race and ethnicity were collected using 2000 US Census questions. White was defined as non-Hispanic white. Nonwhite was defined as non-Hispanic black, Hispanic, or other. Other was defined as Asian or Pacific Islander, Native American, other, or unknown.
Includes 2 or more times per day or any insulin combination (excluding long), 3 or more times per day or any insulin(s) taken once per day, or any insulin combination (excluding long) 2 or more times per day.
Diabetes care measured by frequency of visits with pediatric endocrinology, adult diabetologist, or nurse diabetes educator in the previous 6 months. Other care measured by frequency of visits with nondiabetes caregivers. Data are self-reported.
Based on response to the question, “How would you rate your diabetes care overall?” Possible answers were excellent, good, fair, poor, and not applicable.
Figure 2. Trajectories of Hemoglobin A1c in 1313 Patients With Type 1 Diabetes in the SEARCH for Diabetes in Youth Study .
Group-based trajectory modeling identified 3 distinct hemoglobin A1c trajectories over a mean type 1 diabetes duration of 108 months. To convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
The prevalence of black and Hispanic youth was the highest in group 3 and the lowest in group 1 (non-Hispanic black patients made up 5.1% of group 1, 12.6% of group 2, and 25.3% of group 3; Hispanic patients made up 8.4% of group 1, 12.2% of group 2, and 17.2% of group 3). For non-Hispanic black patients, the difference between group 1 and group 2 was 7.5% (95% CI, 4.2%-10.7%; P < .001); between group 1 and group 3, 20.2% (95% CI, 11.4%-28.9%; P < .001); and between group 2 and group 3, 12.6% (95% CI, 3.7%-21.7%; P = .001). For Hispanic patients, the difference between group 1 and group 2 was 3.8% (95% CI, 0.4%-7.3%; P = .03); between group 1 and group 3, 8.8% (95% CI, 1.0%-16.5%; P = .006); and between group 2 and group 3, 5.0% (95% CI, 3.0%-12.9%; P = .18). Group 3 was composed of 47.5% nonwhite youths (47 patients) (Table 1). Table 2 depicts the odds ratios (ORs) for non-Hispanic black and Hispanic vs non-Hispanic white race/ethnicity and HbA1c trajectory group in a series of sequentially adjusted models. Non-Hispanic black youth had 7.98 higher odds than non-Hispanic white youth of being in the highest HbA1c trajectory group relative to the lowest HbA1c trajectory group (unadjusted OR of non-Hispanic black race in group 3 vs group 1, 7.98; 95% CI, 4.42-14.38). After adjustment for baseline demographic characteristics, clinical factors, and socioeconomic position, non-Hispanic black youth had 4.54 times higher odds than non-Hispanic white youth of being in the highest HbA1c trajectory group relative to the lowest HbA1c trajectory group (adjusted OR [aOR] of non-Hispanic black race in group 3 vs group 1, 4.54; 95% CI, 2.08-9.89). Hispanic youth had 3.29 higher unadjusted odds than non-Hispanic white youth of being in the highest HbA1c trajectory group relative to the lowest HbA1c trajectory group (unadjusted OR of Hispanic ethnicity in group 3 vs group 1, 3.29; 95% CI, 1.78-6.08). Adjustment for baseline demographic characteristics, clinical factors, and socioeconomic position did not fully attenuate the association (aOR of Hispanic ethnicity in group 3 vs group 1, 2.24; 95% CI, 1.02-4.92). Adjustment for clinical variables diminished statistical significance associated with the moderate HbA1c trajectory (aOR of Hispanic ethnicity in group 2 vs group 1, 1.43; 95% CI, 0.90-2.27 vs unadjusted OR, 1.71; 95% CI, 1.17-2.49).
Table 2. Association of Black and Hispanic Race/Ethnicity, Compared With Non-Hispanic White Race/Ethnicity, With Hemoglobin A1c Trajectory Groups in 1011 Patients.
| Modela | Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|
| Black Race (n = 128)b | Hispanic Ethnicity (n = 140)b | |||||
| Group 1: Low Baseline and Mild Increases | Group 2: Moderate Baseline and Moderate Increases | Group 3: Moderate Baseline and Major Increases | Group 1: Low Baseline and Mild Increases | Group 2: Moderate Baseline and Moderate Increases | Group 3: Moderate Baseline and Major Increases | |
| Model 1 | 1 [Reference] | 2.90 (1.88-4.46) | 7.98 (4.42-14.38) | 1 [Reference] | 1.71 (1.17-2.49) | 3.29 (1.78-6.08) |
| Model 2 | 1 [Reference] | 3.00 (1.92-4.67) | 9.94 (5.15-19.20) | 1 [Reference] | 1.67 (1.08-2.58) | 3.56 (1.75-7.21) |
| Model 3 | 1 [Reference] | 2.50 (1.54-4.05) | 7.50 (3.68-15.26) | 1 [Reference] | 1.43 (0.90-2.27) | 3.32 (1.60-6.91) |
| Model 4 | 1 [Reference] | 1.73 (1.04-2.90) | 4.54 (2.08-9.89) | 1 [Reference] | 1.16 (0.71-1.89) | 2.24 (1.02-4.92) |
Model 1 was unadjusted. Model 2 was adjusted for demographic characteristics (age at diagnosis and clinic site). Model 3 further adjusted for body mass index (calculated as weight in kilograms divided by height in meters squared) z score, insulin regimen, insulin dose, and frequency of blood glucose monitoring. Model 4 further adjusted for socioeconomic position (maximum parental education, household structure, and health insurance type).
Self-reported race and ethnicity were collected using 2000 US Census questions and categorized as non-Hispanic white, non-Hispanic black, Hispanic, and other (Asian, Native American, Pacific Islander, other, and unknown). Respondents who self-reported as other were excluded from these analyses due to small sample size (n = 34).
The association of race/ethnicity and HbA1c trajectory was modified by sex (P for interaction = .04) (Table 3). Nonwhite male patients had significantly elevated odds of membership in the highest HbA1c trajectory group (OR of group 3 vs group 1, 5.34; 95% CI, 2.16-13.2) and moderate HbA1c trajectory group (OR of group 2 vs group 1, 2.06; 95% CI, 1.18-3.57) relative to non-Hispanic white male patients. The associations were not significant in female patients (aOR of group 3 vs group 1, 1.48; 95% CI, 0.65-3.39 and aOR of group 2 vs group 1, 1.00; 95% CI, 0.61-1.64). The association of race/ethnicity and HbA1c trajectory was also modified by age at diagnosis (P for interaction = .02) (Table 3). Nonwhite youths diagnosed at or younger than 9 years had significantly elevated odds of membership in the highest HbA1c trajectory group (aOR of group 3 vs group 1, 5.37; 95% CI, 1.91-15.1) and the moderate HbA1c trajectory group (aOR of group 2 vs group 1, 2.04; 95% CI, 1.23-3.37). The association was not significant in youth who were diagnosed when they were older than 9 years (aOR of group 3 vs group 1, 1.65; 95% CI, 0.77-3.51 and aOR of group 2 vs group 1, 0.96; 95% CI, 0.55-1.65).
Table 3. Association of Nonwhite Race/Ethnicity, Compared With Non-Hispanic White Race/Ethnicity, With Hemoglobin A1c Trajectory Group, Stratified by Sex and Age at Diagnosisa.
| Modelb | Odds Ratio (95% CI) | P Value for Interaction | ||
|---|---|---|---|---|
| Group 1: Low Baseline and Mild Increases | Group 2: Moderate Baseline and Moderate Increases | Group 3: Moderate Baseline and Major Increases | ||
| Sex | ||||
| Female (n = 581) | 1 [Reference] | 1.00 (0.61-1.64) | 1.48 (0.65-3.39) | .04 |
| Male (n = 593) | 1 [Reference] | 2.06 (1.18-3.57) | 5.34 (2.16-13.2) | |
| Age at diagnosis, y | 1 [Reference] | |||
| ≤9 (n = 611) | 1 [Reference] | 2.04 (1.23-3.37) | 5.37 (1.91-15.1) | .02 |
| >9 (n = 564) | 1 [Reference] | 0.96 (0.55-1.65) | 1.65 (0.77-3.51) | |
Self-reported race and ethnicity were collected using 2000 US Census questions. White was defined as non-Hispanic white. Nonwhite was defined as non-Hispanic black, Hispanic, Asian or Pacific Islander, Native American, other, or unknown.
Fully adjusted for age at diagnosis, clinic site, maximum parental education, household structure, health insurance type, body mass index (calculated as weight in kilograms divided by height in meters squared) z score, insulin regimen, insulin dose, and frequency of blood glucose monitoring.
Discussion
In a large, population-based multiethnic cohort of youth with T1D, we found 3 distinct HbA1c trajectories that deteriorated over a mean (SD) follow-up of 9.0 (1.4) years (range, 6.1-13.3 years) following diabetes diagnosis, reinforcing that early youth and the transition to adulthood are high-risk periods for worsening glycemic control.3,7,8 Black race and Hispanic ethnicity were associated with membership in the highest and most rapidly increasing (worsening) HbA1c trajectory group.
We tested the association of race/ethnicity with HbA1c trajectory by adjusting for other variables, including clinical factors and socioeconomic position. For example, prescribing practices may vary based on race/ethnicity27 and insulin pump use is known to be higher in white youth than non-Hispanic black or Hispanic youth.28 Lower socioeconomic position has been proposed as a major mediator of the association of race/ethnicity with health outcomes,29,30,31 including T1D complications, due to poorer self-management among persons whose socioeconomic conditions are less favorable.20,46 Despite adjustment for these known risk factors, black race remained significantly associated with HbA1c trajectory. Similarly, adjustment for demographic characteristics, clinical variables, and socioeconomic position did not fully attenuate the association of Hispanic ethnicity with the highest HbA1c trajectory, where the OR remained significantly elevated, suggesting remaining impact of inequity in this group. Evidence of disparity in glycemic control trajectory that exists particularly among nonwhite male patients and nonwhite youth with diabetes diagnosis at an early age (≤9 years) is consistent with previously reported patterns in acute glycemic complications that are more common among the youngest patients and male patients of all ages.47
An important finding of the trajectory analysis was that the highest HbA1c trajectory subgroup also showed the highest mean HbA1c level at baseline, which occurred at a mean (SD) of 9.8 (6.3) months following diagnosis. This suggests that glycemic control obtained in the first year following diagnosis may confer information about longitudinal trends over time. Furthermore, the magnitude of racial/ethnic inequity over the longitudinal data are striking. Group 3 diverged over the follow-up period to give vastly different mean HbA1c measures at the cohort visit that may translate to significant increases in the risk for complications of diabetes based on evidence from the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications Study (EDIC).1,2,3,48 Disparity in glycemic control across trajectory groups in the present analyses even exceeds differences reported across groups of the DCCT/EDIC trial (which compared a median HbA1c of 7% of total hemoglobin in the intensive insulin treatment group with a median HbA1c of 9% of total hemoglobin in the conventional group), suggesting that those risk estimates may be conservative for youth who additionally face a longer period of disease-related exposures.49
Previous studies have shown that the migration status of parents is associated with glycemic control among youth with T1D.50 To address potential differences, we examined a subset of the sample with data on parental nativity (ie, US born vs foreign born) and found no significant differences across HbA1c trajectory groups. Adjustment for parental nativity did not attenuate the association of black race or Hispanic ethnicity with the moderate or highest HbA1c trajectory group, although the analysis is limited by small sample size (data not shown). Differences in youth and parental nativity status likely warrant future study in adequately powered samples.
Given the complexity of the study of race and health outcomes in the United States, in which health risks associated with race/ethnicity are not inherent but instead may signal underlying inequalities,51 we posit that our results may reflect health inequity in T1D operating at multiple levels. The social determinants of health operating outside of the health care system, including aspects of the physical environment, food security, social integration, barriers to health care,52 and complex patterns in health care utilization,53,54 may create race-based groups of individuals for whom glycemic control is challenged by inconsistencies in the availability of resources or support for T1D management. In general, adverse childhood experiences among nonwhite youth have been shown to result in a myriad of psychological and medical sequelae later in life.55
There may also be modifiable aspects within the health care system, including racial/ethnic differences in the interpersonal dynamics of interactions between patients or parents and health care professionals that occur in pediatric clinical settings, extending from implicit bias and microaggressions to stereotyping, prejudice, and macroaggressions.56 Nonwhite youth and families report overtly weakened patient–health care professional communication and decreased participatory decision making.32,33 Implicit bias, the unconscious attitudes that unintentionally influence behavior, may affect health care professionals’ medical management decisions56 and perceptions about black, Hispanic, and young people of color in terms of disease experience57 and patient compliance.58 Higher levels of perceived bias or discrimination have been linked to worse diabetes care.59,60 The direct effect of implicit bias on HbA1c has not been well studied in pediatric diabetes. Finally, while social stigma associated with T1D is known,61,62,63 it may be more pronounced in specific communities where health literacy and resources are lacking or where T1D is significantly less common than type 2 diabetes. Nonwhite youth may struggle with misunderstanding and stigma that act as chronic stressors that indirectly affect glycemic control via psychosocial or behavioral effects,64,65 resulting in impaired self-care strategies or maladaptive coping behaviors that damage health.66
Limitations
A limitation of the study is that the observed inequity after adjustment for other factors may reflect racial and ethnic differences in the validity of HbA1c as a measure of average glycemia owing to racial differences in the glycation of hemoglobin or other factors affecting red blood cell turnover.67,68,69 However, the between-race differences that have been reported are small (0.4 percentage point in HbA1c69) relative to the differences in the present study, where the mean (SD) HbA1c of group 3 was 12.2% (1.5%) of total hemoglobin at the last visit, roughly 2.2% higher than group 2 and 4.4% higher than group 1 at that time. Combining individuals of many races, ethnicities, and cultures into single categories for analysis may result in residual confounding and underemphasize within-group heterogeneity. We are careful to avoid implying that all nonwhite youth have poor control; in our data, nearly a quarter of nonwhite youth had an HbA1c at or below 7.4% of total hemoglobin at the cohort visit (data not shown). Several of the variables measured at baseline may change over time, including health insurance status. Adjustment variables may provide information for future work that will delve into what drives the inequities. For example, measures of socioeconomic position may be improved by including other measures such as the ability to pay for medication, heath literacy, housing security, or food security. We did not control for diet and physical activity in these analyses. A larger sample may identify additional trajectories that capture the experience of smaller subpopulations, such as individuals who initially have low HbA1c that deteriorates later in the course of T1D. The outcome of trajectory group necessitated the use of logistic regression modeling, which may overestimate effect estimates, particularly when the outcome is common.70,71 Finally, there were relatively small numbers of participants across groups in the analyses stratified by sex and age at diagnosis. Larger studies are needed to further explore interactions and identify nonwhite youth who are at the highest risk for poor glycemic control over time. Finally, associations of data-driven trajectory models should be confirmed with future analyses that quantify and compare differences in longitudinal HbA1c across racial/ethnic groups.
However, the study has several strengths, including the large, well-characterized, multiethnic cohort;72 the extended follow-up period; and the use of an analytic approach to characterize multiple common HbA1c trajectories and understand associated individual characteristics from an extensive collection of covariates.
Conclusions
Compared with non-Hispanic white youth with T1D, non-Hispanic black youth, Hispanic youth, and youth with other racial/ethnic backgrounds who are male and diagnosed earlier in life are more likely to show rapid deterioration in glycemic control within 9 years of T1D diagnosis. The findings of this study can be used to inform future research on the identification of factors that contribute to and reinforce racial and ethnic disparity among youth with T1D, particularly nonwhite male patients and nonwhite youth diagnosed earlier in life.
References
- 1.Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125(2):-. doi: 10.1016/S0022-3476(94)70190-3 [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Genuth S, Lachin J, et al. ; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. doi: 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 3.White NH, Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WV; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr. 2001;139(6):804-812. doi: 10.1067/mpd.2001.118887 [DOI] [PubMed] [Google Scholar]
- 4.Pyatak EA, Sequeira PA, Whittemore R, Vigen CP, Peters AL, Weigensberg MJ. Challenges contributing to disrupted transition from paediatric to adult diabetes care in young adults with type 1 diabetes. Diabet Med. 2014;31(12):1615-1624. doi: 10.1111/dme.12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helgeson VS, Snyder PR, Seltman H, Escobar O, Becker D, Siminerio L. Brief report: trajectories of glycemic control over early to middle adolescence. J Pediatr Psychol. 2010;35(10):1161-1167. doi: 10.1093/jpepsy/jsq011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohan JM, Rausch JR, Pendley JS, et al. Identification and prediction of group-based glycemic control trajectories during the transition to adolescence. Health Psychol. 2014;33(10):1143-1152. doi: 10.1037/hea0000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran A, Jacobs DR Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039-2044. doi: 10.2337/diabetes.48.10.2039 [DOI] [PubMed] [Google Scholar]
- 8.Szadkowska A, Pietrzak I, Zmysłowska A, Wyka K, Bodalski J. Insulin resistance in newly diagnosed type 1 diabetic children and adolescents [in Polish]. Med Wieku Rozwoj. 2003;7(2):181-191. [PubMed] [Google Scholar]
- 9.Moore SM, Hackworth NJ, Hamilton VE, Northam EP, Cameron FJ. Adolescents with type 1 diabetes: parental perceptions of child health and family functioning and their relationship to adolescent metabolic control. Health Qual Life Outcomes. 2013;11:50. doi: 10.1186/1477-7525-11-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pyatak EA, Sequeira P, Peters AL, Montoya L, Weigensberg MJ. Disclosure of psychosocial stressors affecting diabetes care among uninsured young adults with type 1 diabetes. Diabet Med. 2013;30(9):1140-1144. doi: 10.1111/dme.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helgeson VS, Reynolds KA, Snyder PR, et al. Characterizing the transition from paediatric to adult care among emerging adults with type 1 diabetes. Diabet Med. 2013;30(5):610-615. doi: 10.1111/dme.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynes L, Byrne M, Dinneen SF, McGuire BE, O’Donnell M, Mc Sharry J. Barriers and facilitators associated with attendance at hospital diabetes clinics among young adults (15-30 years) with type 1 diabetes mellitus: a systematic review. Pediatr Diabetes. 2016;17(7):509-518. doi: 10.1111/pedi.12198 [DOI] [PubMed] [Google Scholar]
- 13.Lawrence JM, Standiford DA, Loots B, et al. ; SEARCH for Diabetes in Youth Study . Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics. 2006;117(4):1348-1358. doi: 10.1542/peds.2005-1398 [DOI] [PubMed] [Google Scholar]
- 14.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. ; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcovecchio ML, Heywood JJ, Dalton RN, Dunger DB. The contribution of glycemic control to impaired growth during puberty in young people with type 1 diabetes and microalbuminuria. Pediatr Diabetes. 2014;15(4):303-308. doi: 10.1111/pedi.12090 [DOI] [PubMed] [Google Scholar]
- 16.Stadler M, Peric S, Strohner-Kaestenbauer H, et al. Mortality and incidence of renal replacement therapy in people with type 1 diabetes mellitus—a three decade long prospective observational study in the Lainz T1DM cohort. J Clin Endocrinol Metab. 2014;99(12):4523-4530. doi: 10.1210/jc.2014-2701 [DOI] [PubMed] [Google Scholar]
- 17.Prince CT, Becker DJ, Costacou T, Miller RG, Orchard TJ. Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). Diabetologia. 2007;50(11):2280-2288. doi: 10.1007/s00125-007-0797-7 [DOI] [PubMed] [Google Scholar]
- 18.Chalew SA, Gomez R, Butler A, et al. Predictors of glycemic control in children with type 1 diabetes: the importance of race. J Diabetes Complications. 2000;14(2):71-77. doi: 10.1016/S1056-8727(00)00072-6 [DOI] [PubMed] [Google Scholar]
- 19.Petitti DB, Klingensmith GJ, Bell RA, et al. ; SEARCH for Diabetes in Youth Study Group . Glycemic control in youth with diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155(5):668-72.e1, 3. doi: 10.1016/j.jpeds.2009.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redondo MJ, Libman I, Cheng P, et al. ; Pediatric Diabetes Consortium . Racial/ethnic minority youth with recent-onset type 1 diabetes have poor prognostic factors. Diabetes Care. 2018;41(5):1017-1024. doi: 10.2337/dc17-2335 [DOI] [PubMed] [Google Scholar]
- 21.Barnard KD, Skinner TC, Peveler R. The prevalence of co-morbid depression in adults with type 1 diabetes: systematic literature review. Diabet Med. 2006;23(4):445-448. doi: 10.1111/j.1464-5491.2006.01814.x [DOI] [PubMed] [Google Scholar]
- 22.Hood KK, Beavers DP, Yi-Frazier J, et al. Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health. 2014;55(4):498-504. doi: 10.1016/j.jadohealth.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003;26(3):631-637. doi: 10.2337/diacare.26.3.631 [DOI] [PubMed] [Google Scholar]
- 24.Naughton MJ, Ruggiero AM, Lawrence JM, et al. ; SEARCH for Diabetes in Youth Study Group . Health-related quality of life of children and adolescents with type 1 or type 2 diabetes mellitus: SEARCH for Diabetes in Youth study. Arch Pediatr Adolesc Med. 2008;162(7):649-657. doi: 10.1001/archpedi.162.7.649 [DOI] [PubMed] [Google Scholar]
- 25.Mayer-Davis EJ, Beyer J, Bell RA, et al. ; SEARCH for Diabetes in Youth Study Group . Diabetes in African American youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth study. Diabetes Care. 2009;32(suppl 2):S112-S122. doi: 10.2337/dc09-S203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallegos-Macias AR, Macias SR, Kaufman E, Skipper B, Kalishman N. Relationship between glycemic control, ethnicity and socioeconomic status in Hispanic and white non-Hispanic youths with type 1 diabetes mellitus. Pediatr Diabetes. 2003;4(1):19-23. doi: 10.1034/j.1399-5448.2003.00020.x [DOI] [PubMed] [Google Scholar]
- 27.Paris CA, Imperatore G, Klingensmith G, et al. ; SEARCH for Diabetes in Youth Study Group . Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155(2):183-9.e1. doi: 10.1016/j.jpeds.2009.01.063 [DOI] [PubMed] [Google Scholar]
- 28.Willi SM, Miller KM, DiMeglio LA, et al. ; T1D Exchange Clinic Network . Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424-434. doi: 10.1542/peds.2014-1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auslander WF, Thompson S, Dreitzer D, White NH, Santiago JV. Disparity in glycemic control and adherence between African-American and Caucasian youths with diabetes: family and community contexts. Diabetes Care. 1997;20(10):1569-1575. doi: 10.2337/diacare.20.10.1569 [DOI] [PubMed] [Google Scholar]
- 30.Walker AF, Schatz DA, Johnson C, Silverstein JH, Rohrs HJ. Disparities in social support systems for youths with type 1 diabetes. Clin Diabetes. 2015;33(2):62-69. doi: 10.2337/diaclin.33.2.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke ABM, Daneman D, Curtis JR, Mahmud FH. Impact of neighbourhood-level inequity on paediatric diabetes care. Diabet Med. 2017;34(6):794-799. doi: 10.1111/dme.13326 [DOI] [PubMed] [Google Scholar]
- 32.Flores G; Committee On Pediatric Research . Technical report—racial and ethnic disparities in the health and health care of children. Pediatrics. 2010;125(4):e979-e1020. doi: 10.1542/peds.2010-0188 [DOI] [PubMed] [Google Scholar]
- 33.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666-668. [PMC free article] [PubMed] [Google Scholar]
- 34.Schwandt A, Hermann JM, Rosenbauer J, et al. ; DPV Initiative . Longitudinal trajectories of metabolic control from childhood to young adulthood in type 1 diabetes from a large German/Austrian registry: a group-based modeling approach. Diabetes Care. 2017;40(3):309-316. doi: 10.2337/dc16-1625 [DOI] [PubMed] [Google Scholar]
- 35.SEARCH Study Group SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25(5):458-471. doi: 10.1016/j.cct.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 36.Hamman RF, Bell RA, Dabelea D, et al. ; SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336-3344. doi: 10.2337/dc14-0574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1-27. [PubMed] [Google Scholar]
- 38.Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2. 2003;(135):1-55. [PubMed] [Google Scholar]
- 39.Mayer-Davis EJ, Bell RA, Dabelea D, et al. ; SEARCH for Diabetes in Youth Study Group . The many faces of diabetes in American youth: type 1 and type 2 diabetes in five race and ethnic populations: the SEARCH for Diabetes in Youth study. Diabetes Care. 2009;32(suppl 2):S99-S101. doi: 10.2337/dc09-S201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones BL, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374-393. doi: 10.1177/0049124101029003005 [DOI] [Google Scholar]
- 41.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109-138. doi: 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 42.Nagin DS, Odgers CL. Group-based trajectory modeling (nearly) two decades later. J Quant Criminol. 2010;26(4):445-453. doi: 10.1007/s10940-010-9113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383-2395. doi: 10.1002/ijc.29981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4(2):139-157. doi: 10.1037/1082-989X.4.2.139 [DOI] [PubMed] [Google Scholar]
- 45.Currie C, Zanotti C, Morgan A, et al. , eds. Social Determinants of Health and Well-Being Among Young People. Health Behaviour in School-aged Children (HBSC) Study: International Report From the 2009-2010 Survey. Copenhagen, Denmark: WHO Regional Office for Europe; 2012. [Google Scholar]
- 46.Secrest AM, Costacou T, Gutelius B, Miller RG, Songer TJ, Orchard TJ. Associations between socioeconomic status and major complications in type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complication (EDC) Study. Ann Epidemiol. 2011;21(5):374-381. doi: 10.1016/j.annepidem.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rewers A, Chase HP, Mackenzie T, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287(19):2511-2518. doi: 10.1001/jama.287.19.2511 [DOI] [PubMed] [Google Scholar]
- 48.Nathan DM; DCCT/EDIC Research Group . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9-16. doi: 10.2337/dc13-2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39(5):686-693. doi: 10.2337/dc15-1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaacks LM, Oza-Frank R, D’Agostino R Jr, et al. Migration status in relation to clinical characteristics and barriers to care among youth with diabetes in the US. J Immigr Minor Health. 2012;14(6):949-958. doi: 10.1007/s10903-012-9617-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardeman RR, Medina EM, Kozhimannil KB. Race vs burden in understanding health equity. JAMA. 2017;317(20):2133. doi: 10.1001/jama.2017.4616 [DOI] [PubMed] [Google Scholar]
- 52.Valenzuela JM, Seid M, Waitzfelder B, et al. ; SEARCH for Diabetes in Youth Study Group . Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164(6):1369-75.e1. doi: 10.1016/j.jpeds.2014.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris MI. Racial and ethnic differences in health care access and health outcomes for adults with type 2 diabetes. Diabetes Care. 2001;24(3):454-459. doi: 10.2337/diacare.24.3.454 [DOI] [PubMed] [Google Scholar]
- 54.Sparud-Lundin C, Öhrn I, Danielson E, Forsander G. Glycaemic control and diabetes care utilization in young adults with type 1 diabetes. Diabet Med. 2008;25(8):968-973. doi: 10.1111/j.1464-5491.2008.02521.x [DOI] [PubMed] [Google Scholar]
- 55.Karatekin C, Ahluwalia R. Effects of adverse childhood experiences, stress, and social support on the health of college students [published online December 5, 2016]. J Interpers Violence. doi: 10.1177/0886260516681880 [DOI] [PubMed] [Google Scholar]
- 56.Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60-e76. doi: 10.2105/AJPH.2015.302903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabin JA, Greenwald AG. The influence of implicit bias on treatment recommendations for 4 common pediatric conditions: pain, urinary tract infection, attention deficit hyperactivity disorder, and asthma. Am J Public Health. 2012;102(5):988-995. doi: 10.2105/AJPH.2011.300621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care. 2008;46(7):678-685. doi: 10.1097/MLR.0b013e3181653d58 [DOI] [PubMed] [Google Scholar]
- 59.Peek ME, Wagner J, Tang H, Baker DC, Chin MH. Self-reported racial discrimination in health care and diabetes outcomes. Med Care. 2011;49(7):618-625. doi: 10.1097/MLR.0b013e318215d925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan AM, Gee GC, Griffith D. The effects of perceived discrimination on diabetes management. J Health Care Poor Underserved. 2008;19(1):149-163. doi: 10.1353/hpu.2008.0005 [DOI] [PubMed] [Google Scholar]
- 61.Reitblat L, Whittemore R, Weinzimer SA, Tamborlane WV, Sadler LS. Life with type 1 diabetes: views of Hispanic adolescents and their clinicians. Diabetes Educ. 2016;42(4):408-417. doi: 10.1177/0145721716647489 [DOI] [PubMed] [Google Scholar]
- 62.Hunter CM. Understanding diabetes and the role of psychology in its prevention and treatment. Am Psychol. 2016;71(7):515-525. doi: 10.1037/a0040344 [DOI] [PubMed] [Google Scholar]
- 63.Jaacks LM, Liu W, Ji L, Mayer-Davis EJ. Type 1 diabetes stigma in China: a call to end the devaluation of individuals living with a manageable chronic disease. Diabetes Res Clin Pract. 2015;107(2):306-307. doi: 10.1016/j.diabres.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 64.Steptoe A, Marmot M. Burden of psychosocial adversity and vulnerability in middle age: associations with biobehavioral risk factors and quality of life. Psychosom Med. 2003;65(6):1029-1037. doi: 10.1097/01.PSY.0000097347.57237.2D [DOI] [PubMed] [Google Scholar]
- 65.Adler NE, Newman K. Inequality in education, income, and occupation exacerbates the gaps between the health “haves” and “have-nots.” In: Bemelmans-Videc M-L, Rist RC, Vedung EO, eds. Carrots, Sticks and Sermons: Policy Instruments and Their Evaluation. Piscataway, NJ: Transaction Publishers; 2002:249-274. [Google Scholar]
- 66.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci. 1998;840(1):33-44. doi: 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- 67.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97(4):1067-1072. doi: 10.1210/jc.2011-1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Selvin E, Sacks DB. Variability in the relationship of hemoglobin A1c and average glucose concentrations: how much does race matter? Ann Intern Med. 2017;167(2):131-132. doi: 10.7326/M17-1231 [DOI] [PubMed] [Google Scholar]
- 69.Bergenstal RM, Gal RL, Connor CG, et al. ; T1D Exchange Racial Differences Study Group . Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. doi: 10.7326/M16-2596 [DOI] [PubMed] [Google Scholar]
- 70.Gail MH, Wieand S, Piantadosi S. Biased estimates of treatment effect in randomized experiments with nonlinear regressions and omitted covariates. Biometrika. 1984;71(3):431-444. doi: 10.1093/biomet/71.3.431 [DOI] [Google Scholar]
- 71.Hauck WW, Neuhaus JM, Kalbfleisch JD, Anderson S. A consequence of omitted covariates when estimating odds ratios. J Clin Epidemiol. 1991;44(1):77-81. doi: 10.1016/0895-4356(91)90203-L [DOI] [PubMed] [Google Scholar]
- 72.Dabelea D, Mayer-Davis EJ, Saydah S, et al. ; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778-1786. doi: 10.1001/jama.2014.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akesson K, Hanberger L, Samuelsson U. The influence of age, gender, insulin dose, BMI, and blood pressure on metabolic control in young patients with type 1 diabetes. Pediatr Diabetes. 2015;16(8):581-586. doi: 10.1111/pedi.12219 [DOI] [PubMed] [Google Scholar]